Potential of epicatechin as antioxidant and antiaging in UV-induced BJ cells by regulating COL1A1, FGF-2, GPX-1, and MMP-1 gene, protein levels, and apoptosis
- Published
- Accepted
- Received
- Academic Editor
- Vladimir Uversky
- Subject Areas
- Biotechnology, Cell Biology, Molecular Biology, Science and Medical Education
- Keywords
- Collagen, Elastin, Epicatechin, Fibroblast, Ultraviolet
- Copyright
- © 2024 Widowati et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Potential of epicatechin as antioxidant and antiaging in UV-induced BJ cells by regulating COL1A1, FGF-2, GPX-1, and MMP-1 gene, protein levels, and apoptosis. PeerJ 12:e18382 https://doi.org/10.7717/peerj.18382
Abstract
Background
Oxidative stress caused by exposure to ultraviolet (UV) light on the skin can damage deoxyribonucleic acid (DNA) and cause keratinocytes to undergo apoptosis. Endogenous antioxidants which play a role in trapping free radicals are also unable to overcome excess reactive oxygen species (ROS) in the body due to UV exposure, so exogenous antioxidants are needed. Polyphenolic compounds extracted from natural ingredients such as flavonoids, quercetin, and epicatechin have quite strong antioxidant activity. This is influenced by the chemical structure of these compounds which are rich in hydroxyl groups and aromatic groups. This structure allows the compound to become an electron donor so that it can neutralize free radicals. In vitro research was used to see the potential effectiveness of epicatechin as an antiaging and antioxidant. The study aims to confirm the potential of epicatechin as an antiaging by in vitro assay.
Methods
The viability test of epicatechin on human skin fibroblast (BJ) cells was carried out using the water-soluble tetrazolium (WST) assay. BJ cells were UV-induced as a cell model of premature aging. Epicatechin 6.25, 12.5, and 25 µg/mL were administered to UV-induced BJ cells. The gene expression of Collagen I Alpha 1 (COL1A1), matrix metalloproteinase-1 (MMP-1), fibroblast growth factor-2 (FGF-2), and glutathione peroxidase-1 (GPX-1) were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). Elastin (ELN), hyaluronidase (HAase), cyclooxigenase-2 (COX-2), 8-hydroxydeoxyguanosine (8-OhdG), and melatonin (MT) protein levels were analyzed by enzyme-linked immunosorbent assay (ELISA). The apoptosis of BJ cells was analyzed using flow cytometry.
Results
Treatment with epicatechin increased relative gene expression including COL1A1 (5.94), FGF-2 (8.34), and GPX-1 (8.09), and also decreased MMP-1 (2.90) relative gene expression compared to the UV-induced BJ cells. Epicatechin also increased levels of ELN (107.7 ng/mg protein) and MT (830 ng/mg protein) levels compared to the UV-induced BJ cells. Epicatechin treatment decreased levels of HAase (505.96 ng/mg protein), COX-2 (33.69 ng/mg protein), and 8-OHdG (97.87 ng/mg protein) compared to the UV-induced BJ cells. Epicatechin also succeeded in maintaining the percentage of live cells and reducing apoptosis, necrotic of UV-induced skin fibroblast cells.
Conclusions
Epicatechin has the potential to be an antiaging agent by in vitro assay.
Introduction
The sun is a source of a full spectrum of ultraviolet (UV) radiation. Continuous exposure to UV rays can cause oxidative stress reactions in the skin. This reaction can directly damage deoxyribonucleic acid (DNA) and cause keratinocytes to undergo apoptosis (De Jager, Cockrell & Du Plessis, 2017). This DNA damage will disrupt the regulation of genes that regulate skin homeostasis, including the matrix metalloproteinase (MMP)-1, Collagen Type I Alpha 1 (COL1A1), and fibroblast growth factor (FGF)-2 genes which regulate the defense of the extracellular matrix (ECM) and fibroblasts in the dermis (Tracy, Minasian & Caterson, 2016). Inflammatory mediators such as mitogen-activated protein kinase (MAPK) and nuclear factor kappa beta (NFκB) can also be activated due to oxidative stress, thereby affecting enzymes such as hyaluronidase (HAase) and cyclooxygenase-2 (COX-2) which contribute to skin aging (Fuller, 2019). The complexity of this damage can cause clinical manifestations on the skin such as wrinkles, fine lines, dark spots, and loss of skin firmness (Thawabteh et al., 2023). Exposure to UV light can also stimulate molecules or atoms in the skin to release extra electrons, creating free radicals, for instance, hydroxyl radicals (OH*), superoxide anion (O2*−), and peroxyl radicals (ROO*) which can react with cellular components namely proteins, lipids, and DNA (Di Meo & Venditti, 2020). These free radicals can cause the oxidation of guanine to form 8-hydroxy-deoxyguanosine (8-OHdG) which, if not treated and allowed to continue, can stimulate mutations in the p53 gene which triggers tumor growth (Anik et al., 2022).
To overcome this, the human body has a unique defense mechanism, namely by releasing endogenous antioxidant enzymes that can capture and convert these free radicals into less reactive atoms or molecules (Eddaikra & Eddaikra, 2021). Some of them are superoxide dismutase (SOD), catalase (CAT), melatonin (MT) and glutathione peroxidase-1 (GPX-1). The SOD enzyme works by catalyzing superoxide radical (O2−*) to become less reactive hydrogen peroxide (H2O2). Then hydrogen peroxide is broken down by catalase into water molecules H2O and oxygen O2 which are safe for cells (Dikilitas, Simsek & Roychoudhury, 2020). Melatonin is a potent endogenously-occurring antioxidant that exhibits unique features, such as cascade reaction with free radicals and inducibility under moderate oxidative stress, making it efficient in defending organisms after oxidative damage (Tan et al., 2015). The GPX-1 enzyme also helps remove H2O2 and lipid radicals by using glutathione, a tripeptide structure that can provide electrons to radical molecules (Sarıkaya & Doğan, 2020). However, the production capacity of these endogenous antioxidants is limited, depending on nutrition and the environment. The emergence of excess free radicals due to environmental influences such as UV radiation, air pollution, smoking, and toxic chemicals cannot be handled completely by endogenous antioxidants alone. Therefore, exogenous antioxidants are needed which can help deal with free radicals in the body.
Exogenous antioxidants can be attained from various natural food sources. Consuming foods rich in antioxidants can aid protect the body from oxidative stress and optimize redox balance. Sources of antioxidants are found in many fruits such as berries, pomegranates, kiwi, and snake fruit, they are also found in green leafy vegetables, nuts, and spices (Pap et al., 2021; Girsang et al., 2022; Pandey et al., 2022; Jideani et al., 2021). This natural ingredient contains various flavonoid compounds such as quercetin, kaempferol, resveratrol, and epicatechin which can react with free radicals and convert them into stable molecules (Zeb, 2020; Gebicka, 2020). Epicatechin (EPI) is a compound that has high attention as a potential anti-aging agent due to its antioxidant properties and ability to modulate various biological pathways in cells (Lv et al., 2021). EC can be extracted from natural ingredients such as cocoa, dark chocolate, apple, green tea, etc. to increase compound purity and compound concentration, thereby providing a stronger and more measurable antioxidant effect (Dower et al., 2016). Additionally, the stability and bioavailability of pure compounds will be better compared to consuming natural ingredients that contain other components, so that the compounds can be absorbed more efficiently by the body (Cosme et al., 2020).
EC was chosen in this study because it had strong antioxidant potential and had been shown to modulate various biological pathways in cells, including those participated in the aging process (Shay et al., 2015). Prior research have shown that EPI increased cell viability and increased the expression of elastin (ELN) and collagen (COL) in skin substitutes, making it a potential candidate for the development of anti-aging agents (Grenier et al., 2021). This study explored the potential of EPI as an antiaging agent on UV-induced BJ cells that has not been widely studied. In addition, this study attempts to uncover the specific molecular mechanisms of EPI as an antiaging agent. Thus, this study’s objective is to confirm the potential of EPI on UV-induced BJ cells as aging cells model by analysis COL1A1, MMP-1, FGF-2, and GPX-1 gene expressions, the level of ELN, Haase, COX-2, 8-OhdG and MT also the apoptosis cell. It is hoped that this research will be the basis for developing new strategies to preserve the skin from the impacts of aging due to UV radiation.
Materials and Methods
Cell culture and EPI preparation
In current study, human fibroblast cells were used as research objects. Human Fibroblast (BJ) (ATCC® CRL-2522™) was obtained from Aretha Medika Utama, Bandung. The cells were cultured in a supplemented minimum essential medium (MEM) (Girsang et al., 2019). The cells were then incubated in an incubator (IH3543; Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C with 5% CO2. After a 24-h incubation period, the viable cell count was determined utilizing a hemocytometer (Neubauer), and trypan blue staining (15250-100; Gibco, Billings, MT, USA) was employed to ensure a sufficient cell number for subsequent cytotoxicity assessments. The research sample, EC was obtained from Chengdu Biopurify Phytochemical (E100615). In this research, EC will be used as a treatment to UV-induced BJ cells. A more complete research flow diagram is presented in Fig. S1.
Viability assay
Cells were seeded in 96-well plates (3596; Costar, Washington, D.C, USA) at a density of 1 × 104 cells per well. Cells were cultured for 24 h at 37 °C. The culture medium was substituted with 90 µL new medium culture, and 10 uL EPI (3.13, 6.25, 12.5, 25, 50, 100, and 200 µg/mL). The used concentrations in the study were adapted from previous research (Widowati et al., 2021). The cells were incubated for 24 h at 37 °C. Briefly 10 µL of Cell Counting Kit-8 (CCK-8) buffer (E-CK-A362; Elabscience, Houston, TX, USA) was added and incubated for 3 h. Spectrophotometry (51119300; Multiskan GO Thermo Scientific, East Lyme, CT, USA) at a wavelength of 450 nm was employed to measure the sample absorbance (Widowati et al., 2021).
qRT-PCR assay
After obtaining the safest concentrations, namely 6.25, 12.5 and 25 µg/mL, BJ cells were cultured on 6-well plates with a density of 5 × 105 by dividing them into six groups, namely negative control (NC), UV-irradiated (PC), UV irradiated + 1% DMSO (CDMSO), UV irradiated + 6.25 µg/mL EPI (EPI6.25), UV irradiated + 12.5 µg/mL EPI (EPI12.5), UV irradiated + 25 µg/mL EPI (EPI25). After 24 h of incubation, cells were exposed to UVB for 75 min, except NC, and given treatment according to the treatment group. After 24 h of incubation, RNA was extracted from BJ cells utilizing the TRI Reagent (R2050-1-200; Zymo Research, Irvine, Murphy Ave, USA) and Direct-zol™ RNA Miniprep Plus (R2073; Zymo Research, Irvine, Murphy Ave, USA) following the manufacturer’s guidelines. Estimation of the total RNA yield was conducted using a spectrophotometer (51119300; Multiskan GO Thermo Scientific, East Lyme, CT, USA) at 260 and 280 nm. The RNA concentration and purity are presented in Table 1. SensiFAST cDNA Synthesis Kit (BIO-65054; Bioline, Tennessee, UK) was used to synthesize cDNA from RNA with a three-stage protocol, priming for 5 min at 25 °C, reverse transcription for 30 min at 42 °C, and reverse transcription inactivation for 5 min at a temperature of 85 °C with polymerase chain reaction (PCR) Gradient Thermal Cycler (GTC96S-230; Cleaver Scientific, Uddingston, Scotland, UK). The primers employed for real-time PCR are outlined in Table 2. PCR amplification was conducted using the AriaMx Real-time PCR System (G8830A; Agilent, Santa Clara, CA, USA) with a total reaction volume of 20 µL (2 µL cDNA, 10 µL SensiFast SYBR No-ROX, 0.8 µL Primer forward, 0.8 µL Primer Reverse and 6.4 uL Nuclease free water) in 96-PCR plate (AB1384W; Thermo-scientific, Waltham, MA, USA). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the internal control. The qPCR conditions comprised an initial pre-denaturation step at 95 °C for 5 min, followed by 40 cycles of qPCR, involving denaturation at 95 °C for 50 s, annealing at 54 °C (MMP-1), 57 °C (COL1A1), 58 °C (GAPDH and FGF-2) and 59 °C (GPX-1) for 50 s, and elongation at 72 °C for 50 s (Widowati et al., 2024). 2^(–delta delta CT) method was use for qPCR analysis (Rao et al., 2013). Isolated RNA and residual cDNA can be stored at −80 °C for long-term use.
| No. | Sample | Concentration (ng/µl) | Purity (λ260/λ280 nm) |
|---|---|---|---|
| 1 | NC | 18.90 | 2.2771 |
| 2 | PC | 15.90 | 2.1200 |
| 3 | KDMSO | 12.60 | 2.0656 |
| 4 | EPI1 | 12.90 | 1.9545 |
| 5 | EPI2 | 13.10 | 1.8194 |
| 6 | EPI3 | 12.50 | 1.9841 |
| Gene | Primer sequences (5′-3′) | Product length (bp) | Annealing (°C) | Cycle | Reference |
|---|---|---|---|---|---|
| FGF-2 (Homo Sapiens) | GGCTTCTTCCTGCGCATCCA | 354 | 58 | 40 | NM_002006.6 |
| GCTCTTAGCAGACATTGGAAGA | |||||
| GPX-1 (Homo Sapiens) | CCAAGCTCATCACCTGGTCT | 127 | 59 | 40 | NM_001329455.2 |
| TCGATGTCAATGGTCTGGAA | |||||
| MMP-1 (Homo Sapiens) | CTGAAGGTGATGAAGCAGCC | 428 | 54 | 40 | NM_001145938.2 |
| AGTCCAAGAGAATGGCCGAG | |||||
| COL1A1 (Homo Sapiens) | CGGCTCCTGCTCCTCTTAG | 137 | 57 | 40 | XM_054315083.1 |
| CACACGTCTCGGTCATGGTA | |||||
| GAPDH (Homo Sapiens) | GGGCTGCTTTTAACTCTGGT | 648 | 58 | 40 | NM_001357943.2 |
| TGGCAGGTTTTTCTAGACGG |
ELISA assay
ELN, HAase, COX-2, 8-OHdG, and MT levels were analyzed according to Priyandoko et al. (2024). Enzyme-linked immunosorbent assay (ELISA) testing follows the protocol according to kit, namely the Human ELN ELISA Kit (E-EL-H1163; Elabscience, Houston, TX, USA), the Human HAase ELISA Kit (E-EL-H2201; Elabscience, Houston, TX, USA), Human COX-2 ELISA Kit (E-EL-H5574), 8-OHdG ELISA Kit (E-EL-0028; Elabscience, Houston, TX, USA), and the Human MT ELISA Kit (E-EL-H2016, Elabscience, Houston, TX, USA). The absorbance was measured using a microplate reader (MultiskanTM Spectrophotometer; Thermo Fisher Scientific, Waltham, MA, USA).
Apoptosis assay
The apoptosis percentage (live cell, necrosis, early and late apoptosis) of BJ cells was analyzed according to Widowati et al. (2023) with modifications. Each treatment group was analyzed using the Annexin V-FITC/PI Apoptosis Detection Kit (ECK-A211; Houston, TX, USA) according to the manufacturer’s instructions. The treated cells were harvested and rinsed using 1 mL of fluorescence-activated cell sorting (FACS) buffer and centrifuged at 1,600 rpm for 5 min. The cell pellet was then added with 500 µL of Annexin binding buffer. Then the samples were stained using 5 µL Annexin V fluorescein isothiocyanate-conjugated (FITC)/propidium iodide (PI) and 5 µL PI (30 min incubation, 4 °C (dark room). Cell apoptosis was measured using MACSQuant 10 flow cytometry.
Statistical analysis
The data is statistically analyzed with Statistical Package for the Social Sciences (SPSS) (version 23.0; SPSS Inc, Chicago, IL, USA) using one-way Analysis of variance (ANOVA), followed by Tukey HSD posthoc test for normally distributed and homogeneous data, while the Independent T-test was performed for normally distributed, but not homogeneous data. P value ≤ 0.05 was considered as the significance value of the data. Data were visualized as mean ± standard deviation from three replicates in histograms using the GraphPad Prism application (version 8.0.244).
Results
Cells viability of BJ cells
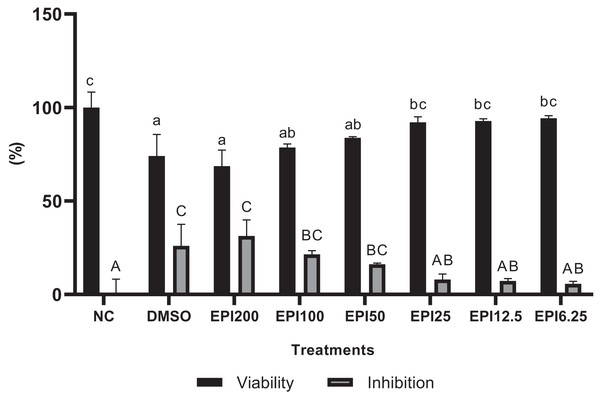
The results of the viability test for BJ cells treated with EPI can be seen in Fig. 1. The results show that EPI was not toxic to BJ cells, based on the data of percentage viability showing the highest concentration, reduced cell viability by 30%. Thus, for further testing, a concentration range was chosen with cell viability results above 90%, namely 25, 12.5, and 6.25 µg/mL.
Figure 1: Results of epicatechin viability and inhibition tests on BJ cells.
*NC, negative control; DMSO, DMSO control; EPI, treatment with epicatechin. All the differences in letters show a significant difference between the treatments based on the independent t-test.Effect of EPI toward FGF-2, GPX-1, MMP-1, COL1A1 genes expression on UV-induced BJ cells
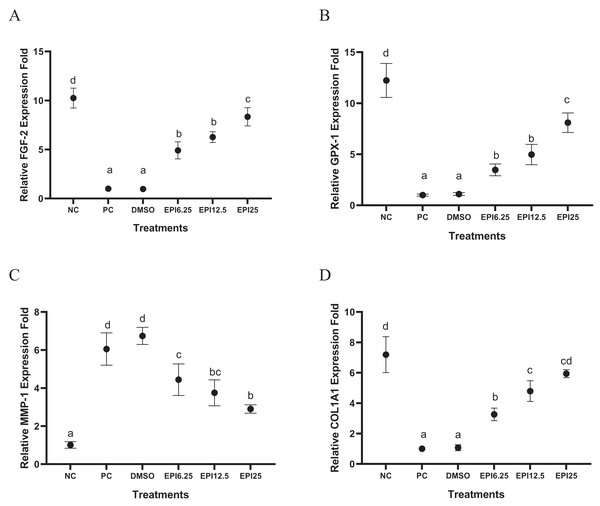
The results of gene expression in UV-induced BJ cells treated with EPI can be seen in Fig. 2. UV induction influenced BJ cells gene expression by the significant differences between the NC (I) and PC (II) groups in each gene expression. Treatment with EPI increased the gene expression of the COL1A1 by 7-fold compared to the positive control. GPX-1 gene, by eight-fold compared to the positive control, and FGF-2 gene by eight-fold compared to the positive control. EPI reduced MMP-1 gene expression in UV-induced BJ cells. The most optimal concentration for increasing COL1A1, GPX-1, and FGF-2 genes expression, as well as decreasing MMP-1 was EPI at concentration 25 µg/mL.
Figure 2: Effect of epicatechin on COL1A1 (A), GPX-1 (B), FGF-2 (C), and MMP-1 (D) gene expression in UV-induced BJ cells.
*NC, negative control; PC, positive control (UV-induced) DMSO, DMSO control; EPI6.25, treatment EPI 6.25 ug/mL; EPI12.5, treatment EPI 12.5 ug/mL; EPI25, treatment EPI 25 ug/mL. The differences in letters show a significant difference between the treatments in COL1A1 and MMP-1 based on the Independent T-test. The differences in letters show a significant difference between the treatments in GPX-1 and FGF-2 based on the independent t-test.Effect of EPI toward ELN, HAase level on UV-induced BJ cells
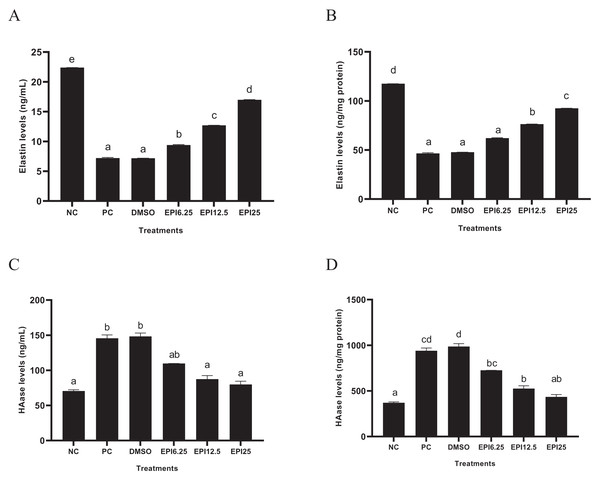
The results of ELN and HAase levels in UV-induced BJ cells treated with EPI are presented in Fig. 3. The results show that UV induction caused a decrease in ELN and an increase in HAase in BJ cells. Treatment with EPI increased ELN and decreased HAase in UV-induced BJ cells. EPI treatment with a concentration of 25 µg/mL was the most active to increase ELN level and decrease HAase level.
Figure 3: Effect of EPI on elastin (A) and hyaluronidase (B) protein expression in UV-induced BJ cells.
*NC, negative control; PC, positive control (UV-induced) DMSO, DMSO control; EPI6.25, treatment EPI 6.25 ug/mL; EPI12.5, treatment EPI 12.5 ug/mL; EPI25, treatment EPI 25 ug/mL. All the differences in letters show a significant difference between the treatments based on the independent t-test.Effect of EPI toward COX-2, 8-OHdG, MT levels on UV-induced BJ cells
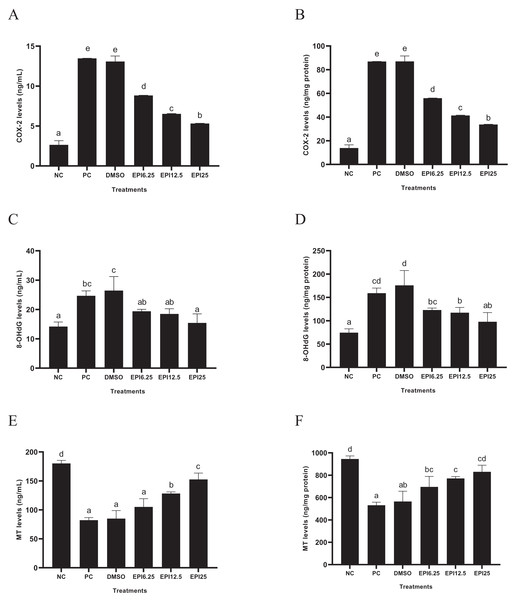
The results of COX-2, 8-OHdG, and MT levels on UV-induced BJ cells treated with EPI are presented in Fig. 4. The results show that UV induction increased COX-2 and 8-OhdG levels and decreased MT levels in BJ cells. Treatment with EPI can reduce COX-2 and 8-OhdG levels and increase MT levels in UV-induced BJ cells. EPI treatment at a concentration of 25 µg/mL reduced COX-2 and 8-OHdG levels and increased MT protein better than other concentrations.
Figure 4: Effect of epicatechin on COX-2 (A), 8-OhdG (B) and MT (C) protein expression in UV-induced BJ cells.
*NC, negative control; PC, positive control (UV-induced) DMSO, DMSO control; EPI6.25, treatment EPI 6.25 ug/mL; EPI12.5, treatment EPI 12.5 ug/mL; EPI25, treatment EPI 25 ug/mL. All the differences in letters show a significant difference between the treatments based on the independent t-test.Apoptosis
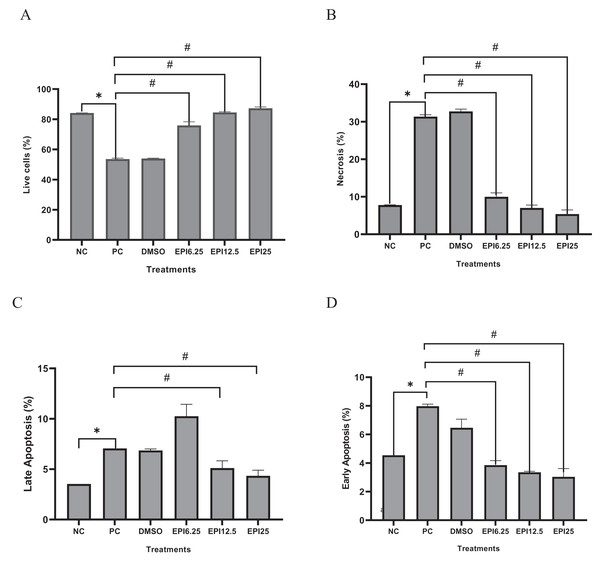
Figure 5 shows the comparison of different treatments of EPI toward live cells, necrosis cells, late apoptosis, and early apoptosis. The findings demonstrated that the administration of EPI increased the number of live cells and inhibited necrosis and apoptosis. Increasing the EPI concentration decreased the percent apoptosis.
Figure 5: Effect of epicatechin treatment on apoptosis in BJ cells UV-induced.
NC, negative control; PC, positive control (UV-induced) DMSO, DMSO control; EPI6.25, treatment EPI 6.25 ug/mL; EPI12.5, treatment EPI 12.5 ug/mL; EPI25, treatment EPI 25 ug/mL. The asterisk (*) indicates significance between NC and PC, while the number sign (#) indicates significance between treatment and PC.Discussion
Oxidative stress caused by exposing the skin fibroblast to UV can damage DNA and apoptosis of keratinocytes (Panich et al., 2016). Endogenous antioxidants which play a role in trapping free radicals are also unable to overcome excess ROS in the body due to UV exposure, so exogenous antioxidants are needed. Polyphenolic compounds extracted from natural ingredients such as flavonoids, quercetin, and epicatechin have quite strong antioxidant activity with IC50 < 50 µg/mL (Kaurinovic & Vastag, 2019). This antioxidant is influenced by the chemical structure of these compounds which are rich in hydroxyl groups and aromatic groups. This structure allows the compound to become an electron donor so that it can neutralize free radicals (Olszowy, 2019). In vitro research was used to see the potential effectiveness of EPI as an antiaging and antioxidant.
The results of the cell viability assay revealed that EPI did not exhibit a toxic effect on BJ cells. These data results show the still high cell viability after the administration of EPI (Fig. 1). For further research, three concentrations were selected based on highly viable cells. These concentrations were 25, 12.5, and 6.25 µg/mL. The results data show that UV induction in BJ cells significantly influenced gene expression and measured protein levels in this study, proven by significant differences (p < 0.05) between NC and PC for each parameter. This finding corresponds with earlier research which showed a rise in 8-OHdG as a marker of oxidative stress due to UV induction in cells (Farooqi et al., 2015). UV exposure increases gene expression which degrades COL, and MMP-1 (Kusumaningrum et al., 2018).
The UV induction significantly decreased FGF-2 gene expression in PC compared to NC (p < 0.05) on UV-induced BJ cells. The results show that EPI could increase FGF-2 gene expression in BJ cells (Fig. 2A). The most effective concentration in increasing FGF-2 gene expression was 25 µg/mL. FGF-2 is a growth factor that is very important for tissue regeneration, by stimulating the proliferation and differentiation of various cell types (Farooq et al., 2021). Other research shows that dalteparin and protamine nanoparticles containing FGF-2 are known to improve UVB-induced photoaging in nude mice skin (Takabayashi et al., 2016), thus FGF-2 is needed to increase in treating skin aging (Takabayashi et al., 2016).
The results show that UV exposure downregulated GPX-1 gene expression significantly in PC compared to NC (p < 0.05) on UV-induced BJ cells. EPI administration could significantly increase GPX-1 gene expression. EPI treatment at a concentration of 25 µg/mL was the most effective to increase GPX-1 gene expression (Fig. 2B), this result was in line with a previous study (Kim et al., 2017) which showed EPI obtained from Momordica charantia can increase levels of Superoxide Dismutase (SOD) and GPX-1. UV exposure is known to increase ROS levels in cells, especially in mitochondria. GPX-1 can maintain mitochondrial function by reducing oxidative damage and reducing the aging process (Handy & Loscalzo, 2022). The EPI can inhibit p38 phosphorylation and the c-Jun N-terminal protein kinases (JNK) pathway as well as downregulate caspase-3 (CASP-3) cleavage and poly (adenosine diphosphate ribose) polymerase (PARP) cleavage (Sharifi-Rad et al., 2020). EPI is higher antioxidant activity compared to quercetin in increasing GPX-1, quercetin can increase GPX-1 expression 4 folds while EPI increases GPX-1 gene expression 7 folds (Wang et al., 2014).
The UV induction significantly increased MMP-1 gene expression in PC compared to NC (p < 0.05) on UV-induced BJ cells. EPI administration could significantly decrease MMP-1 gene expression. EPI treatment at a concentration of 25 µg/mL was the most effective to decrease MMP-1 gene expression (Fig. 2C). UV exposure has been widely studied to cause DNA damage which impairs gene regulation in the skin, especially genes that influence aging such as MMP-1 (Ganguly, Hota & Pradhan, 2021). The in silico study exhibited EPI can bind to MMP-1 with a binding affinity of (−7,862 kcal/mol) greater than epigallocatechin gallate (EGCG) (−6,488 kcal/mol) (Lee et al., 2020). Epicatechin in Nypa fruticans was known can contribute to reducing MMP-1 activity, collagen degradation, and downregulating MAPK/NF-κB/AP-1 signaling in UVB-induced skin aging (Choi et al., 2020).
UV exposure significantly decreased COL1A1 gene expression in the treatment group (PC) compared to the normal control group (NC) (p < 0.05) in UV-induced BJ cells. However, EPI administration was able to significantly increase COL1A1 gene expression. Among the various concentrations tested, treatment with EPI at a concentration of 25 µg/mL showed the highest effectiveness in increasing COL1A1 gene expression (Fig. 2D). This finding is in line with a study conducted by Liu et al. (2018), which reported that epicatechin derived from hawthorn polyphenol extract effectively decreased MMP-1 synthesis and increased type I procollagen formation in skin cells exposed to UVB radiation. This suggests that EPI has the potential to be used as a protective agent against UV-induced collagen damage.
The results showed that UV exposure significantly decreased ELN levels in PC compared to NC (p < 0.05). EPI treatment significantly decreased ELN levels (p < 0.05) in UV-induced BJ cells. Administration of EPI at 25 μg/mL was the most active in increasing ELN levels in UV-induced BJ cells (Fig. 3A). ELN is a very important component in dermis tissue, which this tissue is very sensitive to UV exposure. Other research shows that another member of the catechin compound group, namely EGCG can inhibit intracellular elastase and collagenase activity and maintain ELN levels in the dermis tissue (Wang et al., 2019a, 2019b). Other studies support these results, namely that epicatechin is known to have the ability to inhibit elastase and collagenase activity, helping to maintain elastin levels in the skin (Agudelo et al., 2021).
The UV exposure significantly increased HAase level in PC compared to NC (p < 0.05). EPI treatment significantly reduced HAase levels (p < 0.05) in UV-induced BJ cells. The results showed that administering EPI at a concentration of 25 µg/mL could significantly reduce HAase levels (p < 0.05) in UV-induced BJ cells (Fig. 3B). HAase is an enzyme responsible the hyaluronic acid degradation, which is the main component of the ECM (Buhren et al., 2016). In an in silico study, EPI was non-competitive inhibition (mixed type) against HAase based on the Lineweaver-Burk plot (Tomohara et al., 2017). Another study showed that epicatechin from Ravenala madagascariensis can inhibit HAase activity, leading to increased hyaluronic acid levels (Mohamed et al., 2020)
The exposure of UV increased significantly COX-2 levels in PC compared to PC (p < 0.05) in BJ cells. EPI treatment significantly reduced COX-2 levels (p < 0.05) in UV-induced BJ cells. Administration of EPI at 25 µg/mL was the most active in reducing COX-2 level in UV-induced BJ cells (Fig. 4A). COX-2 is an enzyme that plays a role in inflammatory processes and oxidative stress. Inflammation is known to cause DNA damage and induce cellular dysfunction. Inflammation can also cause various clinical manifestations, including wrinkles, fine lines, decreased skin thickness, hyperpigmentation, and melasma (Silpa-Archa et al., 2017). Previous research exhibited that EPI was found to reduce COX-2 levels in RAW 264.7 cells induced by Lipopolysaccharide (LPS). EPI can also reduce other pro-inflammatory parameters, namely interleukin-1β (IL-1β), IL-6, tumor Necrosis factor-α (TNF-α), Nitric Oxide (NO) and prostaglandin E-2 (PGE-2) (Yang et al., 2015).
The UV exposure significantly increased 8-OHdG level in PC compared to NC (p < 0.05) on UV-induced BJ cells. EPI treatment significantly reduced 8-OHdG level (p < 0.05) on UV-induced BJ cells. The use of EPI at a concentration of 25 µg/mL was the most effective in reducing 8-OHdG level (Fig. 4B). The 8-OHdG is one of the most commonly used biomarkers to measure oxidative DNA damage (Graille et al., 2020). DNA damage plays an important role in aging and the development of various age-related degenerative diseases (Graille et al., 2020). The data of results research was due to the antioxidant activity of EPI which could trap free radicals. In previous research, it was found that EPI and its relatives such as catechin, EGCG, epicatechin gallate (ECG), and catechin gallate (CG) showed higher 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) scavenging capacity and higher iron-reducing antioxidant activity compared to other flavonoids such as morin, naringenin, naringin, and rutin (Grzesik et al., 2018).
The exposure to UV rays reduced MT level in PC compared to NC (p < 0.05) on UV-induced BJ cells. EPI treatment significantly increased MT level (p < 0.05) on UV-induced BJ cells. The use of EPI at a concentration of 25 µg/mL was the most effective to increase MT level (Fig. 4C). MT is a hormone produced by the pineal gland in the brain which plays an important role in regulating the circadian cycle (Kvetnoy et al., 2022). However, MT is a powerful antioxidant capable of neutralizing ROS in the aging process (Reiter et al., 2016). Other research shows a positive correlation between EPI and MT as antioxidants (Lili et al., 2018). Apart from being found endogenously, MT can also be obtained exogenously. The combined results of MT and epicatechin therapy can increase antioxidant capacity in vitro and in vivo (Wang et al., 2018).
The exposure to UV rays decreased live cells, increased necrosis, and apoptosis both late and early apoptosis in PC compared to NC (p < 0.05) on UV-induced BJ cells. Administration of EPI has been proven to be effective in reducing apoptosis and maintaining the viability of UV-induced fibroblast cells (Fig. 5). Like epicatechin, another polyphenol compound, chlorogenic acid, also shows the same effect in maintaining skin fibroblast cells and reducing necrosis and apoptosis in cells (Girsang et al., 2021). Thus, epicatechin compounds have a role in various signaling pathways, both gene and protein expression. The signaling mechanisms in these parameters are interrelated and influence the aging process. The proposed mechanism can be seen in Fig. 6.
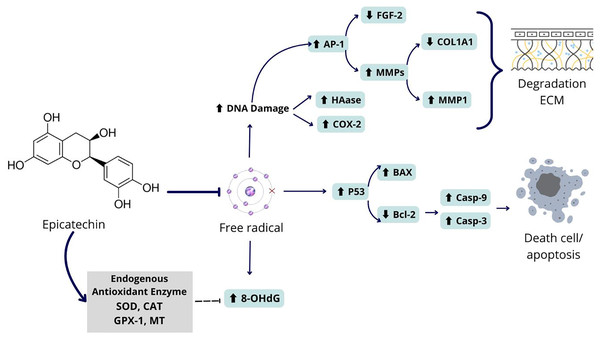
Figure 6: Proposed mechanism of epicatechin as an antiaging and antioxidant.
Epicatechin compounds show potential as an antiaging agents with comprehensive mechanisms. UV exposure to BJ cells can cause oxidative stress in cells due to the formation of free radicals in cells. These free radicals can cause DNA damage which can damage the regulation of genes and proteins related to aging. Among the damage that occurs is increasing the expression of the AP-1 gene which has an impact on increasing MMP1 and decreasing COL1A1, causing ECM degradation. Apart from that, free radicals can also cause activation of the P53 gene which leads to apoptosis in fibroblast cells. Epicatechin can prevent the formation of free radicals by increasing the levels of endogenous proteins in cells that can break down free radicals into safer molecules. Thus, epicatechin can prevent collagen degradation and cell death.Conclusions
Epicatechin can regulate various aging-related genes by increasing COL1A1, FGF-2, and GPX-1 gene expression while reducing MMP-1 gene expression. COL1A1 and FGF-2 are crucial for collagen synthesis and skin repair, while GPX-1 plays a vital role in antioxidant defense. Additionally, epicatechin increases ELN and MT levels and decreases HAase, COX-2, and 8-OHdG levels on UV-irradiated BJ cells, its ability to protect the skin from oxidative damage and extracellular matrix degradation. Epicatechin also reduces apoptosis in BJ cells, highlighting its potential as an anti-aging agent. This study significantly advances the understanding of genes regulation in aging during UV exposure, showing that epicatechin not only acts as an antioxidant but also supports skin repair and maintenance. These findings bridge gaps in previous research and present a potential and possibly more effective alternative to current antioxidant agents, offering new hope for anti-aging therapies.