Serum metabolite profiles of thyroid autoimmunity patients in early pregnancy
- Published
- Accepted
- Received
- Academic Editor
- Daniela Foti
- Subject Areas
- Biochemistry, Internal Medicine, Public Health, Women’s Health, Metabolic Sciences
- Keywords
- Thyroid disease, Autoimmunity, Pregnancy, Serum, Metabolomics
- Copyright
- © 2024 Chen et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Serum metabolite profiles of thyroid autoimmunity patients in early pregnancy. PeerJ 12:e18534 https://doi.org/10.7717/peerj.18534
Abstract
Background
Research on serum metabolite profiles in thyroid autoimmunity (TAI) patients during early pregnancy is currently limited.
Aim & Methods
The current study aimed to identify differential serum metabolites and assess the relationship between pregnancy outcomes and metabolic abnormalities in individuals with TAI. This research included 26 pregnant women with TAI and 30 healthy controls (HC). We employed a liquid chromatograph mass spectrometer (LC-MS) to analyze changes between the two groups.
Results
Newborns in the TAI patients had lower birth weights than those in the control group (P = 0.007). We identified 92 differential metabolites (including 50 upregulated and 42 downregulated) belonging to amino acids, fatty acyls, glycerophosphocholines, steroid and other categories and four significantly enrichment Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways including taurine and hypotaurine metabolism, citrate cycle (TCA cycle), glyoxylate and dicarboxylate metabolism and 2-oxocarboxylic acid metabolism. We further identified 15 characteristic metabolites (6-Methylquinoline, D-erythrose 4-phosphate, 4-Hydroxyisoleucine, phosphatidylcholine (PC)(16:2e/16:0), N3,N4-Dimethyl-L-arginine, N-desmethyltramadol, 3-Methoxybenzaldehyde, sphingomyelin (SM)(d14:3/28:2), gamma-Glutamylleucine, NSI-189, 3-(1-cyano-1,2-dihydroisoquinolin-2-yl)-3-oxopropyl propionate, lysophosphatidylinositol (LPI) 16:0, cis-Aconitic acid, polyamide (PA)(18:1/18:2) and fatty acyl esters of hydroxy fatty acid (FAHFA)(17:0/18:0)) using least absolute shrinkage and selection operator (LASSO) regression. Correlation analyses revealed that 6-Methylquinoline, D-erythrose 4-phosphate, gamma-Glutamylleucine, and LPI 16:0 exhibited a positive correlation with anemia before delivery, while 3-(1-cyano-1,2-dihydroisoquinolin-2-yl)-3-oxopropyl propionate had a negative correlation. LPI 16:0 displayed a positive correlation with uric acid (UA) during both middle and late pregnancy, whereas 3-Methoxybenzaldehyde exhibited a negative correlation with UA in late pregnancy. Cis-Aconitic acid showed a positive correlation with fasting blood glucose (FBG) in middle pregnancy. Conversely, 6-Methylquinoline and 4-Hydroxyisoleucine had a negative correlation with birth weight. Thyroid autoantibodies were found to be associated with 14 metabolites identified using LASSO, with the exception of PA (18:1/18:2).
Conclusions
Our findings provide new evidence supporting the early screening of serum metabolites and their potential for predicting adverse pregnancy outcomes in women with TAI.
Background
Thyroid autoimmunity (TAI) refers to the presence of thyroid autoantibodies in the bloodstream, with thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) being the most prevalent among these autoantibodies. Women of reproductive age exhibit a considerably high prevalence of thyroid autoantibodies, with TPOAb showing a positive rate of approximately 5% to 14%, and TgAb having a positive rate of about 3% to 18% (De Leo & Pearce, 2018). While thyroid antibodies, including TPOAb and TgAb, can cross the placental barrier without affecting the fetus’s thyroid function, numerous studies have found an association between TAI and various adverse pregnancy outcomes. Women with TAI are at a higher risk of infertility, miscarriage, premature delivery, and postpartum thyroid dysfunction (Muller & Berghout, 2003). While the exact cause is still unclear, current research suggests that TAI results from interactions between epigenetics, genetic susceptibility factors, and various environmental factors. The metabolic changes in early pregnancy among TAI women and their potential effects on both the women and their offspring are currently unknown. Therefore, further analysis of serum metabolomics in TAI patients during early pregnancy is required.
The serum metabolome is the omics of the identification and quantification of small molecule metabolites, containing a variety of biomarkers and pathogens of various diseases. Metabolites reflect the metabolic activity of tissues and can affect clinical phenotypes. In recent years, serum metabolomics has shown promise in the preliminary diagnosis and prognosis of autoimmune disorders like myasthenia gravis (MG) (Blackmore et al., 2019), systemic lupus erythematosus (SLE) (Ouyang et al., 2011) and autoimmune thyroid disease (AITD) (Liu et al., 2020) , as well as various pregnancy-related conditions, including obesity (Bandres-Meriz et al., 2023), gestational diabetes mellitus (GDM) (Liu et al., 2021) and preeclampsia (Knapen et al., 1998). So far, there have been no systematic serum metabolite profiles to identify potential biomarkers for distinguishing the underlying pathophysiology of TAI patients from healthy pregnant women.
In the present study, we recruited 56 participants including healthy women and TAI patients in early pregnancy to identify diagnostic biomarkers using LC-MS. Additionally, LASSO regression was employed to identify possible biomarkers that might distinguish TAI patients from healthy pregnant women. Pathway enrichment analysis was also conducted to explore the altered pathways in TAI patients. Our goal was to identify biomarkers associated with pregnant women with TAI and investigate potential pathogenesis and the risk of adverse pregnancy outcomes.
Materials and Methods
Study design and participants
56 naturally pregnant women who were matched for age and body mass index (BMI) within a same geographical region, including 26 TAI patients and 30 healthy controls in early pregnancy, were consecutively recruited between September 2018 and December 2019 from the Obstetric Clinic in the First Hospital of China Medical University. The participants’ characteristics and baseline data are presented in Table 1. Participants classified as healthy controls were defined as follows: in the early pregnancy (7th to 13th week), normal serum levels of free T3 (FT3), free T4 (FT4), and thyroid-stimulating hormone (TSH), negative TPOAb and TgAb. Twenty-six pregnant women in the same early pregnancy with normal serum FT3, FT4 and TSH, but with TgAb and/or TPOAb higher than the normal range, were taken as the TAI group of this study. Based on the following criteria, exclusions were made: age below 20 or above 35 years; thyroid dysfunction (abnormal FT3, FT4 and TSH) and gestational age exceeding 13 weeks; pre-pregnancy BMI ≥ 28.0 kg/m2 or ≤18.0 kg/m2; any gastrointestinal diseases or chronic illness, such as gastric and duodenal ulcer, chronic gastritis, gastric and pancreatic cancer, diabetes, other autoimmune diseases, heart disease, renal or hepatic impairment, bleeding disorders, rheumatic diseases; smoking; alcohol intake; taking antibiotic or probiotics within 3 months. The study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University (No. 2018-22-3). We fully explained the purpose and process of the study to each participant and obtained written informed consents with their signature.
| TAI (n = 26) | HC (n = 30) | P value | |
|---|---|---|---|
| BMI (kg/m2, χ ± s) | 21.67 ± 2.72 | 21.22 ± 2.39 | 0.803 |
| Age (years, χ ± s) | 29.48 ± 3.38 | 30.21 ± 2.29 | 0.317 |
| FT3 (pmol/L, χ ± s) | 4.76 ± 0.62 | 5.01 ± 0.94 | 0.265 |
| FT4 (pmol/L, χ ± s) | 17.33 ± 2.13 | 16.41 ± 2.29 | 0.128 |
| TSH (mIU/L, M, IQR) | 1.89 (1.17–2.86) | 1.57 (0.89–2.26) | 0.175 |
| TPOAb (IU/ml, M, IQR) | 230.65 (48.24–360.95) | 12.54 (8.72–15.72) | <0.001 |
| TgAb (IU/ml, M, IQR) | 372.10 (115.84–528.80) | 10.76 (10.00–13.15) | <0.001 |
| FBG (mmol/L, χ ± s) | 5.01 ± 0.46 | 4.87 ± 0.34 | 0.171 |
| UA (mmol/L, χ ± s) | 229.21 ± 46.61 | 207.67 ± 35.13 | 0.678 |
| TG (mmol/L, χ ± s) | 1.32 ± 0.70 | 1.19 ± 0.51 | 0.416 |
| TC (mmol/L, χ ± s) | 4.19 ± 0.63 | 4.21 ± 0.59 | 0.871 |
| HDL-C (mmol/L, χ ± s) | 1.71 ± 0.35 | 1.75 ± 0.37 | 0.651 |
| LDL-C (mmol/L, χ ± s) | 2.33 ± 0.67 | 2.42 ± 0.51 | 0.628 |
| Anemia before delivery (n)* | 3 | 0 | 0.094 |
| Gestational age at delivery (weeks, χ ± s) | 39.75 ± 1.10 | 38.73 ± 4.50 | 0.266 |
| Normal delivery (n)* | 12 | 13 | 0.729 |
| Birth weight (g, χ ± s) | 3240.38 ± 464.18 | 3584.33 ± 448.23 | 0.007 |
Notes:
The means and median of the two groups were compared with the Student’s t test and Mann–Whitney U test respectively.
Using the spectral bins count from the untargeted analysis of the MetSizeR package (Nyamundanda, Brennan & Gormley, 2010; Nyamundanda et al., 2013), a false discovery rate (FDR) of 0.05, and a PPCA model, we calculated a necessary minimum group size of n = 17 per group (Fig. S1).
Measurement of thyroid parameters
After all participants fasted for more than 8 h, blood was collected from 7.30 am to 9.30 am the following day. A storage temperature of −80 °C was used for all serum samples. Serum TgAb, TPOAb, FT3, FT4 and TSH were determined by electrochemiluminescence immunoassay (Roche Diagnostics Ltd., Rotkreuz, Switzerland). The ranges of normal value of thyroid function were: TgAb: 0–115 IU/mL; TPOAb: 0–34 IU/mL; FT3: 3.10–6.80 pmol/L; FT4: 12.00–22.00 pmol/L; TSH: 0.27–4.20 mIU/L.
Chemicals and reagents
Methanol, formic acid, ammonium acetate and ultra-pure water were purchased from Fisher Scientific (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The purity of the other chemicals was above 99.5% and they were of analytical grade.
Chromatography and mass spectrometry conditions
Chromatographic detection was performed on a chromatograph Vanquish UHPLC (Thermo Fisher Scientific, Inc.). A total of 4 µL aliquot of each sample was injected into an Hypesil Gold column (C18) analytical column (100 × 2.1 mm, 1.9 µm; Thermo Fisher Scientific, Inc.) in a random sequence. The column temperature was kept at 30 °C and flow rate was 0.30 mL/min. The positive ion mobile phase is 0.1% formic acid (A) and methanol (B), and the negative ion mobile phase was 5 mM ammonium acetate (pH 9.0, A) and methanol (B). The gradient elution conditions for serum samples were 2% B at 0–1.5 min, 100% B at 1.6–14 min, and 2% B at 14.1–17 min. Take equal volume samples from each serum sample and mix them as quality control (QC) samples. QC samples shall be added before, during, and after the analysis process, and injected once every 10 samples in order to ensure that the systems were stable and repeatable.
Mass spectrometry was performed on mass spectrometer Q ExactiveTM HF-X (Thermo Fisher Scientific, Inc.). The electrospray capillary voltage was set at 3.2 kV in the electrospray source settings. The mass range was chosen to 100–1500 m/z (mass-to-charge ratio). The capillary temperature was set at 320 °C, with a sheath gas flow of 40 arb and an auxiliary gas flow of 10 arb were fixed.
Differential metabolites analysis
After the on-line detection, input the data file to the CD finder, preliminarily screen the retention duration, mass-to-charge ratio and other characteristics, and align the different peaks according to the consistent standard. Next, set a 5 ppm mass deviation, three signal-to-noise ratio, 30% signal strength deviation, 100,000 minimum signal, and other standards to extract peaks and quantify their areas, and integrate the target ions. Molecular ion peak and ion fragment peak were used to determine the molecular formula, and the results were compared to the database. The quantitative outcomes were standardized after the background ions from the blank sample were eliminated. Eventually, the results of the identification and quantitative data were acquired. Because the total amount of metabolite ion data was large, and it had both positive and negative charges, we used the positive (POS) ion mode and negative (NEG) ion mode to present the outcomes together.
Information analysis
First, mass spectrometry data were collected using the mass spectrometer Q ExactiveTM HF-X. The source file (. Raw) obtained by mass spectrometry was imported into compound discoverer 3.1 software for data processing and searching to determine the qualitative and quantitative results of metabolites, and then quality control was carried out to clarify the dependability and accuracy of the result data. Metabolomics data is characterized by a high degree of variable correlation and dimensionality. As a result, we employed multivariate statistical analysis to reduce the dimensionality of the multivariate data while maximizing preservation. Screening differential metabolites chiefly refers to three parameters: VIP, FC, and P values. VIP refers to the variable importance of the first major component of the supervised partial least squares-discriminant analysis (PLS-DA) model in prediction. VIP value stands for the contribution of metabolites to sample categorization. The ratio of the average value of all metabolites in all biological replicates in the comparison group is known as the differential fold change, or FC. VIP > 1.0, FC > 1.2 or FC < 0.833 and P < 0.05 between two groups was deemed statistically significant. The classification of differential metabolites was found through database searches including the Human Metabolome Database (http://www.hmdb.ca), KEGG database (https://www.genome.jp/kegg/), and Lipid Maps (https://www.lipidmaps.org/). The diagnostic efficacy of each metabolite was assessed by the receiver operating characteristic curve (ROC). The enrichment and pathways analysis were performed with KEGG pathway database. A panel of metabolites that might predict TAI patients in early pregnancy was developed using LASSO regression (R package “glmnetcr”). An independent sample t test was used to compare the two groups for data with normal distribution. A non-parametric Mann–Whitney U test was used for non-normally distributed data. Statistical analysis was conducted using R software (version R 3.4.3; R Core Team, 2017) and Python (version 2.7.6).
Results
Clinical characteristics and pregnancy outcomes
We consecutively recruited 56 participants who consented to this study. The TAI group and the control group were carefully matched for age and BMI, with no statistically significant differences observed in thyroid parameters (FT3, FT4, and TSH), as well as other biochemical indexes including FBG, UA, triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). TPOAb and TgAb in TAI patients showed significant differences compared to HC. There were no significant differences in anemia before delivery and gestational age at delivery. Notably, there was a statistically significant difference in birth weight (BW) (P = 0.007) (Table 1).
Metabolic profiling and KEGG pathways
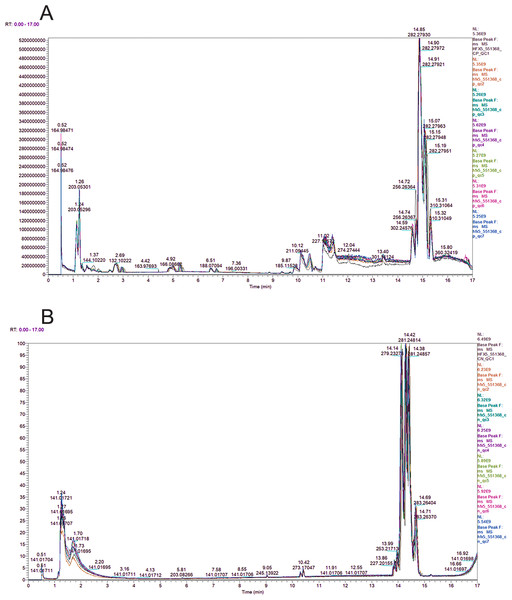
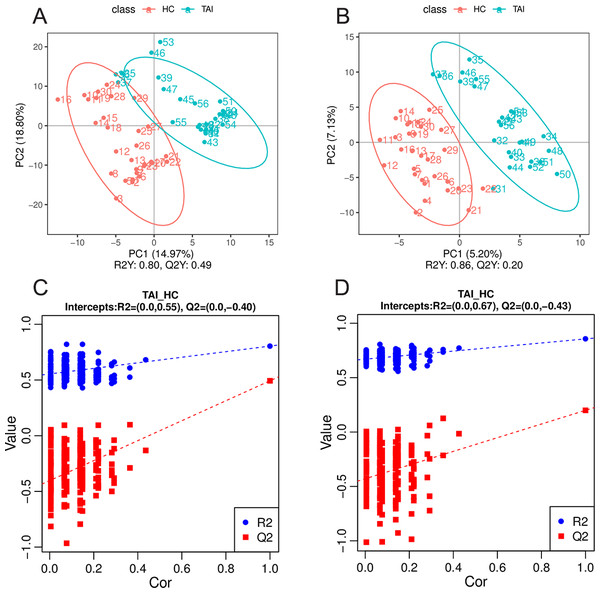
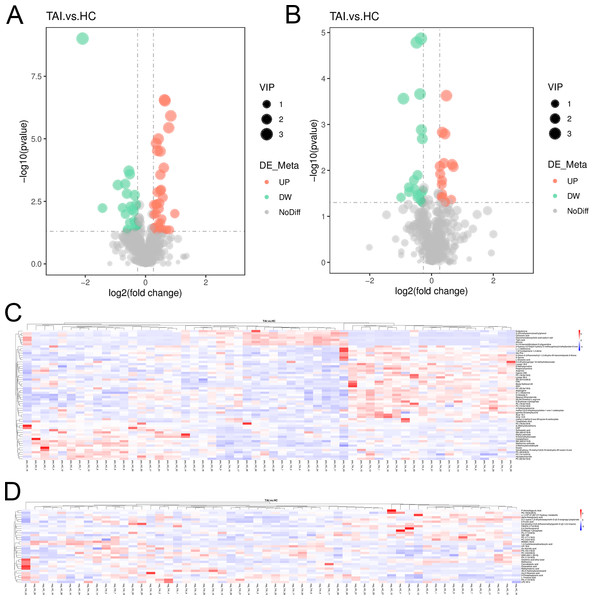
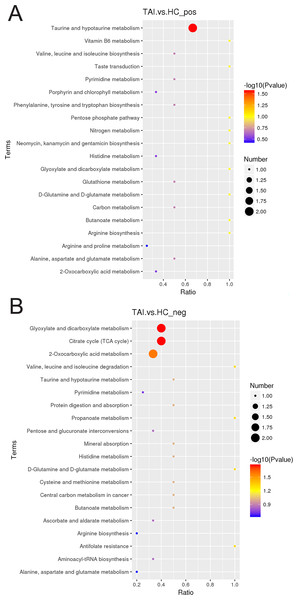
The peak chromatograms (BPC) of QC samples in both POS and NEG ion modes were shown in Figs. 1A and 1B, with good spectral overlap, indicating satisfactory stability of the instrument and reliable detection results. PLS-DA models (Figs. 2A and 2B) clearly separated the TAI group from the HC group, regardless of whether the positive or negative mode was used, demonstrating strong fitness and predictive capability. (R2Y = 0.8, Q2Y = 0.49 in POS mode; R2Y = 0.86, Q2Y = 0.2 in NEG mode). The cross-validation through permutations tests (200 times) of two PLS-DA models verified that the model was not overfitted (generated intercepts of R2 = 0.55, Q2 = −0.4 and R2 = 0.67, Q2 = −0.43 respectively) (Figs. 2C and 2D). We filtered out a total of 92 differential metabolites (50 upregulated and 42 downregulated) in the study case based on the PLS-DA analysis findings and utilizing VIP ≥ 1, FC > 0.2 or < 0.833 and P < 0.05 as the screening criterion. These metabolites include amino acids, fatty acyls, glycerophosphocholines, carboxylic acid, aromatic compounds, carbonyl compounds, carbohydrate derivative and carbohydrate phosphate, steroid, heteromonocyclic compounds such as lactone, furans and pyrimidines, hydroxy compounds such as alcohol and phenols, organonitrogen compounds including amino acid derivative, peptidases and proteinases and other compounds. Details including HMDB/Lipid maps/KEGG ID, VIP, P values and fold change of each metabolite were shown in Tables S1 and S2. Based on the comparison of VIP, P, and FC values, volcano plots were drawn to visually display the overall distribution of differential metabolites (Figs. 3A and 3B). Cluster analysis was performed on statistically significant differential metabolites in both positive and negative ion modes in heatmap (Figs. 3C and 3D). Pathway enrichment analysis identified 8 altered pathways (Table S3), with 4 significantly enriched: taurine and hypotaurine metabolism, citrate cycle (TCA cycle), glyoxylate and dicarboxylate metabolism and 2-oxocarboxylic acid metabolism (Figs. 4A and 4B).
Figure 1: BPC overlap plot of the QC samples in positive (A) and negative (B) ion mode.
Figure 2: Multivariate statistical analysis of serum metabolites in TAI patients and HC in early pregnancy.
(A, B) The serum metabolic profiles in positive and negative ion mode were used to generate PLS-DA scatter plots. (C, D) The corresponding PLS-DA models were statistically verified by permutation tests (200 times).Figure 3: Ninety-two discrepant serum metabolites of TAI patients and HC in early pregnancy.
(A, B) Volcanic map of 92 disparate serum metabolites in positive and negative ion mode. (C, D) An unsupervised heatmap comprising 92 metabolites in positive and negative ion mode.Figure 4: KEGG pathway analysis of TAI patients and HC in early pregnancy in positive (A) and negative (B) ion mode.
Establishment of 15-metabolites-based diagnostic model
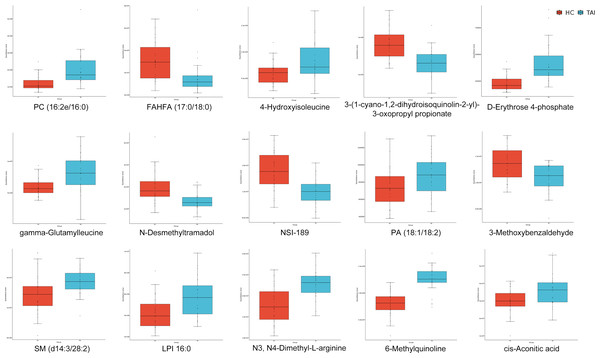
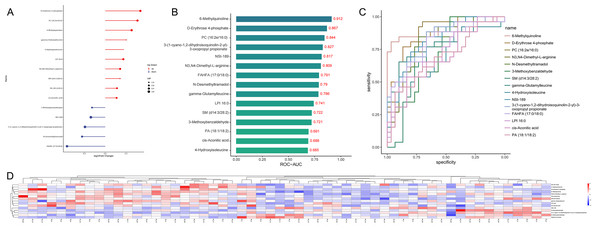
We systematically defined metabolomic profiles and pathways related to TAI. Subsequently, we employed LASSO regression to screen for biomarkers, resulting in a model based on 15 specific metabolites (including 10 upregulated and five downregulated, Figs. 5 and 6A). These metabolites are 6-Methylquinoline, D-erythrose 4-phosphate, 4-Hydroxyisoleucine, PC (16:2e/16:0), N3, N4-Dimethyl-L-arginine, N-desmethyltramadol, 3-Methoxybenzaldehyde, SM (d14:3/28:2), gamma-Glutamylleucine, NSI-189, 3- (1-cyano-1,2-dihydroisoquinolin-2-yl)-3-oxopropyl propionate, LPI 16:0, cis-Aconitic acid, PA (18:1/18:2), and FAHFA (17:0/18:0). We evaluated their diagnostic ability using the AUC in ROC analysis, with AUC values ranging from 0.685 to 0.912 (Figs. 6B and 6C). An unsupervised clustering heatmap demonstrated that the 15 metabolites could effectively differentiate most TAI patients from HC (Fig. 6D). These 15 metabolites were identified as characteristic markers for TAI patients in early pregnancy.
Figure 5: Median concentrations of the 15 specific metabolites selected by LASSO to discriminate TAI patients and HC in early pregnancy.
The differences in metabolite levels between the two groups were compared using Mann-Whitney U test.Figure 6: Fifteen specific metabolites selected by LASSO.
(A) Alterations in the expression of 15 specific metabolites. (B, C) The ROC analysis of 15 specific metabolites. (D) An unsupervised heatmap comprising 15 specific metabolites.Correlation between clinical data and potential biomarkers
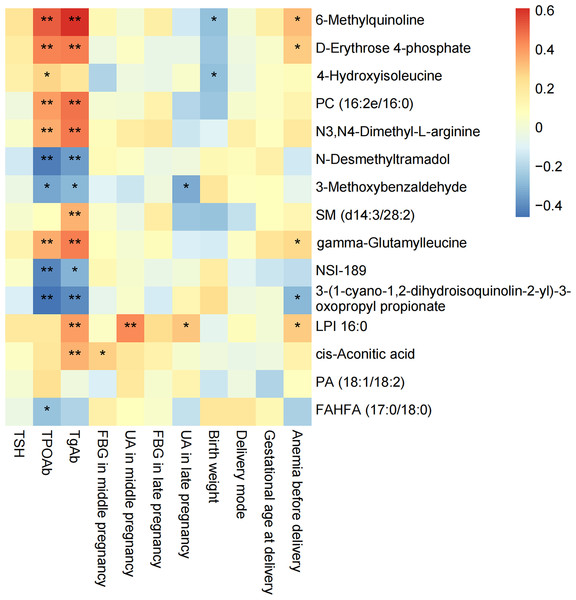
We performed Spearman correlation analyses on the 15 potential biomarkers with clinical data (Fig. 7). 6-Methylquinoline, D-erythrose 4-phosphate, gamma-Glutamylleucine and LPI 16:0 showed a positive correlation with anemia before delivery, while 3- (1-cyano-1,2-dihydroisoquinolin-2-yl)-3-oxopropyl propionate had a negative correlation. LPI 16:0 positively correlated with UA in both middle and late pregnancy, whereas 3-Methoxybenzaldehyde exhibited a negative correlation with UA in late pregnancy. Cis-Aconitic acid positively correlated with FBG in mid-pregnancy. Moreover, 14 out of 15 potential biomarkers were associated with thyroid autoantibodies. 6-Methylquinoline, D-erythrose 4-phosphate, PC (16:2e/16:0), N3, N4-Dimethyl-L-arginine and gamma-Glutamylleucine had a positive correlation with both TPOAb and TgAb, while N-desmethyltramadol, 3-Methoxybenzaldehyde, NSI-189 and 3- (1-cyano-1,2-dihydroisoquinolin-2-yl)-3-oxopropyl propionate had a negative correlation. Furthermore, 14 out of 15 potential biomarkers were associated with thyroid autoantibodies. Specifically, 6-Methylquinoline, D-erythrose 4-phosphate, PC (16:2e/16:0), N3, N4-Dimethyl-L-arginine, and gamma-Glutamylleucine exhibited positive correlations with both TPOAb and TgAb, while N-desmethyltramadol, 3-Methoxybenzaldehyde, NSI-189, and 3- (1-cyano-1,2-dihydroisoquinolin-2-yl)-3-oxopropyl propionate showed negative correlations.
Figure 7: Correlation between 15 specific metabolites selected by LASSO and clinical information.
Discussion
Thyroid autoimmunity (TAI) has witnessed a substantial increase in its global prevalence over recent decades, making it the most common autoimmune disease. Some literatures have summarized the serum metabolic patterns of patients with autoimmune thyroiditis (AIT) or TAI, as detailed in Table S4. While TAI is associated with adverse pregnancy outcomes, there is currently no evidence to recommend routine screening and treatment for euthyroid TAI in pregnant women (Abbassi-Ghanavati, 2011). The impact of TAI on pregnancy outcomes is influenced not only by autoantibodies but also by other potential factors. Therefore, we employed an untargeted technique, LC-MS, to investigate serum metabolite alterations in early pregnancy TAI patients. Comparative analysis of serum metabolomics between TAI patients and healthy controls in early pregnancy revealed 92 significantly different metabolites. Using LASSO, we identified a group of 15 compounds among the 92 metabolites as the best predictors of TAI. Furthermore, correlation analysis demonstrated a strong relationship between these 15 representative differential metabolites, clinical indicators, and pregnancy outcomes, shedding light on the pathophysiology of TAI in early pregnancy.
Previous researches have confirmed that TAI was linked to an increased risk of premature delivery (Korevaar et al., 2018), miscarriage (Liu et al., 2014), low birth weight (Chen et al., 2015) and subfertility (Tanska et al., 2022; Van den Boogaard et al., 2011). The LASSO-selected upregulated combination of 6-Methylquinoline and 4-Hydroxyisoleucine provided a negative correlation with birth weight, and a substantial difference in birth weight between the TAI group and the control group was revealed. 6-Methylquinoline showed toxic potential to the embryos of zebrafish with a LC50 value of 5.6 mg/L (Peddinghaus et al., 2012). 4-Hydroxyisoleucine is one of the key pharmacologically active phytoconstituents found in fenugreek seeds. Several researchers have highlighted that fenugreek seed extract had potential toxic effects on fetal development, which may lead to reduced embryo implantation, increased fetal mortality rate, reduced litter size, and decreased fetal weight in mice and rabbits (Kassem et al., 2006; Khalki et al., 2010).
Amino acids influence maternal metabolism as well as fetal growth and development. In addition to 4-Hydroxyisoleucine, the amino acids selected by LASSO included N3, N4-Dimethyl-L-arginine, gamma-Glutamylleucine and cis-Aconitic acid. Upregulated N3, N4-Dimethyl-L-arginine and gamma-Glutamylleucine may be associated with elevated oxidative stress in TAI patients. Song et al. (2019) found that patients with Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) had higher L-arginine and spermidine than healthy controls. Arginine metabolism leads to enhanced T cell activation, increased cytokines, and increased inflammatory response and oxidative stress. The citrate cycle (TCA cycle) is a metabolic pathway commonly altered in many autoimmune diseases such as TAI and asthma (Quan-Jun et al., 2017; Sarandi et al., 2021), which is consistent with our results. Interestingly, among the four significantly altered pathways, cis-Aconitic acid appears to be a key node. It is interconnected with the citrate cycle (TCA cycle), glyoxylate and dicarboxylate metabolism, and 2-oxocarboxylic acid metabolism. Furthermore, it indirectly influences taurine and hypotaurine metabolism through interactions with other amino acids. While there is no direct research indicating a correlation between TAI and cis-Aconitic acid, findings related to GDM suggest a potential connection. These studies have revealed increased gluconeogenesis to meet energy demands, elevated excretion of hypoxanthine due to hypoxia, and heightened production of cis-Aconitic acid and sugar-generating amino acids. These observations suggest an expansion of the citrate cycle (Diaz et al., 2011). Recent studies indicated that cis-Aconitic acid may be involved in inflammatory, hypoxic, and metabolic signaling (Murphy & O’Neill, 2018). Succinate, an intermediate metabolite of the tricarboxylic acid cycle, was considered to accumulate in macrophages during inflammation, serving as a signal to activate pro-inflammatory gene expression and promoting the production of reactive oxygen species (ROS) through the mitochondrial respiratory chain (Chouchani et al., 2014). In the later stage of inflammation, the inflammatory effect of succinic acid was offset by the derivative of cis-Aconitic acid, itaconic acid (Bambouskova et al., 2018; Lampropoulou et al., 2016). Our study found that cis-Aconitic acid was significantly upregulated in TAI patients in early pregnancy and positively correlated with FBG in middle pregnancy, indicating that it may promote the occurrence of TAI by participating in hypoxia, increased gluconeogenesis, pro-inflammatory, and pro-inflammatory/anti-inflammatory imbalance.
TAI is a multifactorial disease, and lipid metabolism disorders have been correlated with this autoimmune disease in a variety of ways. Liu et al. (2020) reported that HT patients with hypothyroidism were identified to have higher serum levels of some types of PC, SM and PE compared to HC. Cai et al. (2021) and Li et al. (2021) reached similar conclusions in subclinical hypothyroidism (SCH) and hypothyroidism in pregnant women. Moreover, Liu et al. (2020) observed similar alterations in three types of PC and SM in both hyperthyroidism and hypothyroidism groups, suggesting that thyroid hormone alone may not be the exclusive contributing factor. This phenomenon may be associated with thyroid autoimmunity. Consistently, the present study indicated that PC (16:2e/16:0) and SM (d14:3/28:2) were upregulated in pregnant women with TAI. This upregulation is likely attributed to thyroid autoimmunity, as thyroid hormone levels in the TAI group did not significantly differ from those in HC. Moreover, correlation analysis showed a positive correlation between PC (16:2e/16:0), SM (d14:3/28:2) and thyroid autoantibodies. PC and SM have been linked with many other autoimmune diseases such as primary warm autoimmune hemolytic anaemia (Rabelo et al., 2023), type 1 diabetes (Oresic et al., 2013) , multiple sclerosis (Hon et al., 2009) and inflammatory bowel disease (Gersemann, Wehkamp & Stange, 2012). Notably, TAI patients exhibited decreased FAHFA (17:0/18:0) levels compared to healthy controls, and the downregulated FAHFA (17:0/18:0) was also associated with increased TPOAb titers. In vivo, FAHFA attenuated the recruitment and/or activation of T cells as well as expression of pro-inflammatory genes (Th1 and Th17) associated with T cell responses and reduced the expression of cytokines and chemokines, resulting in the reduction of IFN- γ, TNF- α, IL-6 and IL-17 (Lee et al., 2016). In vitro, FAHFA inhibited immune responses by attenuating dendritic cell (DC)-induced CD4+ T cell proliferation and Th1 cell polarization (Lee et al., 2016; Yore et al., 2014). We hypothesize that the reduction in FAHFA levels may be associated with the over-activation of immune cells in TAI patients. Further investigation is needed to uncover the underlying mechanisms of lipids in TAI.
The pentose phosphate pathway (PPP), a major regulator of cellular energy metabolism and biosynthesis, comprises two branches: the oxidative branch (oxPPP) and the non-oxidative branch (non-oxPPP). D-Erythrose 4-phosphate, upregulated in the TAI group, is an intermediate product of the non-oxPPP. This result suggests that there may be an increase in non-oxPPP flux in the TAI group. The PPP produces NADPH, and excessive NADPH in the thyroid may cause oxidative stress via NOXs, resulting in thyroid damage and inflammation (Burek & Rose, 2008).
Organic acids (OAs) have become prominent in recent years in metabonomic analysis and are regarded as potential biomarkers for distinguishing various disease pathologies and clinical features of specific diseases such as asthma and psoriasis. The pathways disrupted in TAI patients, including the citrate cycle, glyoxylate and dicarboxylate metabolism, and 2-oxocarboxylic acid metabolism, are related to OAs and play crucial roles in energy metabolism and immune regulation in the body. The organic acids enriched in these pathways include not only the previously mentioned cis-Aconitic acid but also alpha-Ketoglutaric acid. Alpha-Ketoglutaric acid (citrate cycle intermediate) is catalyzed by aspartate aminotransferase and further catalyzed by isocitrate dehydrogenase 1 and 2 to form R-2-hydroxyglutaric acid. Decreased alpha-Ketoglutaric acid levels in Th17 cells are associated with enhanced DNA methylation of CpG islands at Foxp3, which inhibits Foxp3 transcription and differentiation of Treg cells. More alpha-Ketoglutaric acid enters the citrate cycle and is consumed, which can also promote the proliferation of Th17 cells. The imbalance of Th17/Treg had significant correlation with TAI. In addition, while no direct link has been established between serum alpha-Ketoglutaric acid and inflammation in TAI patients, serum metabolite profiles in psoriatic disease have shown that alpha-Ketoglutaric acid levels were lower in patients with both psoriasis and psoriatic arthritis compared to those with psoriasis alone (Armstrong et al., 2014). The authors believe that the inflammatory response enhanced the consumption of alpha-Ketoglutaric acid (Armstrong et al., 2014), which might also be a contributing factor to the reduced serum alpha-Ketoglutaric acid levels observed in TAI patients.
There are still some limitations to our study: (1) the limited sample size may introduce bias into the findings; (2) the study exhibited regional uniqueness due to factors such as food habits and geographical variances; (3) because serum metabolomics were only conducted on samples from early pregnancy, we could not study the dynamic changes that occur throughout the entire pregnancy. Therefore, more in-depth research is needed: conducting longitudinal studies with a larger sample size and greater geographical diversity, and monitoring the changes of serum metabolites throughout pregnancy, is necessary to further elucidate the mechanisms of TAI during pregnancy and the relationship between differential metabolites and pregnancy outcomes.
Conclusions
In conclusion, this study characterized the serum metabolomic patterns of TAI patients in early pregnancy. We identified 92 altered metabolites and four significantly enrichment KEGG pathways, and we also developed a panel based on 15 metabolites that could differentiate between TAI and healthy pregnant women while also being associated with pregnancy outcomes and autoimmune factors. The changes in the serum levels of metabolites identified can be used in health screening and indicate adverse pregnancy outcomes in TAI patients.
Supplemental Information
Molecular ion peak in the positive ion mode and negative (NEG) ion mode
These were used for statistical analysis to compare pregnant women with TAI and healthy controls.
Group size estimation
Group size estimation using MetSizeR with an estimated significant rate of 20%, FDR 0.05, minimal group size of n = 10, and 903 spectral bins.