Emerging roles of tRNA-derived small RNAs in injuries
- Published
- Accepted
- Received
- Academic Editor
- Rodolfo Aramayo
- Subject Areas
- Biochemistry, Cell Biology, Molecular Biology
- Keywords
- tsRNAs, Stress, Injury, Biomarkers
- Copyright
- © 2024 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Emerging roles of tRNA-derived small RNAs in injuries. PeerJ 12:e18348 https://doi.org/10.7717/peerj.18348
Abstract
tRNA-derived small RNAs (tsRNAs) are a novel class of small noncoding RNAs, precisely cleaved from tRNA, functioning as regulatory molecules. The topic of tsRNAs in injuries has not been extensively discussed, and studies on tsRNAs are entering a new era. Here, we provide a fresh perspective on this topic. We systematically reviewed the classification, generation, and biological functions of tsRNAs in response to stress, as well as their potential as biomarkers and therapeutic targets in various injuries, including lung injury, liver injury, renal injury, cardiac injury, neuronal injury, vascular injury, skeletal muscle injury, and skin injury. We also provided a fresh perspective on the association between stress-induced tsRNAs and organ injury from a clinical perspective.
Introduction
Since their discovery in 1958, transfer RNAs (tRNAs) have been fundamental in decoding genetic information by translating messenger RNAs (mRNAs) into proteins (Hoagland et al., 1958). Our understanding of tRNAs has advanced significantly, allowing us to categorize their effects into canonical and non-canonical roles. In their canonical role, tRNAs are tightly regulated, and even minor alterations can lead to disease states (Orellana, Siegal & Gregory, 2022). In their non-canonical role, tsRNAs, precisely cleaved from tRNAs, have emerged as a fascinating research area (Orellana, Siegal & Gregory, 2022). tsRNAs constitute a novel class of small noncoding RNAs (snoRNAs) with functional fragments ranging from 18 to 40 nucleotides. A comprehensive review illuminates the intricate biology of tsRNAs, covering tRNA and tsRNA sequences, modifications, structures, and interactions with RNA-binding proteins (Kuhle, Chen & Schimmel, 2023). Furthermore, tsRNAs hold promise as potential therapeutic targets and diagnostic biomarkers for various conditions, including cancer (Mao et al., 2023; Yu et al., 2021), neurological diseases (Fagan, Helm & Prehn, 2021), viral infections (Yu et al., 2021), and cardiovascular disorders (Cao, Cowan & Wang, 2020; Wang et al., 2023). However, the topic of tsRNAs in injuries has not been systematically reviewed, to the best of our knowledge.
Stress conditions, including oxidative stress, nutritional deprivation, and hypoxia, play crucial roles in tissue and organ injuries. The cleavage of tRNAs at the anticodon loop in response to amino acid starvation was first discovered in Tetrahymena thermophila using Northern blotting and RNA cloning in 2005 (Lee & Collins, 2005). This phenomenon has since been observed in heat shock, hypothermia, hypoxia, and oxidative stress (Fu et al., 2009; Thompson et al., 2008). The production of tsRNAs can be induced by angiogenin (ANG), a secreted ribonuclease (Fu et al., 2009; Yamasaki et al., 2009). Additionally, ANG-generated tRNA-derived stress-induced RNAs (tiRNAs) can inhibit protein synthesis (Yamasaki et al., 2009; Ivanov et al., 2011) and apoptosis (Saikia et al., 2014). These early studies highlighted the crucial roles of tsRNAs during stress. However, traditional research methods used in early studies cannot accurately quantify tsRNAs due to an average of 13 modifications per tRNA molecule (Pan, 2018). Recently, advanced next-generation sequencing techniques have been used to detect tsRNAs by removing RNA chemical modifications (Li et al., 2022b; Shi et al., 2021). Li et al. (2022b) used an AlkB-facilitated methylation sequencing technique to reveal cellular and extracellular tsRNA abundances across various cell types during different stress responses. Stress is closely related to various injuries, and tsRNAs also play a role in these injuries. For instance, tRF-Gln-CTG-026 shows robust functionality in mitigating liver injury (Ying et al., 2023). In terms of vascular injury, tiRNA-Glu-CTC induces vascular injury through mitochondrial damage (Zhang et al., 2024). Similarly, tRF-Glu-CTC expression increases after vascular injury and inhibits fibromodulin expression in vascular smooth muscle cells (Jiang et al., 2024).
While the topic of tsRNAs in injuries remains largely unexplored, this review provides a fresh perspective. This review will interest readers studying injuries, such as lung, liver, renal, cardiac, and neuronal injuries. Additionally, researchers studying tsRNAs and stress may find this review valuable. This review will help researchers in tsRNA biology or injury diseases fully understand the role of tsRNAs in various injuries.
Survey methodology
We searched related literature on PubMed using the keywords: (“tRFs” OR “tsRNAs” OR “tDRs”) AND (“injury” OR “cell stresses”). We excluded studies unrelated to injury and stress. Articles that were not research articles or reviews were also excluded. Reference lists of related studies were screened to identify additional articles not found in the online search.
An overview of the classification of tsrnas
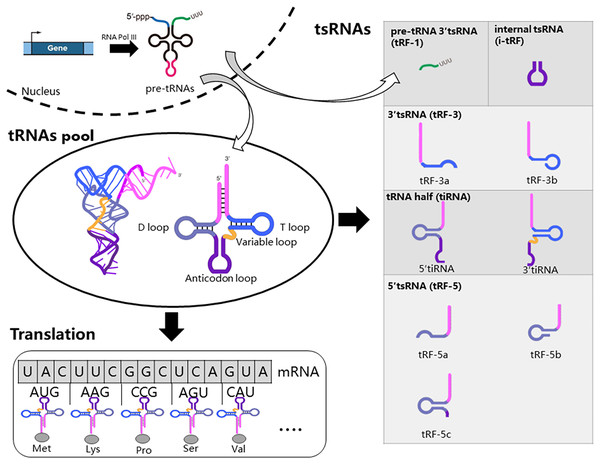
Firstly, we introduced the generation, structure, and function of tRNAs, as tsRNAs are cleaved from tRNAs. RNA Polymerase III actively transcribes tRNA genes into pre-tRNAs. Pre-tRNAs then undergo additional processing steps before maturation, including cleavage of the 5′ PPP-leader and 3′ trailer-UUU sequence, and the addition of the -CCA triplet at the 3′ end (Hu et al., 2022b; Orellana, Siegal & Gregory, 2022). Mature tRNAs typically consist of 70 to 90 nucleotides (nt) and adopt a cloverleaf secondary structure with four loops: the dihydrouracil loop (D-loop), anticodon loop, variable loop, and pseudouridine loop (TψC loop) (Fig. 1) (Hu et al., 2022b; Orellana, Siegal & Gregory, 2022). Furthermore, tRNAs undergo numerous chemical modifications crucial for their function and the biogenesis of tsRNAs (Di Fazio & Gullerova, 2023; Pan, 2018). Mature tRNAs are key molecules in mRNA decoding and protein translation (Fig. 1). Specifically, tRNAs recognize mRNA codons using the anticodon and accurately deliver the corresponding amino acid to the ribosome via their 3′ end. tRNAs are transcribed from multiple genes, and tsRNAs can be generated from various tRNA sources (Li, Xu & Sheng, 2018).
Figure 1: Biogenesis and classification of tsRNAs.
RNA Polymerase III actively transcribes tRNA genes into pre-tRNAs, which then undergo additional processing steps before maturation. Mature tRNAs are key molecules in mRNA decoding and protein translation. Specific nucleases cleave tRNA at distinct sites, resulting in five major types of tsRNAs: tRF-1, tRF-3, tRF-5, i-tRF, and tiRNA. tRF-1 molecules originate from the 3′-trailer sequence of pre-tRNA molecules.The production of tsRNAs involves a regulated cleavage process from both pre-tRNAs and mature tRNAs (Magee & Rigoutsos, 2020). Specific nucleases cleave tRNA at distinct sites, resulting in five major types of tsRNAs: tRF-1, tRF-3, tRF-5, i-tRF, and tiRNA (Fig. 1) (Hu et al., 2022b). tRF-1 molecules originate from the 3′-trailer sequence of pre-tRNA molecules, which includes the termination signal for RNA Pol III transcription (Mao et al., 2023). During tRNA maturation, the 3′-trailer sequence of pre-tRNA is removed, and the CCA sequence is added simultaneously. tRF-3 molecules span from the 3′ end to the T loop of mature tRNAs. tRF-3 can be further classified into tRF-3a and tRF-3b based on their length (18 or 22 nucleotides). tRF-5 molecules begin at the 5′-end of tRNA and end at either the D-loop or the anticodon loop. tRF-5 molecules are further categorized as tRF-5a (14–16 nt), tRF-5b (22–24 nt), and tRF-5c (28–30 nt). i-tRFs originate from internal regions of mature tRNAs, excluding the termini. tiRNAs, ranging from 30 to 40 nucleotides in length, result from cleavage at the anticodon region of mature tRNAs, yielding two fragments: 5′ tiRNAs and 3′ tiRNAs (Di Fazio & Gullerova, 2023; Lee et al., 2023; Mao et al., 2023).
Generation of tsrnas in response to stress
Stress can impact tRNA abundance through processes such as tRNA transcription, stability, chromosomal arrangement, transport, modifications, and fragmentation (Aswathi et al., 2023). These factors may influence the generation of tsRNAs. Here, we focus on how tRNA molecules are cleaved into tsRNAs under stress.
Several types of stress can cause the cleavage of tRNAs, as identified by Northern blotting: nutritional deficiency, heat shock, hypothermia, hypoxia, and oxidative stress (Lee & Collins, 2005; Fu et al., 2009; Thompson et al., 2008). These studies revealed that the cleavage of tRNAs in response to stress, not specific to individual tRNAs, is a conserved phenomenon across different life forms. Specific ribonucleases can induce the cleavage of tRNAs in response to stress. ANG, a member of the RNase superfamily, was found to produce tiRNAs (Fu et al., 2009). Yamasaki et al. (2009) reported that knockdown of angiogenin inhibits arsenite-induced tiRNA production. Mechanistically, ANG is located in the nucleus under growth conditions but relocates to the cytoplasm under oxidative stress, remaining enzymatically active for tiRNA production (Pizzo et al., 2013). However, a study using a global short RNA-Seq approach under ANG knockout or overexpression demonstrated that the majority of stress-induced tRNA halves can be generated by an ANG-independent pathway (Su et al., 2019). tsRNA production also depends on other enzymes, including RNase T2, Dicer, and RNase Z/ELAC2 (Magee & Rigoutsos, 2020), but the relationship between these enzymes and stress requires further research.
tRNA modification can affect the production of tsRNAs. NSUN2 methylates tRNAs site-specifically at either the anticodon or variable loop, protecting them from ANG cleavage. Under oxidative stress, the cytosine-5 RNA methyltransferase NSUN2 is significantly down-regulated, leading to reduced methylation at specific tRNA sites, which significantly impacts the biogenesis of tsRNAs (Gkatza et al., 2019). This study used quantitative mass spectrometry and RNA bisulfite sequencing to confirm remethylation of NSUN2-specific sites. Additionally, the tRNA methyltransferase TRMT2A induces m5U54 tRNA hypomodification. TRMT2A knockdown in human cells induces m5U54 tRNA hypomodification, followed by overexpression of ANG, which cleaves tRNAs near the anticodon, resulting in the accumulation of 5′ tiRNAs (Pereira et al., 2021). On the other hand, the dysregulation of tRNA modifications under hypobaric hypoxia increased the sensitivity of tRNA to RNases, affecting the molecular stability of tissue total tRNA-enriched fragments (Guo et al., 2023). This study used an RNA modification detection platform based on liquid chromatography-tandem mass spectrometry.
Biological functions of tsrnas in response to stress
Translation regulation
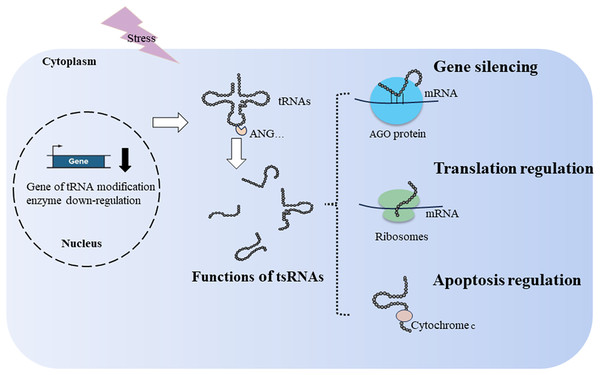
Yamasaki et al. (2009) discovered that transfection of ANG-induced tiRNAs promotes phospho-eIF2α-independent translational arrest. The same group found that transfection of natural or synthetic 5′-tiRNAs induces the assembly of stress granules (Emara et al., 2010). They also found that 5′ tiRNA-Ala and 5′tiRNA-Cys possess terminal oligoguanine motifs (4–5 guanine residues) at their ends, allowing them to fold into G-quadruplex-like structures. These structures can interact with the translational repressor Y-box binding protein 1, replacing eukaryotic initiation factor 4F, leading to stress granule assembly and translation inhibition (Ivanov et al., 2011, 2014). However, recent literature revealed that neither physiological nor non-physiological copy numbers of tsRNAs induced the formation of stress granules (Fricker et al., 2019; Sanadgol et al., 2022), challenging the established role of tsRNAs in stress granule assembly. In summary, 5′tiRNAs can inhibit protein synthesis by interfering assembly of the pre-initiation complex and inducing stress granule assembly. The role of tsRNAs in stress granule assembly should be reconfirmed in future studies.
tsRNAs can bind to ribosomes to regulate translation. In the simple eukaryotic organism Saccharomyces cerevisiae, 3′tsRNAs and 5′tsRNAs directly bind to ribosomes in vitro filter-binding assays (Fig. 2). The binding position of tsRNAs within ribosomes differs from classical A- and P-tRNA binding sites, resulting in protein biosynthesis inhibition during specific environmental stress (Bąkowska-Żywicka, Kasprzyk & Twardowski, 2016). Similarly, Gebetsberger and colleagues confirmed that 5′tsRNA-Val is produced during specific stress in the halophilic archaeon Haloferax volcanii. This tRF-Val can bind to the small ribosomal subunit, displacing mRNA from the initiation complex and leading to global translation attenuation (Fig. 2) (Gebetsberger et al., 2017). In Trypanosoma brucei, 3′tiRNA-Thr enhances translation by associating with ribosomes and facilitating mRNA loading once starvation conditions ceased (Fricker et al., 2019). tsRNAs not only downregulate translation but can also upregulate it, suggesting a diverse role in translation regulation.
Figure 2: Generation and functions of tsRNAs in respond to stress.
tRNA modification and ribonucleases, such as ANG, affect the production of tsRNAs. tsRNAs function in translation regulation, apoptosis regulation, and gene silencing.Apoptosis regulation
During cellular stress, cytochrome c is released from the mitochondria into the cytosol. It then interacts with apoptosis-activating factor-1, resulting in the formation of the heptameric apoptosome. This complex activates downstream caspases and initiates the cell death process (Kalpage et al., 2019). During hyperosmotic stress, ANG-induced tiRNAs can bind cytochrome c, forming a novel ribonucleoprotein complex. This complex inhibits apoptosome formation, thereby increasing cell survival (Fig. 2) (Saikia et al., 2014). In cancer, tsRNA-26576 and tRF-Val were found to inhibit cellular apoptosis and enhance cellular proliferation and migration (Cui et al., 2022; Zhou et al., 2019).
Gene silencing
Similar to miRNAs, tsRNAs achieve gene silencing by assembling the RNA-induced silencing complex, resulting in post-transcriptional gene silencing (Di Fazio & Gullerova, 2023; Jonas & Izaurralde, 2015). In addition to post-transcriptional gene silencing, tsRNAs can downregulate target genes by targeting introns through nascent RNA silencing in the nucleus (Di Fazio et al., 2022). Mechanistically, various types of tsRNAs can bind to Argonaute (AGO)1–4, including tRF-1s, tRF-3s, and tRF-5s (Kumar et al., 2014; Maute et al., 2013; Rosace, López & Blanco, 2020). When tsRNAs load onto AGO proteins, key members of the RNA-induced silencing complex, they guide the degradation of sequence-matched targets (Fig. 2) (Kuscu et al., 2018). For example, tRF5-GluCTC binds AGO4 to form a complex, and AGO1 carries mRNA to provide a target for the AGO4-tRF5-GluCTC complex to silence related genes (Choi et al., 2020). Under stress conditions in Arabidopsis thaliana, tsRNA-AGO1 complex specifically targets and cleaves endogenous transposable element mRNAs, which is detrimental to host fitness (Martinez, Choudury & Slotkin, 2017). In a lymphoma cell line, tRF3-Gly (CU1276) binds AGO1-4, suppressing proliferation and modulating the molecular response to DNA damage (Maute et al., 2013). However, only a limited number of tsRNAs combined with AGO have been identified, and many tsRNAs exhibit weak interactions with AGO proteins (Lee et al., 2023).
Molecules mechanism of organ injury
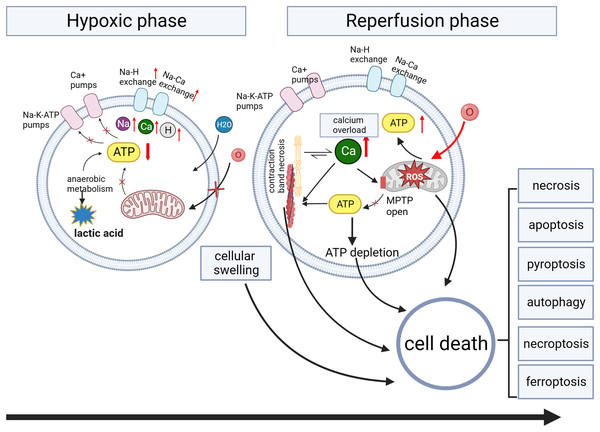
Multiple mechanisms are involved in organ injury, including oxidative stress, metabolic stress, programmed cell death pathways, and inflammatory immune responses (Wang et al., 2024). Oxidative stress plays a crucial role in various injuries, including ischemia-reperfusion injury (Wang et al., 2024), traumatic brain injury (Frati et al., 2017), and radiation injury (Yamaga et al., 2024). Therefore, we will use myocardial ischemia/reperfusion injury as an example to discuss organ injury. The pathophysiology of myocardial ischemia/reperfusion injury is shown in Fig. 3. The lack of oxygen and energy substrates during ischemia leads to cellular acidosis and subsequent accumulation of cytosolic Ca2+ due to the reverse activity of the sodium-calcium exchanger (Bugger & Pfeil, 2020; Comità et al., 2023). Reperfusion can exacerbate ischemia-induced injury in severely ischemic cells by releasing reactive oxygen species (ROS) generated by damaged mitochondria and NADPH oxidase (Monsel et al., 2014). Oxidative stress results from an imbalance between the production of ROS and their removal (Frati et al., 2017). Excessive ROS production reduces membrane fluidity, increases calcium permeability, releases pro-apoptotic proteins, disrupts protein functions, and damages nucleic acids and chromosomes (Galeone, Grano & Brunetti, 2023). These ischemia/reperfusion-induced pathways can lead to various forms of cell death (Davidson et al., 2020) (Fig. 3).
Figure 3: Myocardial ischemia/reperfusion injury.
During the ischemia phase, myocardial cells primarily undergo anaerobic metabolism, resulting in reduced ATP generation. This metabolic shift impairs the function of ion pumps, disrupting membrane ion gradients and mitochondrial membrane potential (ΔΨm). Intracellular conditions manifest as low pH, high sodium and calcium ions, and cellular swelling due to increased osmolarity. Intracellular calcium overload during hypoxia induces the opening of the mitochondrial permeability transition pore (MPTP) activation of phospholipases and calcium-dependent proteases, and contraction band necrosis. During the reperfusion phase, myocardial cells experience the restoration of oxygen and nutrient supply, triggering the production of ROS. The sustained increase in calcium permeability further exacerbates calcium overload, leading to the continued opening of the mitochondrial permeability transition pore and subsequent mitochondrial damage.Tsrnas: new potential markers and therapeutic targets in injuries
Lung injury
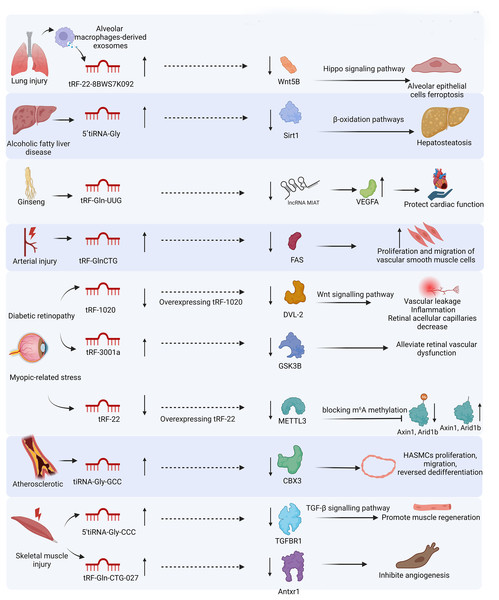
Lung injury encompasses a spectrum of conditions, including acute and chronic lung injury, bronchopulmonary dysplasia, ventilator-induced and ventilator-associated lung injury, radiation-induced lung injury, acute respiratory distress syndrome, chronic obstructive pulmonary disease, asthma, pulmonary fibrosis, and cystic fibrosis(Vishnupriya et al., 2020). These injuries can be fundamentally triggered by exposure to stressors such as hypoxia, oxidative stress (ischemia-reperfusion), and xenobiotics (Vishnupriya et al., 2020). The etiology of lung injury includes pneumonia, infections, trauma, shock, burns, acute pancreatitis, radiation exposure, and blood transfusion (Lan et al., 2023). At the cellular and molecular levels, the mechanism of lung injury involves autophagy (Vishnupriya et al., 2020) and ferroptosis (Yu & Sun, 2023). Wang et al. (2022) performed RNA sequencing in bronchoalveolar lavage fluid exosomes of lipopolysaccharide induced acute lung injury mice and discovered that alveolar macrophage-derived exosomal tRF-22-8BWS7K092 contributes to the pathogenesis of acute lung injury by inducing ferroptosis through the Hippo signaling pathway (Wang et al., 2022). Another study demonstrated that tRF-Gly-GCC inhibits cell proliferation, promotes ROS production, and triggers apoptosis in radiation-induced lung injury (Deng et al., 2022). Furthermore, Lin et al. (2022) established that dexmedetomidine ameliorates pulmonary injury, reduces inflammation, pulmonary edema, and ferroptosis in acute lung injury, resulting in alterations in the tsRNA expression profile. Changes in tsRNA not only reflect lung injury but also have the potential to serve as therapeutic targets for lung injury.
Liver injury
Liver injury can result from conditions such as viral hepatitis, ischemia-reperfusion injury, and drug-induced damage (Liang et al., 2024). Pathological manifestations include necrosis, apoptosis, hepatic steatosis, liver inflammation (Payus et al., 2022), hepatocyte pyroptosis (Xie & Ouyang, 2023), ferroptosis (Liang et al., 2024), and mitochondrial dysfunction (Arumugam et al., 2023). Ying and colleagues knocked down NSun2, a tRNA methyltransferase, to generate tsRNAs, highlighting tRF-1s as essential products capable of mitigating liver injury. Through further screening, they found that tRF-Gln-CTG-026 exhibits robust functionality by suppressing global protein synthesis through the weakened interaction between TSR1 (a pre-rRNA-processing protein homolog) and the pre-40S ribosome (Ying et al., 2023). In alcohol-induced liver injury and steatosis, complement C3 activation products contribute to hepatosteatosis by regulating the expression of tRF-Gly. Mechanistically, tRF-Gly binds with AGO3 to downregulate Sirt1 expression, subsequently impacting downstream lipogenesis and β-oxidation pathways (Zhong et al., 2019). tsRNAs can not only alleviate liver injury but also cause liver injury, indicating their multiple roles in liver injury.
Renal injury
Oxidative stress emerges as a critical pathogenic mechanism in renal diseases (Mishima et al., 2014; Nørgård & Svenningsen, 2023). Ischemia-reperfusion injury can damage mitochondria, leading to decreased mitochondrial DNA, increased ROS, and reduced ATP generation, thus triggering oxidative stress and cell injury (Zhang et al., 2023a). An early study found that circulating tRNA derivatives increased rapidly in various models of tissue damage, even in humans under acute renal ischemia, by detecting tRNA-specific modified nucleoside 1-methyladenosine antibody (Mishima et al., 2014). This study indirectly suggests tsRNAs as potential biomarkers for acute kidney injury. Furthermore, Li and colleagues investigated the tsRNA profiles in healthy controls and moderate/severe ischemia-reperfusion injury kidney tissues in mouse models using tRFs/tiRNAs sequencing. They found that 152 tsRNAs were differentially expressed in the moderate ischemic injury group compared with the normal control group (47 upregulated and 105 downregulated), and 285 tsRNAs in the severe ischemic injury group were differentially expressed (157 upregulated and 128 downregulated) (Li et al., 2022a). tsRNAs serve as promising candidates for biomarkers and therapeutic targets for acute kidney injury, but more studies are needed in the future.
Cardiac injury
Ischemic heart disease is the most prevalent cardiovascular disease. During myocardial ischemia, cardiomyocytes are exposed to nutrient deprivation and hypoxia, ultimately leading to cell death. Timely interventional procedures and thrombolytic agents facilitate the rapid restoration of blood and oxygen following temporary interruption, thereby mitigating myocardial ischemia-reperfusion injury (Peng et al., 2023). The detailed mechanism of myocardial ischemia-reperfusion injury is depicted in Fig. 3. Hu et al. (2022a) identified that tRF-Gln-UUG from ginseng can maintain cytoskeletal integrity and support mitochondrial function. They found that tRF-Gln-UUG targets the lncRNA MIAT/VEGFA pathway. Although the tsRNA profiles of myocardial ischemia-reperfusion injury have not been reported, tsRNAs in myocardial ischemia change significantly. Liu and colleagues reported tsRNA expression patterns in isoproterenol-induced myocardial ischemia and caloric restriction pretreatment. They found that 302 tsRNAs were significantly changed in myocardial ischemia and 55 tsRNAs were significantly regulated by caloric restriction pretreatment, which are potential therapeutic targets for myocardial ischemic injury (Liu et al., 2020). tsRNA expression profiles in different cardiomyocyte injuries may exhibit different changes. For cardiomyocyte injury caused by high glucose, only specific tsRNAs were differentially expressed. Specifically, inhibition of tRF-5014a alleviated cardiomyocyte injury by regulating autophagy under high glucose conditions (Zhao et al., 2022). tsRNAs can be potential therapeutic targets for myocardial injury, and the roles of tsRNAs in different myocardial injuries may vary.
Neuronal injury
The literature on neurological injury mainly focuses on the expression profiles of tsRNAs in various models through RNA sequencing, including traumatic spinal cord injury (Qin et al., 2019), traumatic brain injury (Xu et al., 2022), and therapeutic targets of traditional Chinese medicines like Xuefu Zhuyu Decoction for experimental traumatic brain injury (Yang et al., 2022). In traumatic spinal cord injury, bioinformatics analyses revealed that tiRNA-Gly-GCC-001 might be involved in the MAPK and neurotrophin pathways by targeting BDNF, thereby regulating the pathophysiological processes following spinal cord injury (Qin et al., 2019). For traumatic brain injury, differentially expressed tsRNAs are associated with inflammation and synaptic function (Xu et al., 2022). Yang et al. reported that tsRNAs treated with traditional Chinese medicines could contribute to regulating insulin resistance, the calcium signaling pathway, autophagy, and axon guidance through bioinformatics analysis (Yang et al., 2022). These models exhibit significantly different expressions of tsRNAs in neuronal injury, providing potential therapeutic targets for neuronal injury. Additionally, tiRNAs are associated with cerebral ischemia-reperfusion injury in cell and rat models (Elkordy et al., 2019; Sato et al., 2020).
Vascular injury
Vascular injury encompasses multiple types, involving different mechanisms of cell damage. Here, we discuss the roles of tsRNAs in vascular ischemia injury, diabetes-induced retinal microvascular injury, nanoplastics-induced vascular injury, and vascular trauma. A study found that tiRNA-Val-CAC and tiRNA-Gly-GCC increased in a rat brain ischemic model, a mouse hindlimb ischemia model, and a cellular hypoxia model. These tsRNAs can inhibit cell proliferation, migration, and tube formation in endothelial cells (Li et al., 2016). A tsRNA had a similar function in diabetes-induced retinal microvascular complications. tRF-1020 expression was downregulated in diabetic retinal vessels and retinal endothelial cells of mice during high glucose or H2O2 stress (Ma, Du & Ma, 2022). Additionally, tRF-1020 levels were downregulated in aqueous humor and vitreous samples of patients with diabetic retinopathy (Ma, Du & Ma, 2022). Furthermore, overexpressing tRF-1020 decreased endothelial cell viability, proliferation, migration, and tube formation, and alleviated retinal vascular dysfunction by targeting Wnt signaling (Ma, Du & Ma, 2022). However, tsRNAs can increase the proliferation and migration of endothelial cells in neurovascular dysfunction caused by diabetes. A study reported that tRF-3001a is significantly upregulated under diabetic conditions and in aqueous humor samples of diabetic retinopathy patients (Zhu et al., 2023). Downregulation of tRF-3001a ameliorates retinal vascular dysfunction, suppresses retinal reactive gliosis, improves retinal ganglion cell survival, and preserves visual function and visually guided behaviors by targeting GSK3B (Zhu et al., 2023). tsRNAs can also increase the proliferation and migration of vascular smooth muscle cells in vascular injury, but the mechanisms are different. tiRNA-Gly-GCC is upregulated in the synthetic phenotype of human aortic smooth muscle cells, atherosclerotic vascular tissues and plasma, and the balloon-injured carotid artery of rats (Rong et al., 2023). Inhibiting tiRNA-Gly-GCC effectively represses human aortic smooth muscle cell proliferation, migration, and reversed dedifferentiation by downregulating chromobox protein homolog 3 (Rong et al., 2023). Similarly, tRF-Gln-CTG was found to be overexpressed in the injured rat common carotid artery (Zhu et al., 2021). tRF-Gln-CTG increased the proliferation and migration of rat vascular smooth muscle cells by negatively regulating the expression of the FAS cell surface death receptor (Zhu et al., 2021). tRF-Glu-CTC can also inhibit the expression of fibromodulin to inhibit the TGF-β1/Smad3 signaling pathway, resulting in the promotion of neointimal hyperplasia in vascular injury (Jiang et al., 2024). In vascular injury caused by polystyrene nanoplastics exposure, tiRNA-Glu-CTC induces vascular smooth muscle cells to convert from contractile to synthetic phenotypes and causes vascular injury through mitochondrial damage by targeting Cacna1f (Zhang et al., 2024).
Skeletal muscle injury and skin injury
Skeletal muscle injury can be caused by mechanical trauma, thermal stress, myotoxic agents, ischemia, and neurological damage (Yang & Hu, 2018). In a cardiotoxin-induced skeletal muscle injury model, Shen and colleagues found that 5′tiRNA-Gly-CCC was significantly upregulated after muscle injury through small RNA sequencing (Shen et al., 2023). Mechanistically, 5′tiRNA-Gly-CCC promotes muscle regeneration by facilitating early inflammatory response, satellite stem cell activation, and myoblast differentiation (Shen et al., 2023). It does this by binding AGO1 and AGO3 to directly target Tgfbr1, thereby regulating the TGF-β signaling pathway (Shen et al., 2023). Using the same model, Chen and colleagues found that tRF-Gln-CTG was overexpressed in high abundance and inhibited angiogenesis by directly targeting Antxr1 (Chen et al., 2023). These two research results seem contradictory, as tsRNAs promote muscle regeneration but inhibit angiogenesis, indicating the complex regulatory role of tsRNAs.
Currently, tsRNAs in irradiation-induced skin injury and diabetic wounds have been reported. A study showed expression profiles of tsRNAs in an animal model of ultraviolet irradiation-induced skin injury and found that 10 tsRNAs had significantly different expression levels. They speculated that tRF-Gly-CCC-019 is an important target for regulating the ras-related C3 botulinum toxin substrate 1 gene in the WNT signaling pathway (Fang et al., 2021). For diabetic wounds, Zhang et al. (2023b) performed small RNA sequencing of skin tissues from patients with diabetic foot ulcers and confirmed that tRF-Gly-CCC-039 expression was upregulated in the diabetic model and impaired HUVEC function.
Clinical value of tsrnas in organ injury-associated diseases
To date, a proportion of tsRNAs in organ injury-associated diseases have been identified, and the mechanisms of organ injury have been validated. tsRNAs are expected to be developed as RNA therapeutics in the future. RNA interference is a common RNA therapy technology that can reduce the activity and levels of various RNA species, such as miRNA, mRNA, and lncRNA (Robinson & Port, 2022). In theory, this strategy is an invaluable RNA therapeutic to knock down tsRNAs, as most currently verified tsRNAs are harmful in organ injury-associated diseases. However, few studies in this review employ this strategy as investigational interventions. Moreover, it is still unknown whether this strategy will interfere with the tRNA pool. RNA mimetics were often used in the cited studies in this review to overexpress tsRNAs. Nevertheless, their pharmacokinetic properties have hindered their progress to clinical trials compared with RNA interference (Robinson & Port, 2022).
Our review revealed that tsRNAs are dysregulated in various types of organ injury. tsRNAs are better indicators of different stress responses than miRNAs (Li et al., 2022b). Therefore, tsRNAs can be promising candidates for biomarkers in organ injury-associated diseases. The exploration of the potential of tsRNA-based liquid biopsy is at an early stage (Li et al., 2022c). A study investigated the comprehensive expression profiles of plasma tsRNAs in children with fulminant myocarditis. They identified a target, tiRNA-Gln-TTG-001, which was overexpressed during the acute phase. This particular tsRNA was positively associated with highly sensitive cardiac troponin T, C-reactive protein, and procalcitonin (Wang et al., 2021). Li et al. (2022c) conducted a multicenter prospective study and found that exosomal tsRNAs have the potential for diagnosis, prognosis, and pre-operative biomarkers for esophageal carcinoma. A large number of studies on tsRNAs as biomarkers in organ injury-associated diseases are needed in the future. Prediction models of different organ injuries based on tsRNAs may be developed in the future.
Conclusions
In this review, we systematically discuss the classification of tsRNAs, their generation, and biological functions in response to stress, and their potential as biomarkers and therapeutic targets in various injuries, including lung, liver, renal, cardiac, neuronal, vascular, skeletal muscle, and skin injuries. We provide a fresh perspective on the biogenesis of stress-induced tsRNAs and their functions in cell behavior under stress. We establish the association between stress-induced tsRNAs and organ injury from a clinical perspective. We also summarize the functions of tsRNAs in injuries in Table 1 and the miRNA-like mechanisms of tsRNAs in different injuries in Fig. 4.
| Injury types | tsRNA | tsRNA types | Expression in injury | Mechanism | Reference |
|---|---|---|---|---|---|
| Lung injury | tRF-22-8BWS7K092 | tRF-3 (tRF-Gln-TTG) | Up | Alveolar macrophage-derived exosomal tRF-22-8BWS7K092 contribut to ALI by inducing ferroptosis through the Hippo signaling pathway. | Wang et al. (2022) |
| tRF-Gly-GCC-1 | tRF-5 (1–28) bases |
Up | tRF-Gly-GCC inhibits cell proliferation and promotes ROS production, and apoptosis in RILI. | Deng et al. (2022) | |
| Liver injury | tRF-Gln-CTG-026 | tRF-1 | __ | Ameliorates liver injury by suppressing global protein synthesis through the weakened association between TSR1 and pre-40S ribosome. | Ying et al. (2023) |
| tRF-Gly | 5′tiRNA | Up | tRF-Gly contributes to hepatosteatosis in alcoholic fatty liver disease by binding with AGO3 to downregulate Sirt1 expression to affect downstream lipogenesis and β-oxidation pathways. | Zhong et al. (2019) | |
| Cardiac injury | tRF-Gln-UUG (HC83) |
22 mer tRF-3 | __ | HC83 protects cardiac function and maintains both cytoskeleton integrity and mitochondrial function of cardiomyocytes by targeting the lncRNA MIAT/VEGFA pathway. | Hu et al. (2022a) |
| Vascular injury | tRNAVal tRNAGly |
5′tiRNA | Up | Inhibit cell proliferation, migration, and tube formation in endothelial cells when exposed to ischemic injuries. | Li et al. (2016) |
| tRF-GlnCTG | i-tRF | Up | Increase the proliferation and migration of rat vascular smooth muscle cells through negatively regulating the expression of FAS cell surface death receptor. | Zhu et al. (2021) | |
| tRF-1020 | tRF-1 (tRF- Phe-GAA) | Down | Decrease endothelial cell viability, proliferation, migration, and tube formation and, meanwhile, decrease retinal acellular capillaries, vascular leakage, and inflammation by targeting Wnt signaling. | Ma, Du & Ma (2022) | |
| tRF-22-8BWS72092 | tRF-3 (tRF-Gln-CTG) | Down | Improve choroidal vascular dysfunction by interacting with METTL3 and then blocking m6A methylation of Axin1 and Arid1b mRNA transcripts to increased expression of Axin1 and Arid1b | Liu et al. (2023) | |
| tRF-3001a | tRF-3 (tRF-Leu) | Up | Alleviate retinal vascular dysfunction, suppress retinal reactive gliosis, improve retinal ganglion cell survival, and preserve visual function and visually guided behaviors by targeting GSK3B | Zhu et al. (2023) | |
| tiRNA-Gly-GCC | 5′tiRNA | Up | Inhibition of tiRNA-Gly-GCC can alleviate vascular intimal hyperplasia by repressing human aortic smooth muscle cell proliferation, migration, and reversed dedifferentiation via downregulating chromobox protein homolog 3. | Rong et al. (2023) | |
| Skeletal muscle injury | 5’tiRNA-Gly-CCC | 5′tiRNA | Up | Promotes muscle regeneration by facilitating early inflammatory response, satellite stem cell activation, and myoblast differentiation via binding AGO1 and AGO3 to directly target Tgfbr1 to regulate the TGF-β signaling pathway. | Shen et al. (2023) |
| tRF-Gln-CTG-027 | (Length 19 nt) | Up | Inhibites angiogenesis by directly targeting Antxr1 | Chen et al. (2023) | |
| Skin injury | tRF-Gly-CCC-039 | tRF-5 | Up | Impair HUVECs function by the suppression of proliferation, migration, tube formation, and the expression of Coll1a1, Coll4a2, and MMP9. | Zhang et al. (2023b) |
Figure 4: miRNA-like mechanism of tsRNAs in different injuries.
MicroRNAs (miRNAs) can assemble with AGO proteins into miRNA-induced silencing complexes to direct post-transcriptional silencing of complementary mRNA targets. tsRNAs have a similar function of gene silencing by assembling the RNA-induced silencing complex.Currently, the literature on tsRNAs is rapidly increasing, gradually unveiling their mysteries. However, a knowledge gap still exists regarding the relationship between tsRNAs, stress, and injury, and tsRNA research is in its infancy. Stress conditions can activate cellular protective responses, including stress neutralization, cell cycle pausing, translation alterations, and damage repair. When the damage is too great for cellular survival, the response to stress results in death pathways (Thompson et al., 2008). The most well-known types of tsRNAs in response to stress are tiRNAs, which conserve energy for repairing stress-induced damage (Yamasaki et al., 2009) and inhibit apoptosis (Saikia et al., 2014). However, recent research suggests modifying current experimental stress paradigms to separate the function of tsRNAs during the acute stress response from their role as a consequence of ongoing cell death (Sanadgol et al., 2022). More work is needed to explore the association between tsRNAs and various types of cell death, such as apoptosis, pyroptosis, autophagy, necroptosis, and ferroptosis. Other aspects of the injury mechanism are also worth exploring, such as the relationship between tsRNAs and inflammatory immune responses.
Although several reviews (Kuhle, Chen & Schimmel, 2023; Magee & Rigoutsos, 2020; Aswathi et al., 2023) have summarized research methods for tsRNAs, we will supplement some points about tsRNAs in organ injury-associated diseases as follows. Firstly, the development of sequencing technology, especially when combined with artificial intelligence, will advance tsRNA research. Secondly, we can study the function of tsRNAs by artificially generating them. Some studies manipulate ribonucleases to generate tsRNAs, such as ANG (Ivanov et al., 2014) and Dicer (Di Fazio et al., 2022). One study generated tsRNAs by altering tRNA modification through the knockdown of NSun2, a tRNA methyltransferase (Ying et al., 2023). Thirdly, miRNA-like effects and research methods are often used in studies focusing on the functions of tsRNAs in injury. Other mechanisms, such as translation regulation, are rarely studied in tsRNA research. This may be due to the extensively researched functions of miRNAs and the mature tools for target prediction based on sequence complementarity. Nevertheless, future studies need to supplement the research on other mechanisms in injury.