MALAT1 promotes colonic epithelial cell apoptosis and pyroptosis by sponging miR-22-3p to enhance NLRP3 expression
- Published
- Accepted
- Received
- Academic Editor
- Jincheng Wang
- Subject Areas
- Cell Biology, Molecular Biology, Immunology
- Keywords
- Fetal human colon cell, Long non-coding RNAs, Proliferation, Inflammasome
- Copyright
- © 2024 Yan et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. MALAT1 promotes colonic epithelial cell apoptosis and pyroptosis by sponging miR-22-3p to enhance NLRP3 expression. PeerJ 12:e18449 https://doi.org/10.7717/peerj.18449
Abstract
Background
Colonic epithelial cell apoptosis and pyroptosis had a close relationship with the pathological progression of ulcerative colitis (UC). LncRNA play a crucial role in the progression of UC. However, the role of the lncRNA MALAT1 in colonic epithelial cell apoptosis and pyroptosis remains unclear.
Methods
UC colitis cell model was established through lipopolysaccharide (LPS) treatment. MiR-22-3p and MALAT1 expression in fetal human colon (FHC) cells were analyzed by qRT-PCR. Proliferation and apoptosis of FHCs were measured using CCK-8 assay and flow cytometry, respectively. Pyroptosis indicators including interleukin (IL)-1β, IL-18, tumor necrosis factor-α (TNF-α), NLR family pyrin domain containing 3 (NLRP3), caspase-1, and N-gasdermin D (N-GSDMD) in FHCs were detected using ELISA, qRT-PCR, western blotting, and immunofluorescence.
Results
In this study, apoptosis was facilitated, IL-1β, IL-18, and TNF-α levels were enhanced, NLRP3, caspase-1, N-GSDMD protein were increased, and MALAT1 expression was markedly increased in LPS-treated FHCs (LTFs). MALAT1 knockdown remarkably facilitated proliferation and suppressed apoptosis, reduced IL-1β, IL-18, and TNF-α levels, and decreased the protein of NLRP3, caspase-1, N-GSDMD. Furthermore, NLRP3 overexpression remarkably reversed the effect of MALAT1-downexpression in LTFs. In addition, miR-22-3p could bind with MALAT1 and NLRP3 3′ UTR. Furthermore, miR-22-3p inhibition remarkably reversed the effect of MALAT1 overexpression in LTFs.
Conclusions
These findings suggest that MALAT1 represents a promising therapeutic target for the treatment of UC by modulating the miR-22-3p/NLRP3 pathway, potentially leading to novel strategies for reducing inflammation and cell death in the colon.
Introduction
Ulcerative colitis (UC) is an inflammatory condition affecting the rectum and colonic mucosa of the colon (Kobayashi et al., 2020). Asian countries are important regions where the UC epidemic is growing rapidly (Ng et al., 2017). Long-term irritation of UC can increase the risk of colorectal cancer (Choy et al., 2019; Olén et al., 2020). Although increased types and effectiveness of drugs including mesalazine, budesonide, and methylprednisolone for UC have been updated, unfortunately, the drugs for treating UC are not satisfactory, which manifests the high recurrence rate (Eder et al., 2023; Sun et al., 2020). Thus, to ameliorate the clinical outcomes of UC patients, it is crucial to elucidate latent molecular mechanisms of this disease and develop valid therapeutic drugs for UC patients.
Currently, inflammation of intestinal epithelial cells can lead to apoptosis or pyroptosis of intestinal epithelial cells and impairment of intestinal absorption function, which was believed to be induced the processed of UC (Huang et al., 2022; Liu et al., 2021; Porter, Kalla & Ho, 2020). Inhibiting colonic epithelial cell apoptosis and pyroptosis helps improve ulcerative colitis (Niu et al., 2024; Shen, Zhao & Cui, 2024). Hence, inhibition of colorectal epithelial cell apoptosis and pyroptosis is a therapeutic strategy for improving UC. NLR family pyrin domain containing 3 (NLRP3) was the target for the inhibition of pyroptosis and inflammatory responses (Wang & Hauenstein, 2020). NLRP3 inflammasome activation cleaves pro-caspase-1 and converts it to active caspase-1, which activates the downstream effector proteins of interleukin (IL)-1β, IL-18, and N-gasdermin D (N-GSDMD) (Strowig et al., 2012). There studies suggested that NLRP3, caspase-1, N-GSDMD IL-1β, and IL-18 were important indicators for assessing pyroptosis. In addition, NLRP3 inflammasome was closely related to the pathogenesis of UC via regulating apoptosis and pyroptosis (Niu et al., 2024; Zhen & Zhang, 2019). Hence, NLRP3 was a target for treatment UC. Studying the upstream genes of NLRP3 can help uncover new mechanisms and targets, contributing to the advancement of treatment strategies for ulcerative colitis.
Currently, lncRNAs are involved in various cellular functions, including cell apoptosis, pyroptosis, and inflammatory cytokine expression, play the crucial role in UC progression (Ray, Fenton & Paulssen, 2022; Yu et al., 2021). For instance, Tian et al. (2021) reported that lncRNA TUG1 overexpression enhanced proliferation of TNF-α-stimulated intestinal epithelial cells by blocking apoptosis. Upregulation of MEG3 alleviates severe colonic ulcers in UC rats by targeting miR-98-5p to increase IL-10 level (Wang et al., 2021). MALAT1, a widely investigated lncRNA, was found to involved in apoptosis, inflammation, and pyroptosis (Kumar et al., 2024; Shoeib et al., 2023). In addition, MALAT1 expression was upregulated in UC patients, and MALAT1 overexpression can induce the apoptosis of colon epithelial cell (Zhu & Xie, 2020). However, it is currently unclear whether MALAT1 is involved in regulating the apoptosis and pyroptosis of colonic epithelial cells in patients with ulcerative colitis. Furthermore, previous studies have shown that MALAT1 can regulate apoptosis and pyroptosis by promoting the expression of NLRP3 (Liu et al., 2020; Song et al., 2019). However, the relationship between them in ulcerative colitis (UC) remains unclear.
Therefore, this project aims to investigate the role of MALAT1 in the apoptosis and pyroptosis of colon cells during the development of UC, and to further explore whether MALAT1 can improve the mechanisms of colon cell apoptosis and pyroptosis by regulating the expression of NLRP3. In our study, a colitis cell model was first established via using 10 ng/mL lipopolysaccharide (LPS) treated fetal human colon (FHC) cells. Next, MALAT1 expression levels in colitis model cells were analyzed following which the effects of MALAT1 on the proliferation, apoptosis, and pyroptosis of LPS-treated FHC cells (LTFs) were assessed. Finally, the possible molecular regulatory mechanisms of MALAT1 involvement were investigated. This study provided that a deeper exploration of the specific mechanisms by which MALAT1 operates in this process may provide new insights into the pathogenesis of ulcerative colitis.
Materials and Methods
Cell culture and UC cell model
FHCs (ATCC), the normal colon cell line, were soaked in RPMI-1640 (Gibco, Waltham, MA, USA) supplemented with 10%FBS (Gibco, Waltham, MA, USA) and then incubated at 37 °C with 5% CO2. To establish the UC cell model, FHCs were treated with 10 ng/mL LPS (L8880; Solarbio, Beijing, China) (Yan, Liang & Hu, 2023; Zhao, Chen & Yue, 2024).
Cell transfection
NLRP3 overexpression (ov-NLRP3) and MALAT1 overexpression (ov-MALAT1) were synthetized (GenePharma, Suzhou, China) and inserted in pcDNA3.1 plasmids, and empty pcDNA3.1 plasmids acted as negative control (NC). siRNAs against MALAT1 (si-MALAT1, 5′-TAGCGTTAAGTTTTTAACGTAAT-3′) and si-NC (5′-TTCTCCGAACGTGTCACGTTT-3′) were purchased from GenePharma. After 24 h of LPS treatment, FHCs were transfected with plasmids and siRNAs using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the method provided by the manufacturer.
qRT-PCR
Total RNA was separated from transfected FHCs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) after treated LPS at 24 h. Subsequently, 1 μg RNA was converted to cDNA and measured by qPCR which performed using an ABI PCR system (Applied Biosystems, Foster City, CA, USA). The 20 μl qPCR reaction system consists of 5 μl cDNA, 0.5 μl upstream primer, 0.5 μl downstream primer, 10 μl 2× SYBR Green qPCR SuperMix, and 4 μl H2O. The reaction conditions are as follows: 95 °C for 5 min; followed by 40 cycles of 95 °C for 15 s and 60 °C for 32 s. Fold changes in the transcripts were computed using the 2−ΔΔCT method, with GAPDH serving as an internal reference (Singaravelan, Sivaperuman & Calambur, 2022). Primer sequences: MALAT1 forward, 5′-GGTTGAGATGAAGCTTCTT-3′, reverse: 5′-GCACTTCTTGTGTTCTCTT-3′; NLRP3 forward: 5′-TGGTCTGCTGGATCGTGTGC-3′, reverse: 5′-CGGGGCTGCAGCAAACTGGA-3′; GAPDH forward: 5′-GCTCATTTGCAGGGGGGAG-3′, reverse: 5′-GTTGGTGGTG CAGGAGGCA-3′; miR-22-3p forward: 5′-ACACTCCAGCTGGGAGAACTGT-3′, reverse: 5′-CTCAACTGGTGTCGTGGA-3′, U6 forward: 5′-CTCGCTTCGGCAGCACA-3′, reverse: 5′-AACGCTTCACGAATTTGCGT-3′.
Proliferation assays
The transfected FHCs were treated 10 ng/mL LPS and inoculated into plates. Subsequently, FHCs were cultured for 0, 24, 48, and 72 h at 37 °C and added the 10 µL of CCK-8 solution (CCK-8 kit, Solarbio, Beijing, China) in each well at the corresponding time points. The OD450 nm of each well was analyzed using a microplate reader (51119670DP, Thermo Scientific, Waltham, MA, USA) after 2 h of incubation.
Apoptosis assay
In brief, the transfected FHCs were treated 10 ng/mL LPS at 24 h and rinsed with ice-cold PBS and suspended, then incubated with Annexin V-FITC and PI at 37 °C for 15 min in the dark according to Annexin V-FITC/PI Cell Apoptosis Detection Kit (KeyGen Biotech, Nanjing, China). Cell apoptosis was analyzed by flow cytometry (FCM; BD Biosciences, San Jose, CA, USA) used Flow Jo v10.8.1 (BD).
ELISA, western blotting, and immunofluorescence assay
IL-1β, TNF-α, IL-18 concentrations in FHCs after transfection or treatment were analyzed using the human IL-1β (PI305), TNF-α (PT518), IL-18 (PI558) ELISA Kit (Beyotime, Shanghai, China) as per the method provided by the manufacturer. The western blot and immunofluorescence assays were done as described in our previous study and antibody sourceand dilution ratio were done as described in our previous study (Yan, Liang & Hu, 2023).
Bioinformatics and luciferase reporter assays
The miRNAs combined with MALAT1 were analyzed using starbase and RNAInter while the miRNAs combined with NLRP3 3′-UTR were analyzed using targetscan and RNAInter. Dual luciferase reporter assays were carried out to confirm bound between miR-22-3p and MALAT1/NLRP3 3′-UTR. Wild type (WT) and mutant (mut) sequences of MALAT1 and NLRP3 3′-UTR which bind to miR-22-3p were synthetic and inserted to psi-CHECK2. Then the psi-CHECK2 and miR-22-3p were co- transfected into LTFs. After 24 h, the luciferase activity was assessed using the Dual-Light Chemiluminescent Reporter Gene Assay System (Applied Biosystems, Foster City, CA, USA) and Renilla/firefly luciferase activity rate were calculated.
Statistical analysis
The data in this study were analyzed using IBM SPSS 19.0 (Armonk, NY, USA). All data are expressed as mean ± standard deviation (SD). Statistical differences were assessed using t-test (two groups) or one-way ANOVA followed by pairwise analysis using LSD analysis (diverse groups). Statistical differences were set at P values < 0.05.
Results
MALAT1 expression affected proliferation and apoptosis of LTFs
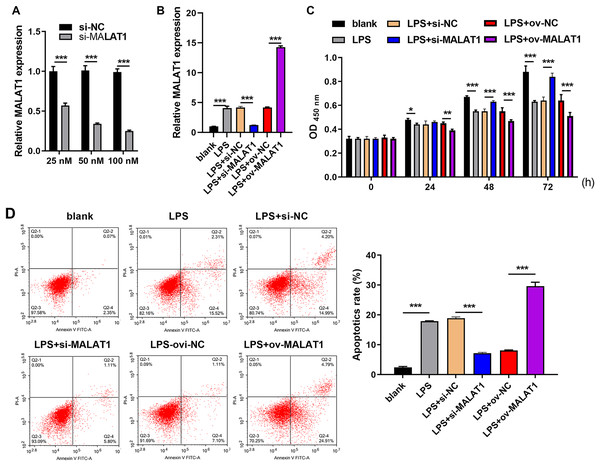
Frist, MALAT1 expression was significantly reduced in 100 nM si-MALAT1 group compared with si-NC and 20 nM si-MALAT1 groups, suggested that 100 nM si-MALAT1 transfection could reduce MALAT1 expression in FHC cells without LPS treatment (Fig. 1A). MALAT1 expression was markedly increased in LTFs (LPS group) compared to that in blank group (Fig. 1B). To explore the functional effects of MALAT1 on FHCs, MALAT1 were overexpressed or downexpressed in LTFs. MALAT1 was remarkably upregulated in LPS+ov-MALAT1 group while it was remarkably decreased in LPS+si-MALAT1 group compared to the corresponding control group (Fig. 1B). Functionally, LPS treatment significantly suppressed FHC proliferation while promoted apoptosis compared to that in blank group (Figs. 1C and 1D). Proliferation was remarkably inhibited in LPS+ov-MALAT1 group at 48 and 72 h while remarkably enhanced in LPS+si-MALAT1 group compared to the corresponding control group at 24, 48 and 72 h (Fig. 1C). Conversely, apoptosis was remarkably enhanced in LPS+ov-MALAT1 group while it was remarkably reduced in LPS+si-MALAT1 group (Fig. 1D).
Figure 1: MALAT1 expression affected proliferation and apoptosis in LPS treated FHCs (LTFs).
(A) The MALAT1 expression in FHC cells and after transfection with si-MALAT1 were measured through qRT-PCR. (B) The MALAT1 expression in LTFs alone and after transfection with ov-MALAT1 or si-MALAT1 were measured through qRT-PCR. (C) Cell proliferation of LTFs and LTFs transfected with ov-MALAT1 and si-MALAT1 were studied using CCK-8 assay. (D) Cell apoptosis of LTFs and LTFs transfected with ov-MALAT1 and si-MALAT1 were evaluated through flow cytometry (*P < 0.05, **P < 0.01, and ***P < 0.001).MALAT1 downexpression inhibits the pyroptosis of LTFs
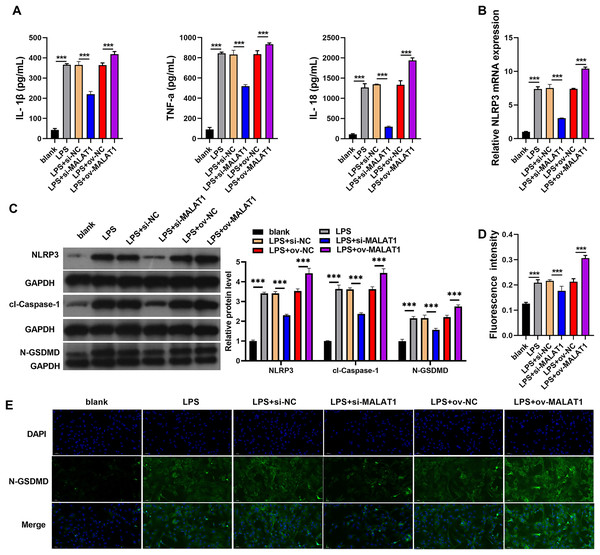
Inflammation of intestinal epithelial cells can lead to apoptosis or pyroptosis of intestinal epithelial cells and Impairment of intestinal absorption function, which was believed to be induced the processed of UC (Huang et al., 2022; Liu et al., 2021; Porter, Kalla & Ho, 2020). Therefore, we further analyzed the effects of MALAT1 on cell pyroptosis and inflammation. In addition, LPS induction (LPS group) remarkably increases the levels of IL-1β, TNF-α, IL-18, and the protein of pyroptosis marker including NLRP3, caspase-1, and N-GSDMD in FHCs compared to normally cultured FHCs (blank group) (Fig. 2). IL-1β, TNF-α, IL-18 levels were remarkably enhanced in LPS+ov-MALAT1 group while those were remarkably reduced in LPS+si-MALAT1 group (Fig. 2A). Consistently, the mRNA of NLRP3 and the protein of NLRP3, caspase-1, and N-GSDMD was remarkably enhanced in LPS+ov-MALAT1 group while it was remarkably reduced in LPS+si-MALAT1 group (Figs. 2B and 2C). In addition, N-GSDMD fluorescence intensity was remarkably enhanced in LPS+ov-MALAT1 group while it was remarkably reduced in LPS+si-MALAT1 group (Figs. 2D and 2E).
Figure 2: The interference of MALAT1 expression inhibited the pyroptosis of LPS treated FHCs (LTFs).
(A) The secretion levels of inflammatory cytokine were examined through ELISA in LTFs. (B) The NLRP3 mRNA in LTFs were measured by qRT-PCR (C) the pyroptosis biomarker NLRP3, caspase-1, and N-GSDMD levels in LTFs were measured by western blot analysis. (D and E) Immunofluorescence was used to measure N-GSDMD protein in LTFs (***P < 0.001).NLRP3 overexpression could reverse the effect of MALAT1 interference in LTFs
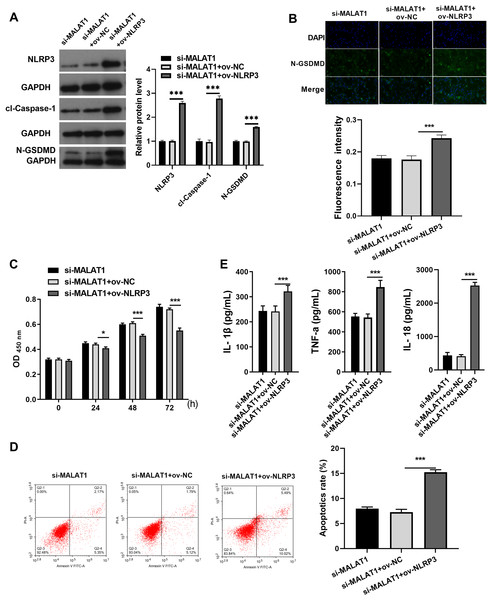
Next, to expound whether MALAT1 plays the effect in LTFs via NLRP3, ov-NLRP3 was transfected along with si-MALAT1 into LTFs. Overexpression efficiency results show NLRP3 to be significantly elevated in LTFs transfected with ov-NLRP3+si-MALAT1 (Fig. 3A). In addition, cl-caspase-1 and N-GSDMD expression levels to be significantly elevated in LTFs transfected with ov-NLRP3 plus si-MALAT1 (Fig. 3A). The immunofluorescence assay also verified that NLRP3 overexpression could facilitate the N-GSDMD protein in LTFs, even in the presence of MALAT1 interference (Fig. 3B). NLRP3 overexpression significantly blocked the proliferation while enhanced the apoptosis and the levels of inflammatory cytokine of LTFs even in presence of MALAT1 interference (Figs. 3C and 3E).
Figure 3: NLRP3 overexpression could block proliferation while promote apoptosis and pyroptosis of LPS treated FHCs (LTFs) in the presence of MALAT1 interference.
(A) The NLRP3, cl-caspase-1, and N-GSDMD expression levels in LTFs transfected with ov-NLRP3 +si-MALAT1 were assessed by western blot. (B) N-GSDMD protein in LTFs was analyzed through Immunofluorescence after transfected with ov-NLRP3+si-MALAT1. (C and D) The proliferation and apoptosis of LTFs transfected with ov-NLRP3 plus si-MALAT1 were analyzed. (E) ELISA was used to examine the secretion of inflammatory cytokine in LTFs transfected with ov-NLRP3 plus si-MALAT1 (*P < 0.05 and ***P < 0.001).MALAT1 exerts regulatory effects on LIFs by competitively adsorbing miR-22-3p with NLRP3
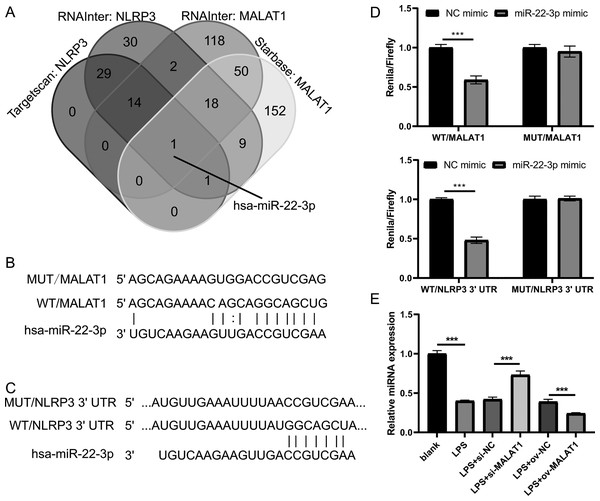
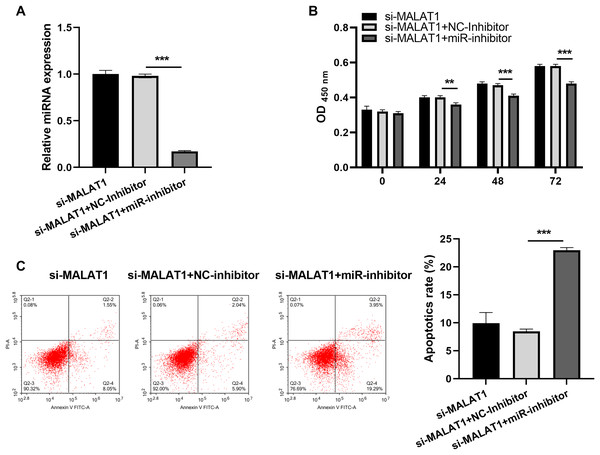
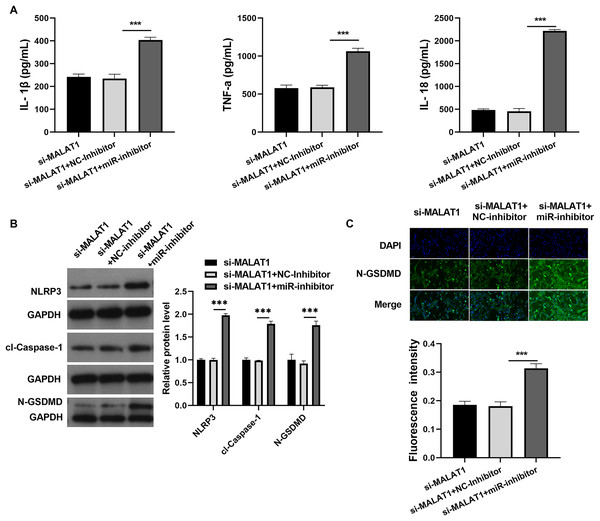
Next, to investigate the miRNAs that interact with both MALAT1 and the 3′-UTR of NLRP3, this study employed two online platforms, Starbase and RNAInter, to analyze the potential miRNAs associated with MALAT1. Additionally, Targetscan and RNAInter were used to evaluate the potential miRNAs binding to the 3′-UTR of NLRP3. Ultimately, the intersection of four sets of results was only found the miR-22-3p (Fig. 4A). The potential binding sites between miR-22-3p and MALAT1/NLRP3 3′-UTR were shown (Figs. 4B and 4C). Then, the renilla/firefly values of miR-22-3p+WT (WT/MALAT1 and WT/NLRP3 3′-UTR) groups were significantly lower than that of NC mimic+WT groups. Compared with the NC mimic+MUT (MUT/MALAT1 and MUT/NLRP3 3′-UTR) groups, there were no significant change in the renilla/firefly value of the miR-22-3p+MUT groups (Fig. 4D). The results showed that miR-22-3p can bind with MALAT1 and NLRP3 3′-UTR (Fig. 4D). MiR-22-3p expression was markedly enhanced in LPS group compared to blank group (Fig. 4E). Compared with the corresponding control group, miR-22-3p expression was significantly enhanced in the LPS+si-MALAT1 group, while decreased in the LPS+ov-MALAT1 group (Fig. 4E). To explore function of miR-22-3p on MALAT1/NLRP3, a rescue experiment was performed by transfecting miR-22-3p inhibitor along with si-MALAT1 into LTFs. miR-22-3p expression could be significantly inhibit in LTFs transfected with miR-22-3p inhibitor+si-MALAT1 (Fig. 5A). Compared with NC inhibitor+si-MALAT1 group, the proliferation was reduced while the apoptosis was enhanced in miR-22-3p inhibitor+si-MALAT1 group (Figs. 5B and 5C). Compared with NC inhibitor+si-MALAT1 group, the levels of inflammatory cytokine were increased in miR-22-3p inhibitor+si-MALAT1 group (Fig. 6A), and NLRP3, cl-caspase-1, and N-GSDMD protein also obviously elevated (Fig. 6B). The immunofluorescence assay verified that compared with NC inhibitor+si-MALAT1 group, the N-GSDMD fluorescence intensity in LTFs was facilitated in miR-22-3p inhibitor+si-MALAT1 group (Fig. 6C).
Figure 4: MALAT1 and NLRP3 3′- UTR combine miR-22-3p through competing endogenous.
(A) The miRNAs combined with MALAT1 were analyzed using starbase and RNAInter while it combined with NLRP3 3′-UTR were analyzed using targetscan and RNAInter, then take the intersection of the four sets of results. (B and C) Wild type and mutant sequences bind to miR-22-3p. (D) Double Luciferase experiment confirmed that both MALAT1 and NLRP3 3′- UTR could bind to miR-22-3p. (E) miR-22-3p expression was measured by qRT-PCR in LPS treated FHCs (LTFs) after MALAT1 overexpression and downexpression (***P < 0.001).Figure 5: miR-22-3p interference could block proliferation while promote apoptosis of LPS treated FHCs (LTFs) in the presence of MALAT1 interference.
(A) miR-22-3p expression in LTFs was assessed by qRT-PCR after co-transfected with si-MALAT1+miR-inhibitor. (B and C) The proliferation and apoptosis of LTFs transfected with si-MALAT1+miR-22-3p inhibitor were analyzed after co-transfected with si-MALAT1+miR-inhibitor (**P < 0.01 and ***P < 0.001).Figure 6: miR-22-3p interference could promote pyroptosis of LPS treated FHCs (LTFs) even in the presence of MALAT1 interference.
(A) ELISA was used to examine the secretion levels of inflammatory cytokine in LTFs co-transfected with si-MALAT1+miR-22-3pinhibitor. (B) Proteins in LTFs co-transfected with si-MALAT1+miR-22-3p inhibitor were assessed by western blot. (C) N-GSDMD protein in LTFs was analyzed through immunofluorescence (***P < 0.001).Discussion
UC has become the fast-growing, serious, chronic inflammatory disorder worldwide (Rao et al., 2021). Although UC was not directly fatal, it was the long-term chronic condition that affects quality of life and can cause serious complications (Du et al., 2020). Recent studies have found that key lncRNAs associated with UC provide novel and useful strategies for UC treatment (Li et al., 2021). MALAT1 is highly conserved lncRNA which regulated inflammation. Zhu & Xie (2020) revealed that MALAT1 expression was increased in UC patients, and this promoted FHC apoptosis. Similar to this, this study found that MALAT1 was expressed at high levels in LTFs. Dai et al. (2018) reported that MALAT1 knockdown played the protective role in LPS-induced ALI via reducing inflammatory responses. Similarly, MALAT1 knockdown suppressed apoptosis and pyroptosis in LTFs. These results confirm that MALAT1 knockdown plays a crucial role in the development of UC via promoting FHC apoptosis and pyroptosis, which provide theoretical support for MALAT1 as a promising biomarker for clinical treatment of UC in the future.
NLRP3 was the target for the inhibition of pyroptosis and inflammatory responses (Wang & Hauenstein, 2020). NLRP3 inflammasome activation cleaves pro-caspase-1 and converts it to active caspase-1, which activates the downstream effector proteins of IL-1β, IL-18, and N-GSDMD (Strowig et al., 2012). NLRP3 inflammasome was closely related to the pathogenesis of UC (Zhen & Zhang, 2019). This study indicated that MALAT1 interference facilitates proliferation and suppresses apoptosis and pyroptosis of LTFs by blocking NLRP3 expression. Similar to previous studies, this study indicates that NLRP3 is a target for activating LTFs apoptosis and pyroptosis, promoting the development of UC. Moreover, MALAT1 enhanced NLRP3 expression to mediate pyroptosis and apoptosis in LPS-treated HK-2 cells (Huang & Xu, 2021), which found that the regulatory relationship between MALAT1 and NLRP3 is similar to our findings. These results confirm that MATAL1 functions by regulating NLRP3 activated apoptosis and pyroptosis in LTFs.
MALAT1 can act as a sponge for miRNAs by regulating the expression of downstream genes (Zhou et al., 2021). MALAT1 promotes the expression of NLRP3 by adsorbing miR-224-5p, thereby activating the inflammatory response of microglial cells, particularly in patients with diabetes complicated by obstructive sleep apnea (Du et al., 2020). Additionally, MALAT1 binds to miR-133 to enhance the expression of NLRP3 inflammasome in ischemia-reperfusion injury of the heart (Yu et al., 2018). Furthermore, MALAT1 interacts with miR-22 to influence NLRP3 expression, promoting hyperglycemia-induced pyroptosis in endothelial cells and the progression of atherosclerosis (Song et al., 2019). Moreover, MALAT1 targets NLRP3 by adsorbing miR-30c, facilitating pyroptosis in HK-2 cells and advancing diabetic nephropathy (Liu et al., 2020). The above studies indicate that MALAT1 promotes the expression of NLRP3 and cell pyroptosis by adsorbing different miRNAs. Similarly, this study found that MALAT1 promotes the expression of NLRP3 and cell pyroptosis by adsorbing miR-22-3p. miR-22-3p had the therapeutic potential for treating UC and colorectal cancer (Guo et al., 2022). In addition, miR-22-3p can directly bind NLRP3 3′- UTR to attenuate its protein and inflammation (Li et al., 2018; Wu et al., 2021). Here, we also found that miR-22-3p interference can enhance NLRP3 protein, inflammatory levels, inflammasome, and apoptosis, reversing the effect of MALAT1 interference on LTFs. These results indicated that MALAT1/miR-22-3p/NLRP3 plays the key regulatory role in LTFs.
Nevertheless, our study has some limitations. First, we did not verify whether MALAT1 interference had similar effects in vivo. Second, we did not make further efforts to explore the signaling pathway by which MALAT1 may participate in pyroptosis in LTFs.
In conclusion, our study illustrated that MALAT1 was dramatically decreased in LTFs, and that lncRNA MALAT1 inhibited colonic epithelial cell apoptosis and pyroptosis to relieve ulcerative colitis by silencing NLRP3 expression. Thus, MALAT1 may be a novel biomarker for treatment of UC which is the potentially leading to novel strategies for reducing inflammation and cell death in the colon and UC.