Genome-wide survey and expression analysis of the OMT gene family in Stephania japonica
- Published
- Accepted
- Received
- Academic Editor
- Vladimir Uversky
- Subject Areas
- Bioinformatics, Genomics, Molecular Biology, Plant Science
- Keywords
- Stephania japonica, O-methyltransferase, Gene family, Genome-wide survey
- Copyright
- © 2025 Bi et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Genome-wide survey and expression analysis of the OMT gene family in Stephania japonica. PeerJ 13:e18600 https://doi.org/10.7717/peerj.18600
Abstract
Background
O-methyltransferase (OMT) is an important rate-limiting enzyme that plays a vital role in synthesizing various key metabolites, such as benzylisoquinoline alkaloids (BIA). Nevertheless, there is a dearth of extensive research on the analysis of the OMT gene family in Stephania japonica, a main source of cepharanthine with an anti-coronavirus effect.
Methods
Two OMT family genes, SjCCoAOMT and SjCOMT, were identified from the high-quality genome of S. japonica during this investigation. Further analysis of SjCCoAOMT and SjCOMT genes involved chromosome distribution, gene structure, phylogenetic relationship, conserved motif, expression profile, quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) experiments an d cis-acting elements analysis.
Results
There are six SjCCoAOMT members and fifty-two SjCOMT members in the genome of S. japonica, which are unevenly distributed on 11 chromosomes. OMTs could be clustered into SjCCoAOMT and SjCOMT subfamilies through phylogenetic relationship, consistent with the conserved motif and gene structure analysis results. The expression profile revealed SjCOMT11 and SjCOMT13 showed specific expression levels mainly in root. SjCOMT21, SjCOMT33 and SjCOMT37 were significantly expressed in the root and slightly expressed in the stem, bud and leaf. SjCOMT15 and SjCOMT45 were not only significantly expressed in root, but also expressed highly in leaf. Significantly enhanced expression of SjCOMT11, SjCOMT13, SjCOMT15, SjCOMT21, SjCOMT33, SjCOMT37, and SjCOMT45 suggested these OMTs are essential for cepharanthine synthesis in the S. japonica roots. Cis-acting element analysis revealed the potential roles of OMTs in S. japonica in growth, development, and resistance to stress. These findings provide insight into understanding the functions and characterization of OMTs from S. japonica and lay a foundation for further revealing the role of the OMT genes in the biosynthesis of cepharanthine.
Introduction
In the plant genome, O-methyltransferase (OMT) serves as a crucial biological regulatory factor governing protein loci methylation. OMT is widely acknowledged to play a decisive role in the synthesis of diverse essential metabolites with strict substrate specificity (Lam et al., 2007). According to the molecular weight, OMTs are usually composed of two major subfamilies, including caffeic acid OMTs (COMT with a molecular weight of 38–43 kD) and caffeoyl-CoA OMTs (CCoAOMT with a molecular weight of 23–27 kD) (Joshi & Chiang, 1998; Ibrahim, Bruneau & Bantignies, 1998). The O-methylation reaction is mainly accomplished by the transfer of methyl from s-adenosyl-L-methionine by OMT to nucleophilic receptors, such as nitrogen, oxygen, sulfur, or carbon (Roje, 2006). The abundant OMTs contribute to the structural and functional diversity of secondary metabolites in plants, which have given rise to a rapid exploration into the function and members of OMT genes, such as grape, Gossypium species and Ophiorrhiza pumila (Hafeez et al., 2021; Shi et al., 2022). The OMT gene family plays an essential role in the biosynthesis of alkaloids in Stephania, such as tetrandrine (Li et al., 2020; Tu et al., 2022). However, the function of OMT gene family in Stephania japonica has not yet been explored.
S. japonica is a sustainable source of cepharanthine, belonging to Menispermaceae (Bailly, 2019). As a benzylisoquinoline alkaloid (BIA), cepharanthine is mainly distributed in the root of S. japonica and is widely used to increase white blood cell levels in cancer patients after chemotherapy. Since the outbreak of the novel coronavirus disease 2019 (COVID-19), cepharanthine has attracted worldwide attention because of its ability to inhibit novel coronavirus mutations and different types of coronaviruses (Fan et al., 2022). In addition, cepharanthine has been proven to possess various biological activities, such as anti-tumour (Rattanawong et al., 2017), anti-inflammatory (Nagano et al., 2003) and anti-parasitic (Desgrouas et al., 2014). These important biological activities are expected to be further developed and applied by emerging biotechnology (Chen et al., 2022, 2023b). As the medicinal value of cepharanthine is widely recognized, the demand for it is gradually increasing. However, due to the unclear synthetic pathway and the complex chemical structure, cepharanthine is mainly extracted from S. japonica. Relying on plant resources alone makes it difficult to meet the growing market demand and increases the risk that S. japonica will become an endangered species for overcollection. Therefore, revealing the pathways and key genes for the biosynthesis of cepharanthine is beneficial for promoting resource conservation.
Gene sequencing at the chromosome level and transcriptome analysis at the single cell level provide insight into the biosynthetic pathways of key secondary metabolites in medicinal plants (Liao et al., 2022; Sun et al., 2022; Chen et al., 2023a). The biosynthesis of cepharanthine begins with the condensation of dopamine and 4-hydroxyphenyl acetaldehyde (4HPAA) via norcoclaurine synthase (NCS) to yield (S)-norcoclaurine (Samanani, Liscombe & Facchini, 2004; Lee & Facchini, 2010). After that, 6-OMT and coclaurine N-methyltransferase are involved in catalyzing the conversion of (S)-N-methycoclaurine, which is an important intermediate for producing different BIAs (Liu et al., 2017; Payne, Valentic & Smolke, 2021). Although the synthetic pathway from (S)-N-methycoclaurine to cepharanthine has not been elucidated, it is undeniable that OMT genes are crucial in the synthesis of cepharanthine.
The main objective of this research was to analyze and characterize the function of the OMT genes family, aiming to enhance our comprehension of the function of OMTs in S. japonica and to offer a reference for elucidating the synthesis of cepharanthine.
Materials and Methods
Plant materials
The S. japonica plants were cultivated and harvested in Wuhan, China and the ages of S. japonica plants are 1 year. The stems, leaves, roots, and shoots of S. japonica were collected for transcriptome sequencing and quantitative real-time polymerase chain reaction experiment.
Identification and sequence analysis of OMT gene family members in S. japonica
The genome assemblies and annotations were obtained from the National Center for Biotechnology Information (NCBI) GenBank accession number PRJNA888087 (Leng et al., 2024). The OMT genes sequences of the S. japonica were acquired from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) with the accession numbers of OR240135 (SjCOMT) and OR240192 (SjCCoAOMT). In order to obtain the protein sequence of the candidate OMT gene, the amino acid sequences of 24 OMT members in Arabidopsis thaliana (https://www.arabidopsis.org/) were used as the reference for Blastp search (e-value < 1e−5).
The methyltransferase domains, including COMT (PPF00891) and CCoAOMT (PF01596), were obtained from Pfam (http://pfam.xfam.org) and were used as the query in Hidden Markov Model (HMM) searches using TBtools with the cutoff at 0.05. Further, all putative proteins were sent to NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for blast-p. Proteins with non-significant similarity were sent to to Softberry (http://linux1.softberry.com/) for correction and proteins without target domain were manually removed. Finally, the remaining candidate genes containing the complete OMT domain were regarded as OMT genes in S. japonica and renamed according to their relative location on chromosomes.
ExPASy (https://web.expasy.org/compute_pi/) was used to acquire the physical and chemical properties of OMT protein, such as isoelectric points (PI) and molecular weight (MW). WoLF PSORT (https://wolfpsort.hgc.jp/) was utilized to predict subcellular localization of OMT protein (Horton et al., 2007). Finally, the chromosomal location image of OMT genes was visualized using TBtools (Chen et al., 2020).
Multiple sequence alignment and phylogenetic analysis
The OMT protein sequences of A. thaliana and Vitis vinifera were obtained from the EnsemblPlants database (http://plants.ensembl.org/index.html) and reference (Lu et al., 2022), respectively. Multiple sequence alignments (MSA) of these protein sequences were performed using the MEGA11. With 100 bootstrap replications, the phylogenetic tree of COMT and CCoAOMT in S. japonica was constructed using the MEGA11 and Maximum-likelihood (ML) methods.
Investigation of gene structure and conserved motif
The MEME online tool (https://meme-suite.org/meme/tools/meme) was employed to identify the conserved motifs of OMT proteins in S. japonica, with the following parameters: maximum number of motifs = 6; optimum motif length= 6–50 residues (Bailey et al., 2006). By analyzing the S. japonica genome sequences, the exon-intron structure of the OMT members was investigated, and TBtools was used to visualize the gene structure.
RNA extraction and qRT-PCR analysis
RT-qPCR was performed to confirm the expression levels of selected OMT genes (SjCOMT11, SjCOMT13, SjCOMT21, SjCOMT37, SjCOMT33, SjCOMT15, and SjCOMT45). The Plant Total RNA Isolation Kit (FOREGENE, Chengdu, China) was used to extract total RNA from different samples following the instruction. The cDNA was synthesized from total RNA using the RT Easy TM II (FOREGENE, Chengdu, China). Table S1 contained the primer sequences for qRT-PCR analysis.
The ChamQ Universal SYBR qPCR Master Mix (FOREGENE, Chengdu, China) was used to conduct qRT-PCR reactions. The total amplification method was 10 μL, which consist of 2 × SYBR Master Mix (5 μL), ddH2O (3.8 μL), forward and reverse primer (0.4 μL, 10 mM), cDNA (0.4 μL, 2.5 ng/μL). The amplification process consisted of the pre-denaturation at 95 °C for 180 S, denaturation at 95 °C for 10 S, and renaturation at 55 °C for 30 S. A total of 40 cycles were executed. The melting curve analysis was used to verify the specificity of the amplicon for each primer pair. The comparative Ct (2−ΔΔCt) method (Livak & Schmittgen, 2001) was used to calculate transcripts amounts. Normalization was performed using GAPDH as the internal reference (Jin et al., 2019). Three biological replicates per sample were used for the analysis. ANOVA analysis was performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). P < 0.05 was considered statistically significant.
Expression analysis of OMTs in different tissues
The expression profiles of SjCCoAOMTs and SjCOMTs in five tissues (root, leaf, flowers, bud and stem) were analyzed to verify expression levels using the RNA-seq data. The log2 (FPKM+1) values were used for normalization and the expression heat map was generated by TBtools.
Analysis of cis-acting elements of OMTs
The 1,400 bp upstream sequences of OMT genes in S. japonica genome were extracted as promoter sequences for prediction. The cis-acting elements related to plant growth and development, hormone response and biotic stress, abiotic stresses and TF binding sites were identified using PlantCare (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). The cis-acting elements results were further visualized by TBtools.
Results
Identification and classification of OMTs in S. japonica
According to the sequence comparison, six CCoAOMT genes and fifty-two COMT genes were identified from the genome data of S. japonica after a series of screens (including BLASTP searching, Pfam analyses and eliminating short sequences). These OMT genes were named SjCCoAOMT1 to SjCCoAOMT6 and SjCOMT1 to SjCOMT52. The characteristics of SjCCoAOMTs and SjCOMTs were further analyzed and summarized in Table S2, including molecular weight (MW), cDNA sequence length, chromosome location, deduced protein sequence length, isoelectric point (pI), and subcellular localization. The length of encoded protein sequences in the SjCCoAOMT genes showed significant variation, ranging from 684 (SjCCoAOMT2) to 852 bp (SjCCoAOMT1) and 227 to 283 amino acids. As for the SjCOMT genes, the length of encoded protein sequences ranged from 256 to 393 amino acids, with the cDNA length ranging from 771 (SjCOMT25) to 1,182 bp (SjCOMT31). The MW of SjCCoAOMT proteins ranged from 25,522.7 (SjCCoAOMT2) to 31,330.55 Da (SjCCoAOMT1), and SjCOMT proteins ranged from 28,019.58 (SjCOMT25) to 43,948.65 Da (SjCOMT48). Most of the pI of SjCCoAOMT had values from 4.85 (SjCCoAOMT4) to 6.00 (SjCCoAOMT2), and only one alkaline protein exhibited a pI value higher than 8, namely SjCCoAOMT1 (8.96). As for SjCOMT, all pI ranged from 4.88 (SjCOMT33) to 6.63 (SjCOMT31). The subcellular localization predictions revealed that only SjCCoAOMT1 was situated in the chloroplast, and other SjCCoAOMTs were located in the cytoplasm region. As for SjCOMTs, SjCOMT4, SjCOMT10, SjCOMT17, SjCOMT31, SjCOMT36, and SjCOMT37 were located in the chloroplast; SjCOMT39 was located in the endoplasmic reticulum; SjCOMT13, SjCOMT23, SjCOMT28, SjCOMT30, SjCOMT40, and SjCOMT43 were located in the cytoskeleton. The remaining SjCOMTs were all located in the cytoplasm region.
Phylogenetic, orthologues analysis and chromosome distribution of OMTs in S. japonica
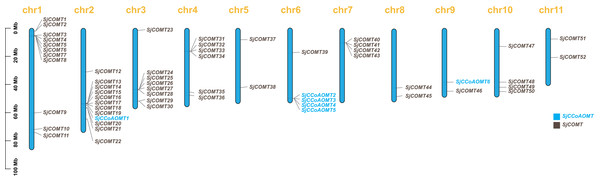
The physical localization map (Fig. 1) showed the distribution of SjCCoAOMT genes and SjCOMT genes on 11 chromosomes of S. japonica. It was obvious that the distribution of OMT genes was not homogeneous. Notably, four SjCCoAOMTs were concentrated on chromosome 6, while SjCCoAOMT1 and SjCCoAOMT6 were distributed separately on chromosome 2 and chromosome 9, respectively. SjCOMT genes were widely distributed on all chromosomes. Among them, 46% of SjCOMT genes were concentrated on chromosome 1 and chromosome 2, whereas chromosome 6 and chromosome 9 each had only one SjCOMT gene. Several duplicate gene pairs of SjCOMT genes could be found in chromosomes 1, 2, 3, 4 and 7.
Figure 1: Chromosomal localization of OMTs in S. japonica.
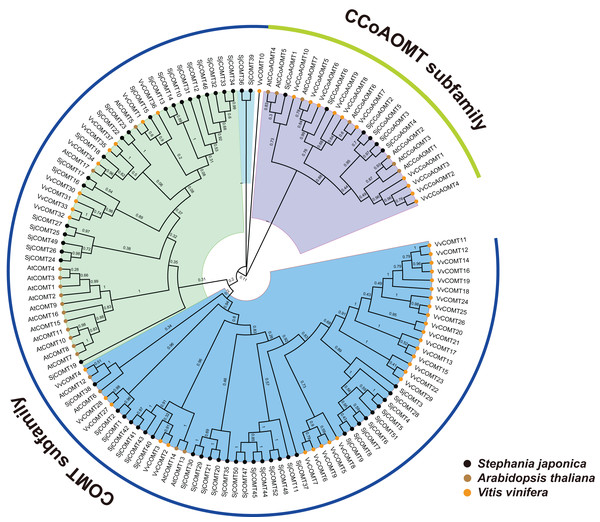
The scale on the left was based on megabytes (Mb). The number of chromosomes was indicated at the top of each column.In order to gain a comprehensive understanding of the evolutionary relationship of SjCCoAOMT genes and SjCOMT genes, the phylogenetic tree was created using the alignment of the full-length protein sequences of V. vinifera (10 CCoAOMT and 37 COMT), A. thaliana (seven CCoAOMT and 17 COMT), and S. japonica (six CCoAOMT and 52 COMT). It was found that OMT members were divided into two major subfamilies, CCoAOMT and COMT, based on their sequence similarity and topology structures (Fig. 2). Based on the sequence similarity and topology structures of the phylogenetic tree, OMT proteins were classed into two major subfamilies, One subfamily contained CCoAOMT members from the three species, while the other subfamily contained COMT members from the three species (Fig. 2). This finding suggested that the OMT genes remained highly conserved throughout plant evolution, spanning various species.
Figure 2: Phylogenetic relationship of OMTs from S. japonica, Arabidopsis and Vitis vinifera.
Subclades were marked by different backgrounds. The numbers beside the branches represent bootstrap support values from 100 replications.Orthologous OMT genes in A.thaliana, S. japonica and V. vinifera were identified through orthofinder. Finally, 26 of orthologous genes from A.thaliana and S. japonica, 27 from A.thaliana and V. vinifera, and 34 from S. japonica and V. vinifera were identified. The COMT genes had 5, 10 and 11 of orthologous genes in A.thaliana, S. japonica and V. vinifera. The CCoAOMT genes had seven, six and nine pairs of orthologous genes in A.thaliana, S. japonica and V. vinifera.
Gene structure and protein motif analysis of OMTs in S. japonica
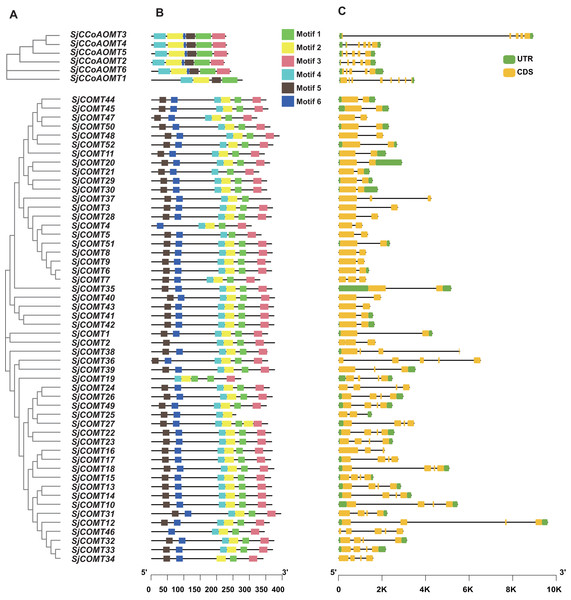
Phylogenetic, MEME online tool and gene structure analysis were used to further illustrate the structural diversity of the SjCCoAOMT and SjCOMT in S. japonica, respectively (Fig. 3A). Six conserved motifs (motif 1–motif 6) were predicted, which exhibited specificity to individual groups (Fig. 3B). Different OMT groups exhibited varying compositions of structural motifs, whereas the same group contained similar motifs. The MEME results revealed that 85% of SjCOMT proteins contained six motifs, while SjCOMT proteins including SjCOMT2, SjCOMT4, SjCOMT5, SjCOMT19, SjCOMT21, SjCOMT25, SjCOMT37, and SjCOMT46 have four or five motifs. Notably, there were two motif 1 existed in SjCOMT19. Motif 1, motif 2, motif 4, and motif 5 were distributed in SjCCoAOMT1 to SjCCoAOMT6, which indicated that these motifs might play an essential role in the function regulation. While motif 3 and motif 6 were only absent in the SjCCoAOMT1.
Figure 3: Phylogenetic tree, conserved motif, and gene structure of OMTs in S. japonica.
(A) Phylogenetic tree constructed by MEGA analysis. (B) Conserved motifs identified by MEME analysis. Each motif was represented with a different color. The protein length could be estimated using the scale at the bottom. (C) Exon/intron structures of OMTs.OMT genes that were closely associated within the same phylogenetic cluster exhibited identical exon-intron arrangements, and those members belonging to the same group were more similar in their number and length of exons and introns (Fig. 3C). The number of exons in SjCCoAOMT2, SjCCoAOMT3, SjCCoAOMT4, SjCCoAOMT5, and SjCCoAOMT6 were mostly five or six, only SjCCoAOMT1 possessed eight exons. While the number of exons in SjCOMTs varied from two to five. The size of the exons and the length of the intron varied widely. These findings demonstrated that SjCCoAOMT and SjCOMT possess different structural patterns based on their features.
Expression profiles of OMTs in S. japonica
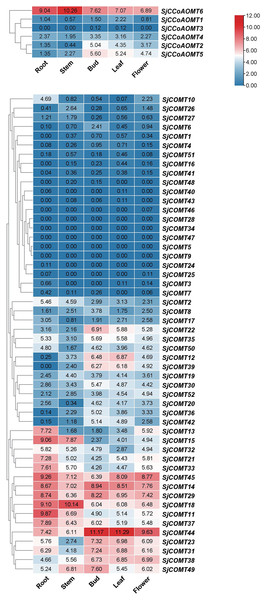
Investigating the expression of SjCCoAOMT and SjCOMT in S. japonica is essential to elucidate their potential function. We examined the expression patterns of SjCCoAOMTs and SjCOMTs in the root, bud, flower, leaf, and stem based on RNA-seq data which have been deposited under the National Center for Biotechnology Information (NCBI) GenBank accession number PRJNA888087 (Fig. 4). According to the clustering analysis, there was significant variability in the heat map of SjCCoAOMT and SjCOMT members in five tissues. SjCCoAOMT6 was highly expressed in five tissues, but SjCCoAOMT1, 3 and 4 were barely expressed in all tissues. In buds, only SjCCoAOMT5 and 2 were moderately expressed. Interestingly. As for SjCOMT members, there were 12 members (SjCOMT13, SjCOMT15, SjCOMT21, SjCOMT33, SjCOMT45, SjCOMT14, SjCOMT29, SjCOMT18, SjCOMT11, SjCOMT37, SjCOMT44, SjCOMT31) highly expressed in root. SjCOMT15, SjCOMT45, SjCOMT14, SjCOMT29, SjCOMT18, SjCOMT11, SjCOMT37, SjCOMT44, and SjCOMT49 were significantly expressed in the stem. Furthermore, 22 SjCOMT members were barely expressed in the root, bud, flower, leaf, and stem, such as SjCOMT46, SjCOMT28 and SjCOMT34. While SjCOMT45, SjCOMT14, SjCOMT29, SjCOMT18 and SjCOMT44 exhibited significant expression in five tissues. SjCOMT22, SjCOMT12 and SjCOMT39 were only highly expressed in bud and leaf.
Figure 4: Subclades were marked by different backgrounds.
The numbers beside the branches represent bootstrap support values from 1,000 replications. The bars on the right represent log2 (FPKM + 1) values, and different colors indicated different levels of transcripts. Red indicated relatively high transcripts amounts and blue indicated relatively low transcripts amounts.Gene expression analysis of OMTs in S. japonica
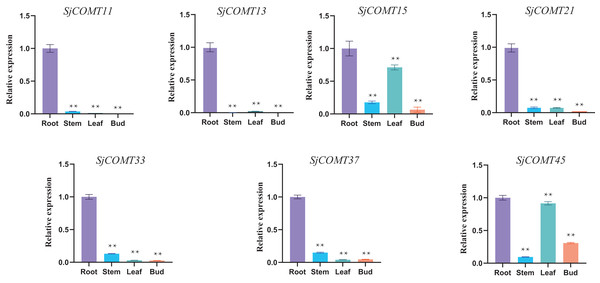
Due to the high content of cepharanthine in the root of S. japonica, seven OMT members specifically expressed in the root were selected for qRT-PCR validation according to their expression in the transcriptome analysis (Table S3, Fig. 5). SjCOMT11 and SjCOMT13 showed specific expression levels mainly in root. SjCOMT21, SjCOMT33 and SjCOMT37 were significantly expressed in the root and slightly expressed in the stem, bud and leaf. SjCOMT15 and SjCOMT45 were not only significantly expressed in root, but also expressed highly in leaf. The findings were generally in line with the transcriptome information in the root.
Figure 5: Relative expression of SjCOMTs in different tissues.
Transcript levels were analyzed using quantitative real-time RT-PCR. GAPDH was used as the reference gene. qRT-PCR was performed in triplicates for each independent biological replicate. Transcript levels in roots were arbitrarily set to 1, and levels in other tissues were given relative to this. One-way ANOVA was performed using GraphPad Prism v9.0.1 software. Double asterisks indicate that the value is highly significantly different from that of the Root (**, P < 0.01). Error bars represent the standard deviations for three biological replicates.Analysis of cis-acting elements of OMTs in S. japonica
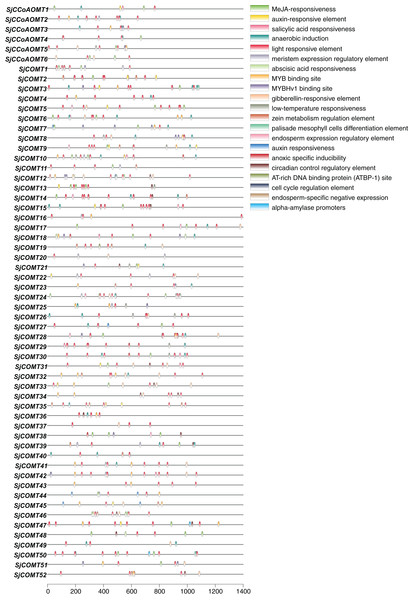
The promoter, a key structure affecting the binding affinity of RNA polymerase, can influence the level of gene expression (Liu et al., 2019). Analyzing the cis-acting elements in the promoter sequence was an important way to understand the regulatory functions of OMT genes. Thus, the cis-acting elements in the promoters (1,400 bp upstream from the transcription start sites) of OMT members in S. japonica were further analyzed (Fig. 6). The cis-acting elements could be categorized into four primary groups based on their function: plant growth and development, phytohormone responsive, abiotic and biotic stress and TF binding sites. Among them, light-responsive elements, including ATCT-motif, Box4, CAG-motif, chs-CMA2a, GA-motif, GATA-motif, G-box, GC-motif, GTGGC-motif, I-box, LAMP-element, GT1-motif, Sp1, TCCC-motif, TCT-motif and ATC-motif, were the most abundant elements and distributed in most members, which was consistent with the distribution among the cis-acting elements in blueberry (Liu et al., 2021). These findings suggest these identified motifs might be important for light mediated regulation in S. japonica. Many cis-acting elements related to phytohormone responsive, such as CGTCA-motif and TGACG-motif related to MeJA-responsiveness, ABRE related to abscisic acid responsiveness elements, were present in large numbers in OMT genes. Furthermore, ARE, a cis-acting element necessary for anaerobic induction, was also found. CCAAT-box and MBS were the binding sites of the MYBHv1 and MYB transcription factors. Each CCoAOMT possessed 8–12 cis-acting elements. As for COMT, the number of cis-acting elements in each gene ranged from 5 (SjCOMT20, SjCOMT37) to 23 (SjCOMT24). In CCoAOMT, light-responsive elements were mainly distributed in SjCCoAOMT2 and SjCCoAOMT5, and MeJA-responsiveness was abundant in SjCCoAOMT1 and SjCCoAOMT6. Among them, SjCOMT15 contained the most abundant light-responsive elements (9), while only SjCOMT38 did not have light-responsive elements. In summary, OMT genes were involved in the growth, development and resistance to stress in S. japonica.
Figure 6: Predicted cis-acting elements in the promoters of OMT genes in S. japonica (1,400 bp upstream from the transcription start sites).
Discussion
Due to involvement in the synthesis of many vital secondary metabolites, such as alkaloids, flavonoids and phenylpropanoids, OMT genes play an important role in plant adaptation and response to stresses (Noel et al., 2003; He et al., 2017). Until now, systematic and comprehensive genome-wide analyses of the OMT gene family have been summarized in a variety of plants. However, a systematic study of the OMT gene family has not been conducted in the cepharanthine-producing species. As cepharanthine possesses an anti-coronavirus activity (Fan et al., 2022) and its synthesis pathway is unclear, this study aimed to identify the members of the OMT gene in S. japonica, which might lay the foundation for elucidating the OMT members involved in cepharanthine biosynthesis.
In this work, sequence similarity searches of S. japonica genomes enabled the identification of 58 OMTs that were unevenly distributed on the chromosomes in S. japonica. The expansion of the OMT gene family appears to be driven largely by gene duplication, which is widely regarded as a significant driver of gene evolution (Qiao et al., 2019). The OMT genes in Grapes (Lu et al., 2022) and Arabidopsis (Kim et al., 2005) present similar findings where OMT genes were duplication in tandem or segmental duplication.
According to phylogenetic analysis, caffeoyl-CoA OMT (CCoAOMT) and caffeic acid OMT (COMT) were the two major subfamilies of OMTs in S. japonica. The reliability of group classification could be effectively demonstrated by combining phylogenetic analysis of OMT proteins with the similarity of gene structures and conserved motifs within the same subclade. OMT genes with similar gene structures and motif compositions were always clustered in the same class.
Exploring the expression profiles of SjCCoAOMT, and SjCOMT in five tissues is necessary to provide insight into the diverse OMTs that perform different functions on specific secondary metabolites (Ibrahim, Bruneau & Bantignies, 1998). By examining the gene expression data, we can better elucidate the roles of the genes in different tissues. And through this method, we can infer the genes most likely to be associated with the biosynthesis of S. japonica. We noticed that thirteen OMTs were highly expressed in roots, such as SjCCoAOMT6, SjCOMT11, SjCOMT45, and SjCOMT18, which might be related to the synthesis of cepharanthine in the root (Semwal et al., 2010). qRT-PCR data also supported the high expression levels of SjCOMT11, SjCOMT13, SjCOMT15, SjCOMT21, SjCOMT33, SjCOMT37 and SjCOMT45 in root.
Motif analysis indicated that almost all SjCCoAOMTs and SjCOMTs contained six motifs, but there were still some members without specific motifs. For example, motif 6 was not detected in SjCCoAOMT1, motif 5 and motif 6 were not observed in SjCOMT19, while SjCOMT34 did not contain motif 4. Although most motifs in OMT family genes were highly conserved, differences in some motifs might result in different functions in S. japonica, which need to be further verified. The cis-acting elements in the promoters play a central role in regulating gene transcription as the binding site between OMT genes and various proteins. Analyzing the cis-acting elements in S. japonica found that a large number of light-response elements and hormone-response elements existed in the promoters of OMT genes, which might be related to the growth and development of S. japonica and the synthesis of cepharanthine (Wong et al., 2017).
Overall, this comprehensive analysis of OMT family genes from S. japonica provided insights into the characteristics of SjOMT genes and may improve our understanding of the mechanisms regulating cepharanthine biosynthesis in S. japonica.
Conclusions
In summary, we identified and characterized fifty-eight OMTs in S. japonica. Comprehensive study of phylogenetic relationship, conserved motifs, and gene structures allowed for a clear classification and understanding of these OMTs. The tissue preference of OMT genes was identified by expression profile, indicating functional divergence of SjCCoAOMTs and SjCOMTs in different tissues. Several SjCOMTs genes were highly expressed in the root according to qRT-PCR, which might be related to the synthesis of cepharanthine in the root. Cis-acting elements analysis contributed to understanding the expression and regulation of OMT genes in S. japonica. These results provide a reference for revealing the OMT genes involved in the BIA synthesis pathway in S. japonica.
Supplemental Information
Protein sequence information of the OMT gene family in S. japonica.
Details of 58 OMT genes, including their gene ID, chromosome position, protein length, molecular weight, isoelectric point, and predicted subcellular location, were listed.
The qRT-PCR data of seven SjCOMT genes.
The expression level of each gene in the root was normalized to 1.