Risk factors associated with air embolism following computed tomography-guided percutaneous lung biopsy: a retrospective case-control study
- Published
- Accepted
- Received
- Academic Editor
- Francesco Nucera
- Subject Areas
- Oncology, Radiology and Medical Imaging, Respiratory Medicine
- Keywords
- Air embolism, Lung, Puncture biopsy, Retrospective case-control study, Risk factors
- Copyright
- © 2024 Wu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Risk factors associated with air embolism following computed tomography-guided percutaneous lung biopsy: a retrospective case-control study. PeerJ 12:e18232 https://doi.org/10.7717/peerj.18232
Abstract
Background
Retrospective analysis to identify the risk factors for air embolism following computed tomography (CT)-guided percutaneous transthoracic needle biopsy (TNB).
Methods
A retrospective analysis of patients who underwent CT-TNB at The First Affiliated Hospital of Zhengzhou University and Xuzhou Cancer Hospital from January 2017 to December 2021 was performed. A total of 21 factors relevant to air embolisms were collected. Risk factors associated with air embolisms were determined by the least absolute shrinkage and selection operator (LASSO). The receiver-operator characteristic (ROC) was used to assess the ability of these factors to identify air embolisms.
Results
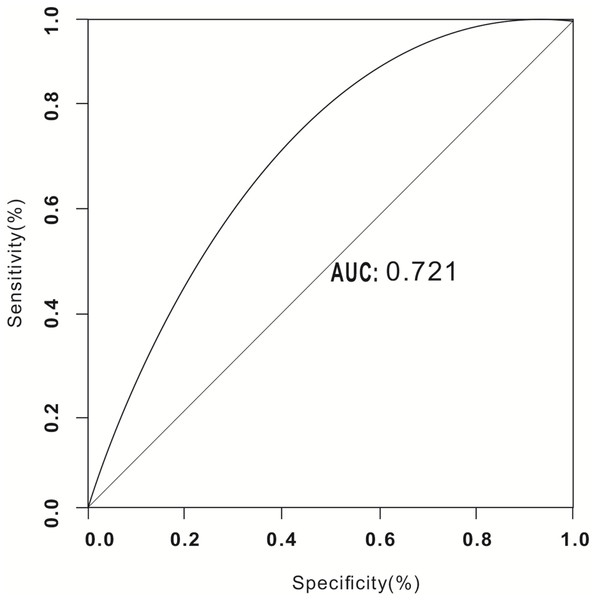
Of these 32,748 patients, 28 experienced air embolisms (19 at The First Affiliated Hospital of Zhengzhou University (incidence, 1.46%) and nine at Xuzhou Cancer Hospital (incidence, 0.69%); total incidence, 2.16%). Only seven patients exhibited symptoms (symptom rate, 25.00%). A total of 21 patients were asymptomatic at the time of swept-source CT. No deaths occurred. We found through univariate and multivariate analysis that eight out of these 21 factors are associated with the occurrence of air embolism. The area under the ROC curve was 0.721, indicating good predictive power (P < 0.05).
Conclusion
Cough during the procedure, hemoptysis during the procedure, the distance between the mass and the pulmonary vein, the presence of a cavity in the lesion, lesion location, number of samples, abnormalities in the patient’s coagulation mechanism, and the puncture position may be the risk factors for air embolism in CT-TNB.
Introduction
Computed tomography (CT)-guided percutaneous transthoracic needle biopsy (TNB) is a widely utilized method for the pathological diagnosis of lung diseases (Wang, Tu & Chen, 2019). It holds significant importance for the clinical diagnosis and treatment of these conditions (Esakov et al., 2022). This technique is increasingly adopted in clinical settings. Although computed tomography-guided percutaneous transthoracic needle biopsy (CT-TNB) is minimally invasive (Heerink et al., 2017), it is associated with some complications (Han et al., 2018) including bleeding (Sabatino et al., 2021), pneumothorax (Bae, Ha & Jeon, 2020), chest pain (Beck et al., 2021), and air embolisms (Liu et al., 2020).
Most of these complications have a good prognosis and do not require special treatment. If air embolism occurs in the coronary or cerebral arteries, it may lead to acute myocardial infarction or cerebral infarction, and even death (Ring et al., 2024; Yang et al., 2023). Therefore, air embolism demands the attention of clinical physicians, as its onset of air embolism is rapid and the symptoms are complex. If not managed properly or promptly, it can endanger life (Michels et al., 2021), necessitating urgent attention.
CT-TNB has demonstrated high accuracy in the diagnosing of pulmonary lesions, leading to its widespread adoption in clinical settings. Consequently, the likelihood of some complications increases, such as air embolism also increases. Previous studies indicate that its incidence ranges from 0.02% to 0.07% (Richardson et al., 2002; Tomiyama et al., 2006); however, recent findings suggest a growing awareness of this issue, with the highest recorded incidence rate reaching 3.8% (Lee et al., 2021). Despite the relatively low incidence, the potential risks associated with air embolism are significant and cannot be overlooked. When it occurs, it can pose a severe risk and may even be life-threatening. Numerous studies have explored the diagnosis and localization of complications such as hemorrhage, pneumothorax, and air embolisms using CT post CT-TNB (Anzidei et al., 2017). Nonetheless, there is limited research on preventive strategies for CT-TNB-induced air embolism. The occurrence of air embolism may be influenced by various factors, including the patient’s specific health condition (e.g., cardiopulmonary function), the practitioner’s technical expertise, and the precision of the equipment. During CT-TNB procedures, physicians should carefully assess the patient’s risk and select the optimal puncture site and trajectory to minimize the risk of complications such as air embolism. Concurrently, healthcare facilities must develop thorough emergency response plans to manage and effectively treat severe complications such as air embolism.
With the widespread application of CT-TNB in clinical practice, attention and research on complications such as air embolism are gradually increasing. By continuously improving technology and operating standards, it is anticipated that the incidence of these complications will decrease, thus enhancing patient safety. Therefore, during this study, we reviewed and analyzed our own clinical data and identified risk factors for air embolisms associated with CT-TNB to provide guidance on reducing their occurrence.
Materials & Methods
Ethics
All patients were from two tertiary hospitals (The First Affiliated Hospital of Zhengzhou University and Xuzhou Cancer Hospital) and underwent CT-TNB. This study was conducted in accordance with the Declaration of Helsinki revised in 1964. This study was approved by the ethics committees of The First Affiliated Hospital of Zhengzhou University (Ethical Application Ref:2023-1243) and Xuzhou Cancer Hospital (Ethical Application Ref:2023-CS-021). Written informed consent was obtained from all patients who underwent CT-TNB.
Data collection
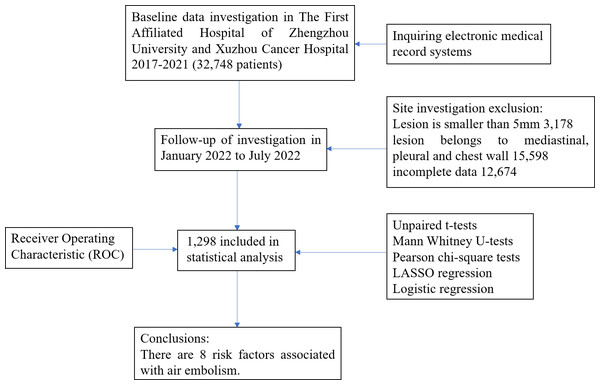
A retrospective collection of data from 32,748 patients who underwent CT-TNB at The First Affiliated Hospital of Zhengzhou University and Xuzhou Cancer Hospital between January 2017 and December 2021 was analyzed. These patients had previously undergone chest enhanced CT, which revealed lung nodules of indeterminate nature. Following departmental discussion, it was recommended that the patients undergo CT-TNB to ascertain the pathological characteristics of the nodules and to obtain informed consent. Patients were excluded if they had pulmonary nodules with a maximum diameter of less than 5 mm, nodules located in the mediastinum, pleura, or chest wall, or if their data were incomplete. Ultimately, 1,298 patients were included in the study. (Fig. 1).
Figure 1: CT-TNB flow diagram of study design.
Biopsy technique
Six clinicians, each with 3 to 10 years of experience, conducted all CT-TNB procedures according following standard protocols. At The First Affiliated Hospital of Zhengzhou University, CT was performed using a 16-detector GE scanner (120 mA, 120 kV, 3.75-mm layer thickness), while Xuzhou Cancer Hospital utilized a 64-detector Phillips Medical Systems scanner (120 mA, 120 kV, 3.0-mm layer thickness) for image acquisition. Biopsies were executed using a coaxial approach with a 17-G coaxial guide needle (MCXS1815BP; Argon, Fairfax, VA, USA) and an 18-G soft tissue biopsy needle (MFN1806; Argon, Fairfax, VA, USA). All patients undergoing CT-TNB received local anesthesia. Initially, a routine CT plain scan was performed to establish the puncture point. Following successful anesthesia, a 17G coaxial needle was carefully inserted into the lesion guided by the previously determined puncture point. Upon removal of the internal needle core, an 18G biopsy needle was promptly inserted. To prevent air entry, the coaxial needle was sealed with fingers during each transition between the needle core and the biopsy needle. Patients were instructed to either hold their breath or breathe calmly throughout this procedure. To ensure safety, at least 2 samples were collected for pathological examination, depending on the lesion size. After the coaxial needle’s removal, the patient remained in the same position for a subsequent CT scan of the entire chest to check for any surgery-related complications.
Measurement variables
By combining the literature and our own experience, 21 risk factors associated with patients, lesions, and procedures were identified, and data were collected for analysis. The patient-related risk factors included sex, age, smoking, abnormal coagulation mechanisms, intraoperative cough, and intraoperative hemoptysis. Risk factors associated with lesions included lesion site, lesion size (maximum short-axis diameter), distance between the lesion and pulmonary veins, cavitation within the lesion, necrosis within the lesion, distance between the lesion and pleura (puncture path), emphysema, and pleural adhesions. Risk factors associated with the procedure included puncture angle, patient position, sampling method, procedure time (time from needle insertion to removal), number of samples obtained, postoperative pneumothorax, and bleeding caused by the puncture needle.
Statistical analyses
Statistical analyses were performed using R version 4.2.0 (https://www.R-project.org). Summary statistics were used to describe the characteristics of the subjects. Continuous data were represented as median (interquartile range), while statistical data were represented as numbers and percentages. Unpaired t-tests, Mann Whitney U-tests, and Pearson chi-square tests were employed to compare features between cohorts. Potential risk factors were screened using the least absolute shrinkage and selection operator (LASSO) regression analysis with the glmnet package, logistic regression analysis with the rms package, and receiver-operating characteristic (ROC) analyses with the pROC package. Discrimination was evaluated by calculating the area under the ROC curve (AUROC) curve. A P-value of <0.05 was considered statistically significant.
Results
Clinical features
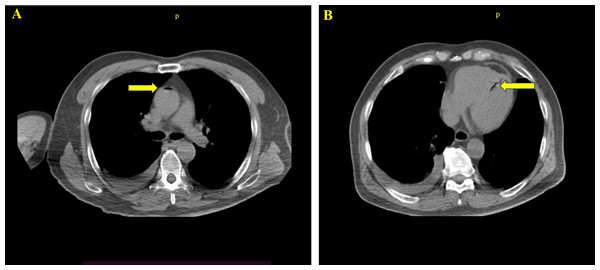
At The First Affiliated Hospital of Zhengzhou University and Xuzhou Cancer Hospital, 32,748 patients underwent CT-TNB. After excluding 3,178 patients with lesion smaller than five mm, 15,598 patients with lesions in the mediastinum, pleura, and chest wall, and 12,674 patients with incomplete data, a total of 1,298 patients were included in the statistical analysis (Fig. 1). Following CT-TNB, these patients received comprehensive chest CT scan. Among them, 28 experienced air embolisms (19 at The First Affiliated Hospital of Zhengzhou University (incidence, 1.46%) and nine at Xuzhou Cancer Hospital (incidence, 0.69%); total incidence, 2.16% (28/1298) with a 95% confidence interval (CI) of 1.29–4.45%). Only seven patients exhibited symptoms (symptom rate, 25.00%). Including chest tightness, breathing difficulties, dizziness, headache, restlessness, and hemiplegia. Following active management, all patients were advised bed rest and oxygen therapy, three also received hyperbaric oxygen therapy. Within 1 h of CT-TNB, 25 patients fully recovered, the remaining three recovered after two sessions of hyperbaric oxygen therapy within 48, with no sequelae. A total of 21 patients were asymptomatic and monitored using swept-source CT. There were no fatalities (Fig. 2).
Figure 2: One patient developed air embolism after CT-TNB, and air appeared in the ascending aorta and left ventricle.
| Variables | Air embolism | Non-air embolism | P |
|---|---|---|---|
| 28 | 1,270 | ||
| Patients’ characteristics | |||
| Age | 59.93 ± 10.99 | 55.08 ± 14.78 | 0.209 |
| Gender (Male/Famale) | 16/12 | 657/613 | 0.355 |
| Smoke (Yes) | 15 | 642 | 0.451 |
| Intraoperative cough (Yes) | 23 | 752 | 0.010 |
| Intraoperative hemoptysis (Yes) | 22 | 710 | 0.012 |
| Coagulation mechanism (Yes) | 20 | 674 | 0.040 |
| Lesion characteristics | |||
| Pleural adhesion (Yes) | 13 | 650 | 0.379 |
| Emphysema (Yes) | 17 | 653 | 0.206 |
| Distance between lesion and pleura | 4.148 ± 3.015 | 4.107 ± 2.740 | 0.986 |
| Pathological necrosis (Yes) | 16 | 598 | 0.194 |
| Lesion size (mm) | 26.4 ± 10.6 | 27.5 ± 11.8 | 0.543 |
| Lesion location (Left upper/lower lung/Right upper/middle/lower lung) | 12/1/10/4/1 | 402/130/221/427/90 | 0.034 |
| Lesion cavity (Yes) | 21 | 705 | 0.029 |
| Distance between lesion and pulmonary vein | 0.300 ± 0.332 | 4.820 ± 2.555 | 0.000 |
| Procedure characteristics | |||
| Postoperative pneumothorax (Yes) | 13 | 476 | 0.219 |
| Puncture angle | 45.640 ± 28.058 | 40.870 ± 28.543 | 0.308 |
| Puncture position(supine/prone/Lateral) | 11/5/12 | 612/365/293 | 0.046 |
| Sampling type (CNB/FNA) | 19/9 | 876/394 | 0.522 |
| Sampling numbers | 4.790 ± 2.470 | 2.860 ± 1.565 | 0.000 |
| Procedure time | 9.500 ± 3.967 | 9.960 ± 2.698 | 0.376 |
| Puncture needle to bleeding (Yes) | 17 | 562 | 0.122 |
Risk factors
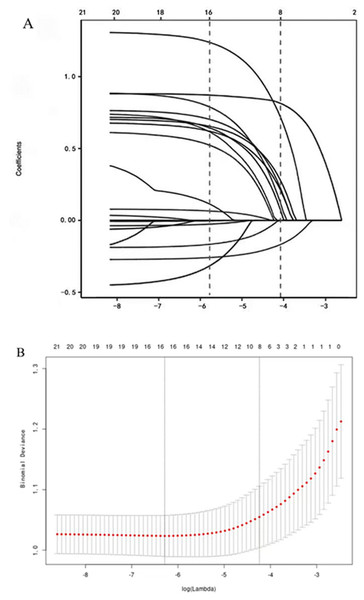
Screening of 21 factors was performed using the least absolute shrinkage and selection operator regression analysis (Table 1, Fig. 3). Table 1 presents the univariate analysis results of patients with air embolism and those without air embolism. There were statistical differences between the two groups in terms of intraoperative cough, hemoptysis, coagulation mechanism indicators, lesion location, lesion cavity, distance between lesion and pulmonary vein, puncture position, and sampling numbers (P < 0.05). However, there were no statistically significant differences in age, gender, smoke, pleural adhesion, emphysema, distance between lesion and pleura, pathological necrosis, lesion size, postoperative pneumothorax, puncture angle, sampling type, puncture time, and puncture needle to bleeding (P > 0.05). Furthermore, Table 2 presents the Logistic regression analysis results, and revealed that the aforementioned 8 predictive factors are independently associated with the risk of air embolism following CT-TNB. Among them, the most important risk factors for air embolism were lesion cavity (OR 28.029, 95% CI [9.511–82.598]), sampling numbers (OR 6.576, 95% CI [3.145–14.269]), intraoperative cough (OR 5.496, 95% CI [1.907–15.839]) and coagulation mechanism (OR 4.972, 95% CI [1.931–12.800]). Puncture position (OR 1.683, 95% CI [1.029–2.752]), distance between the lesion and pulmonary vein (OR 1.113, 95% CI [0.631–6.873]), lesion location (OR 0.639, 95% CI [0.455–0.897]) and intraoperative hemoptysis (OR 0.350, 95% CI [0.128–0.952]) also had an important prognostic value of air embolism. The AUROC of the air embolism risk prediction was 0.721 (95% confidence interval, 0.565–0.773), indicating good discrimination (Fig. 4). These eight risk factors were closely linked to the incidence of air embolisms in patients who undergoing CT-TNB.
Figure 3: Variable selection by the LASSO binary logistic regression model.
A coefficient profile plot was constructed against the log(lambda) sequence.| Multivariate analysis | ||||
|---|---|---|---|---|
| OR | 95% CI | P | ||
| Intraoperative cough | 5.496 | 1.907 | 15.839 | 0.002 |
| Intraoperative hemoptysis | 0.350 | 0.128 | 0.952 | 0.040 |
| Coagulation mechanism | 4.972 | 1.931 | 12.800 | 0.001 |
| Lesion location (Left upper/lower lung / Right upper/middle/lower lung) | 0.639 | 0.455 | 0.897 | 0.010 |
| Lesion cavity | 28.029 | 9.511 | 82.598 | 0.000 |
| Distance between lesion and pulmonary vein | 1.113 | 0.631 | 6.873 | 0.048 |
| Puncture position(supine/prone/Lateral) | 1.683 | 1.029 | 2.752 | 0.038 |
| Sampling numbers | 6.576 | 3.145 | 14.269 | 0.045 |
Figure 4: Receiver operating characteristic curve (ROC) validation of the air embolism risk prediction.
Discussion
Among the various complications of CT-TNB, air embolisms are rare and serious and can be fatal; however, their incidence is low (Zhang et al., 2023), and the mortality rate is even lower, reported to be 0.0002% (Pigaiani et al., 2024). Notably, our retrospective analysis revealed an incidence of air embolism at 2.16%, which is significantly higher than that reported in previous studies. This discrepancy may be associated with the comprehensive chest CT scans performed post-CT-TNB, whereas many prior studies have limited their scans to the level of puncture masses (Kukuljan et al., 2018), with fewer scanning layers, potentially leading to an underreported incidence.
Although the incidence of air embolism in our study is relatively high, the majority are asymptomatic. On the entry of a small volume of air, no clear symptoms were observed, with only gas signs apparent on CT images (Pietersen, Jørgensen & Christiansen, 2021). Conversely, the introduction of a large volume of air elicited symptoms necessitating immediate intervention (Arora & Burks, 2021). Of the 28 patients with air embolism, only seven exhibited symptoms, primarily including palpitations, chest tightness, difficulty breathing, restlessness, and limb movement disorders. There were no fatal outcomes; an immediate left-side lying position was implemented, along with interventions such as positioning with a lowered head and raised feet, oxygen therapy, and hyperbaric oxygen treatment, resulting in recovery without any residual effects. Importantly, air embolism, while potentially severe, can be effectively managed with early detection and prompt treatment.
Numerous studies have investigated the postoperative complications of CT-TNB (Yiminniyaze et al., 2022), primarily focused on prevalent issues such as pneumothorax and hemoptysis (Chiu et al., 2022). A risk model has been established for predicting emphysema and hemoptysis (Wu et al., 2021; Wang, Dong & Chen, 2020). However, the clinical prediction model for air embolism remains unexplored, largely due to the rarity of this complication and the challenges in developing such a model. Most existing studies are characterized by small sample sizes, are conducted at single centers, are retrospective, and lack comprehensive analysis (Lee et al., 2021). Furthermore, the underlying mechanism of air embolism remain elusive. It is hypothesized that if the puncture needle damages the pulmonary vein and remains exposed to the atmosphere after the needle core is removed, external atmospheric pressure could force air into the compromised pulmonary vein, particularly when the pressure within the pulmonary vein is lower than that of the atmosphere. Additionally, during the biopsy, simultaneous damage to small airways (such as alveoli and trachea) and pulmonary veins could lead to the formation of a bronchopulmonary venous fistula.
When a patient coughs or takes deep breaths, the pressure in the airway increases, which may encourage air to enter the damaged blood vessels. A very small number of patients still have congenital arteriovenous fistula, which leads to air entering the arteriovenous fistula during the puncture process, increasing the risk of air embolism, although this situation is relatively rare (Pan et al., 2023; Kang, 2023; Park et al., 2024).
In this study, we extracted 21 indicators based on the risk factors reported in the literature and derived from our clinical experience. By examining patient characteristics, lesion characteristics, and surgical processes, we identified the risk factors associated with CT-TNB and air embolism. We performed logistic univariate and multivariate regression analyses on the 8 performance indicators most closely to the occurrence of air embolism, namely cough, hemoptysis, the distance between the mass and pulmonary vein, cavity within the lesion, lesion location, sampling frequency, whether the patient’s coagulation mechanism status, and puncture position. The Multivariate analysis indicated that these 8 factors are related to the occurrence of air embolism. Furthermore, we employed AUROC to assess these risk factors’ predictive capacity for the occurrence of air embolism, achieving an AUROC of 0.721, which suggests a strong association with air embolism.
Studies have shown that these risk factors were easily overlooked during clinical practice, increasing the risk of air embolism. For example, abnormal coagulation mechanism, cough, hemoptysis, and damage to the peripheral pulmonary veins during CT-TNB can lead to increased pulmonary pressure (Nicolini et al., 2018). If no measures are taken to control it before surgery, air embolism is highly likely to occur during the puncture process. During the sampling process, to gather more pathological specimens, increasing the sampling frequency artificially may enhance the risk of air entry into damaged pulmonary vein through the puncture needle, thus increasing the incidence of air embolism (Jang et al., 2019). If there is a diseased cavity containing air, and the pulmonary vein is compromised during the CT-TNB, air may directly enter the pulmonary vein directly through the puncture needle, leading to air embolism (Bouaggad et al., 2021). When the lesion location is situated above the left atrium, the pulmonary vein pressure around the lesion lower than in other regions, and the pressure within the alveolar cavity may exceed that in the pulmonary vein. Consequently, air can more easily infiltrate the injured pulmonary vein, causing air embolism (Maehara et al., 2023). Furthermore, being in a prone position can decrease chest compliance, altering the intrathoracic pressure ratio and result in external atmospheric pressure exceeding the pulmonary vein pressure. Thus, the prone position increases the risk of air embolisms (Glodny et al., 2017).
Among the 8 key risk factors identified in this study, one was associated with the procedure, the number of samples taken, while four were linked to patient symptoms, cough, hemoptysis, coagulation mechanism, and puncture position. This indicates that the risk of air embolisms can be minimized by optimizing the procedure and managing patient symptoms prior to surgery. Thus, the risk of air embolisms may be further reduced by modifying controllable factors such as sampling frequency, and by addressing symptoms like cough and hemoptysis, as well as enhancing postoperative management for high-risk patients.
Our research presented some limitations. First, some factors such as the experience of the puncturing physician, size of the puncture needle, degree of cough, the extent of hemoptysis, severity of emphysema, and size of the cavity were not included. Moreover, the risk factors proposed in this study can be used only to predict the possibility of postoperative air embolisms; they cannot predict the severity of their occurrence. Second, this was a retrospective study, and few research institutions were included, which may have created bias. Third, some variables with potential predictive value, such as vascular abnormalities, blood flow direction, blood vessel diameter, and local tension of blood vessels, were not analyzed, which may have created deviations in the results. Finally, a forward-looking study is necessary to conduct in-depth research on these factors, expand the research institutions, and increase the sample size to further understand the relationship between these factors and the occurrence of air embolisms.
Conclusions
Identifying the risk of air embolisms associated with CT-TNB is of great significance. The eight indicators analyzed in this study can help predict the risk of air embolisms associated with CT-TNB. Furthermore, they can be used to make preemptive decisions regarding patients who are at high risk for air embolisms, improve patient management, and provide guidance to clinically reduce air embolisms.