Pdk3’s role in RANKL-induced osteoclast differentiation: insights from a bone marrow macrophage model
- Published
- Accepted
- Received
- Academic Editor
- Fanglin Guan
- Subject Areas
- Cell Biology, Orthopedics, Sports Injury, Sports Medicine
- Keywords
- Osteoporosis, Inflammation, Osteoclasts, RANKL, Osteoclastogenesis
- Copyright
- © 2024 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Pdk3’s role in RANKL-induced osteoclast differentiation: insights from a bone marrow macrophage model. PeerJ 12:e18222 https://doi.org/10.7717/peerj.18222
Abstract
Background
Osteoporosis (OP) is a chronic disease characterized by decreased bone mass, loss of skeletal structural integrity and increased susceptibility to fracture. Available studies have shown that the pyruvate dehydrogenase kinase (PDK) family is associated with osteoclastogenesis and bone loss, but the specific role of Pdk3 in bone pathology has not been systematically investigated.
Methods
A cell OP model was established in receptor activator for nuclear factor-κB Ligand (RANKL)-induced bone marrow macrophages (BMMs). Hereafter, the expression levels of Pdk3 and osteoclastogenesis feature genes including nuclear factor of activated T cells 1 (Nfatc1), Cathepsin K (Ctsk), osteoclast associated Ig-like receptor (Oscar) in BMMs-derived osteoclasts were examined based on real-time quantitative PCR and western blotting methods. Further, the phosphorylation of ERK, P65 and JAK/STAT and their correlation was Pdk3 was gauged. In particular, changes in the activity of these signaling pathways were observed by silencing experiments of the Pdk3 gene (using small interfering RNA). Finally, the effects of Pdk3 gene silencing on signaling pathway activity, osteoclastogenesis, and related inflammatory and apoptotic indicators were observed by transfection with PDK3-specific siRNA.
Results
Following RANKL exposure, the levels of Pdk3 and osteoclastogenesis feature genes were all elevated, and a positive correlation between Pdk3 and osteoclastogenesis feature genes was seen. Meanwhile, ERK, P65 and JAK/STAT phosphorylation was increased by RANKL, and Pdk3 was confirmed to be positively correlated with the phosphorylation of ERK, P65 and JAK/STAT. Additionally, in RANKL-exposed osteoclasts, Pdk3 knockdown diminished the phosphorylation of ERK, P65 and JAK/STAT, reduced the expressions of osteoclastogenesis feature genes. Importantly, knockdown of Pdk3 also reduced the expression of inflammatory cytokines and resulted in elevated levels of Bax and Casp3 expression, as well as downregulation of Bcl2 expression.
Conclusion
This study reveals for the first time the role of Pdk3 in RANKL-induced osteoclastogenesis and OP. These findings provide a foundation for future studies on the role of Pdk3 in other bone diseases and provide new ideas for the development of OP therapeutics targeting Pdk3.
Introduction
Osteoporosis (OP) refers to a chronic condition which is featured by the decrease in bone mass, the loss of skeletal integrity and the increase of susceptibility to fracture, which, according to some relevant data, affects 10.2% of adults over 50 years old and is expected to increase to 13.6% by the year 2030 (Ramchand & Leder, 2024; Harris, Zagar & Lawrence, 2023). Currently, pharmacotherapy has been recommended as the primary therapeutic option for patients who have suffered from the fragility fracture, including stimulants of bone matrix formation, inhibitors of bone resorption and dual-action drugs (Iolascon et al., 2020; Khosla & Hofbauer, 2017; Marie, 2006). However, despite the effectiveness in the therapy of OP, the early diagnosis of OP remains a challenge and a concern (Yalaev et al., 2022). Currently, the main tools used clinically for the treatment of OP include synovitis ointment, bisphosphonates, hormone replacement therapy, and biologics, and although these therapies are effective in reducing bone loss, they are often accompanied by side effects and have limited long-term efficacy (Muñoz, Robinson & Shibli-Rahhal, 2020; Yu & Wang, 2022; Zhang et al., 2023). Molecular insights on the pathogenesis of OP, therefore, are required so as to work out the clinically viable therapy regimens.
The principal reason accounting for the development of processes underlying the progression of OP has been categorized to the imbalance between the functions of osteoblasts and osteoclasts (Kaur, Nagpal & Singh, 2020). Osteoblasts are those remarkably versatile cells building up our skeleton which require tight regulation in all phases of their differentiation in order to ensure proper skeletal development and homeostasis (Ponzetti & Rucci, 2021). However, osteoclasts are derived from the monocyte/macrophage lineage and are responsible for the resorption of aging bone. These cells fuse to form multinucleated giant cells with bone-resorbing capabilities (Chen et al., 2018; Da, Tao & Zhu, 2021). The bone formation and resorption is in a stable at physiological conditions; however, the triggering of bone metabolic diseases can lead to an imbalance of abnormal bone structure or function (Zaidi, 2007). Currently, evidence suggests that a direct interaction between osteoblasts and osteoclasts facilitates the bidirectional transmission of activation signals via EFNB2-EPHB4, FASL-FAS, or SEMA3A-NRP1, which plays a vital role in regulating the differentiation and survival of both cell types. Additionally, osteoblasts secrete various molecules such as M-CSF, RANKL/OPG, WNT5A, and WNT16, which can either encourage or inhibit the differentiation and maturation of osteoclasts (Kim et al., 2020; Tonna et al., 2014; Wang et al., 2015). Hence, these findings prompt us to explore the underlying mechanisms by which osteoclasts exert their effects in osteoporosis or other bone diseases. Pyruvate dehydrogenase kinases (PDKs, four genes: PDK1-PDK4) are the major regulatory enzymes of glucose metabolism due to their negative role in the regulation of pyruvate dehydrogenase complex (PDC) via phosphorylation (Anwar et al., 2021). While linking PDKs with bone, some existing studies have suggested that PDK4 induction leads to bone loss via promoting osteoclastogenesis (Wang et al., 2012). In the meantime, Pdk1 was shown to be required for the function of bone marrow hematopoietic stem and progenitor cells in transplantable mice and to be able to influence osteoclast differentiation in ankylosing spondylitis (Halvarsson, Eliasson & Jönsson, 2017; Sun et al., 2021). Additionally, some researchers have demonstrated that the prevention of osteoporosis in mice lacking estrogen is achieved through the inhibition of Pdk2, which likely works by diminishing irregular activation of osteoclasts, potentially through the suppression of the nuclear factor-κB ligand (RANKL)-CREB-cFOS-nuclear factor of activated T cells 1 (Nfatc1) signaling pathway (Lee et al., 2021). Notably, unlike other members of the PDK family, the specific role of Pdk3 in bone pathology has not been systematically investigated.
Here, this study preliminarily initiates with the aim to delve into the specific involvement of Pdk3 in osteoclasts. We explored the regulation of osteoclastogenic signature gene expression, signaling pathway activation, inflammatory response and apoptosis by Pdk3 through gene silencing experiments, thus initially revealing the potential mechanism of Pdk3 in OP. Our study not only provides new insights into the specific role of Pdk3 in osteoclast function but also lays the theoretical foundation for the future development of Pdk3-targeted therapeutic strategies for OP.
Materials and Methods
Bone marrow macrophages
Bone marrow macrophages (BMMs) were purchased from Shanghai Fuheng Biotechnology Co., LTD. (Shanghai, China). Hereafter, the non-adherent cells were layered onto a Ficoll density gradient solution and centrifuged at 440 g for 30 min at ambient temperature. The cells were cultured in α-minimum essential medium (12000063, Gibco, Waltham, MA, USA) with 10% bovine calf serum (F2442, Sigma, Burlington, MA, USA) and 1% penicillin-streptomycin (P4333, Sigma) at 37 °C under 5% CO2.
Cultured BMMs were inoculated in 6-well plates with 5 × 105 cells per well. Macrophage colony-stimulating factor (M-CSF, M9170, Sigma) at 30 ng/mL was added to the culture medium to promote cell survival and proliferation. Subsequently, 50 ng/mL of RANKL (R0525, Sigma) was added 48 h after the initial culture and the culture was continued for 5 days to induce the differentiation of BMMs to osteoblasts. This is mainly based on the ability of RANKL to activate osteoclast differentiation and activity by binding to the RANK receptor, leading to an increase in bone resorption and thus triggering OP (Xiao et al., 2015).
The small interfering RNAs (siRNAs) specific to Pdk3 (hereafter si-Pdk3#1 and si-Pdk3#2) as well as the corresponding negative control were synthesized by Guangzhou RiboBio Co., Ltd (Guangzhou, China). Next, the transfection of cells was performed with the Lipofectamine 2000 reagent (11,668; Thermo Fisher, Waltham, MA, USA) following the guidelines provided by the manufacturer. The relevant target sequence was displayed in Table 1.
Western blot
The total protein in our cultured BMMs were isolated using a commercial RIPA lysis buffer (R0010, Solarbio, Beijing, China), followed by the quantification of the concentration. Protein concentration was determined using the BCA Protein Quantification Kit (Pierce, Appleton, WI, USA) to ensure a consistent amount of protein was loaded into each sample. Equal amounts of protein were separated by SDS-PAGE, transferred to a polyvinylidene fluoride membrane (YA1701, Solarbio) (Millipore, Bredford, USA) and probed with primary antibodies against phosphorylated-ERK1/2 (1:2000, CST), ERK1/2 (1:10000, Abcam), phosphorylated-P65 (1:1000, CST), P65 (1:1000, Abcam), phosphorylated-JAK1 (1:10000, Abcam), JAK1 (1:10000, Abcam), phosphorylated-STAT1 (1:10000, Abcam), STAT1 (1:10000, Abcam) and housekeeping control GAPDH (1:10000). Then the membranes were further incubated with a solution containing horseradish peroxidase (1:5,000, GE Healthcare, Chicago, IL, USA)-labeled secondary antibody at ambient temperature for 2 h and exposed to the electrochemical luminescence reagent (PE0010, Solarbio) to develop protein bands. ImageJ 1.42 quantified the density of protein bands.
Total RNA extraction and real-time quantitative PCR
The total RNA was isolated using the TriZol assay kit (15596026, Invitrogen, Waltham, MA, USA) and then reverse transcribed into cDNA using the PrimeScript RT Reagent Kit (RR037Q, Takara, Shiga, Japan). Then the PCR was conducted using the QuantiTect SYBR Green RT-PCR Kit (204243, Qiagen, Hilden, Germany) at the following conditions: 95 °C for 15 s, and 35–45 cycles of 94 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s. The experiment was carried out in triplicate. The primer sequences used are shown in Table 2. The relative gene expression was calculated by the 2−ΔΔCt method with GAPDH as the housekeeping control (Livak & Schmittgen, 2001).
| Target | Target sequence (5′–3′) |
|---|---|
| si-NC | AGAGGAAATAATAATCATGAAGG |
| si-Pdk3#1 | AAGGGATAATGCATGTGAAAAAA |
| si-Pdk3#2 | AGGGATAATGCATGTGAAAAAAC |
Statistical analysis
SPSS 21.0 (SPSS, Inc., Armonk, NY, USA) software was applied in statistical analysis. The data of three independent trials were expressed as mean ± standard deviation. The student’s t-test was applied for two-group comparison throughout the study, and the Pearson’s correlation test was applied in correlation analyses. In this study, statistical significance was set at P < 0.05.
Results
Involvement of Pdk3 in RANKL-induced osteoclastogenesis in vitro
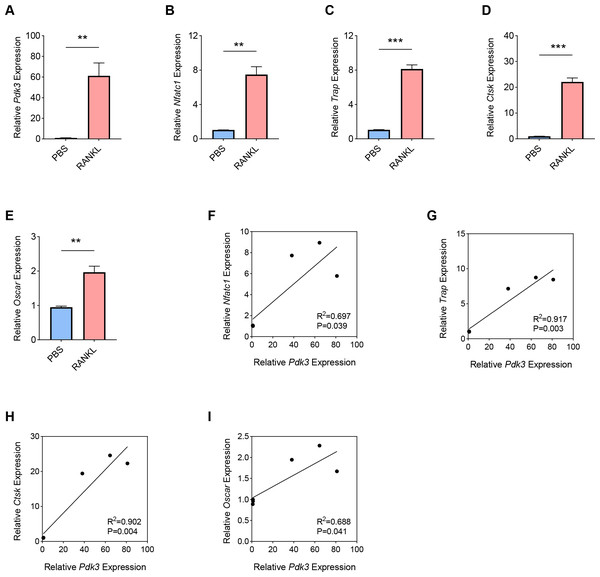
RANKL has been shown to be a key regulator of osteoclast differentiation, survival and activity. RANKL attaches to its receptor, RANK, to trigger the primary signaling pathways responsible for osteoclast formation, thereby promoting both transcriptional and epigenetic processes essential for osteoclastogenesis (Park-Min, 2018; Yasui et al., 2011; Bae et al., 2023). To this end, we used an in-vitro OP model was constructed using the RANKL as the inducer in BMMs, and the expression levels of Pdk3 as well as osteoclastogenesis feature genes were the gauged. A sharp increase in Pdk3 level was clearly seen following RANKL exposure (Fig. 1A, P < 0.01), concurrent with the elevation of osteoclastogenesis feature genes Nfatc1 (Fig. 1B, P < 0.01), Trap (Fig. 1C, P < 0.001), Cathepsin K (Ctsk, Fig. 1D, P < 0.001), osteoclast associated Ig-like receptor (Oscar, Fig. 1E, P < 0.01). We observed a positive correlation between Pdk3 and osteoclastogenesis feature genes Nfatc1 (Fig. 1F, R2 = 0.697, P = 0.039), Trap (Fig. 1G, R2 = 0.917, P = 0.003), Ctsk (Fig. 1H, R2 = 0.902, P = 0.004), and Oscar (Fig. 1I, R2 = 0.688, P = 0.041). These findings support a possible important regulatory role for PDK3 in osteoclastogenesis, which provides a basis for further investigation of its role in OP.
| Target | Primer sequence (5′–3′) | |
|---|---|---|
| Forward | Reverse | |
| Pdk3 | CTATCAAACAGTTCCTGGAC | CTTTAACCACATCAGCTACA |
| Nfatc1 | TACTTGGAGAATGAACCTCT | CAGTAAAAACCTCCTCTCAG |
| Trap | GCACAGATTGCATACTCTAA | GCTGGTCTTAAAGAGTGATT |
| Ctsk | AGACTCACCAGAAGCAGTAT | CTGGAGTAACGTATCCTTTC |
| Oscar | ATACTCCAGCTGTCGACTC | AGCAGTTCCAGAACATTACT |
| Bax | TGAACAGATCATGAAGACAG | TCTTGGATCCAGACAAGC |
| Bcl2 | CATTATAAGCTGTCACAGAGG | GGAGAAATCAAACAGAGGTC |
| Casp3 | AAGAACTTCCATAAGAGCAC | AGGTGCTGTAGAGTAAGCAT |
| Il1b | CTGAACTCAACTGTGAAATG | AAGTCAATTATGTCCTGACC |
| Il6 | GTCTTCTGGAGTACCATAGC | TATCTGTTAGGAGAGCATTG |
| Tnf | CTCACACTCAGATCATCTTCTC | TTCTCCTGGTATGAGATAGC |
| Gapdh | GCTTAGGTTCATCAGGTAAA | TGACAATCTTGAGTGAGTTG |
Figure 1: Involvement of Pdk3 in RANKL-induced osteoclastogenesis in vitro.
(A) Expression level of Pdk3 in RANKL-induced osteoclastogenesis in vitro. (B–E) Expression levels of osteoclastogenesis feature genes Nfatc1 (B), Trap (C), Ctsk (D), and Oscar (E). (F–I) Correlation between Pdk3 and osteoclastogenesis feature genes Nfatc1 (F), Trap (G), Ctsk (H), and Oscar (I). All experimental data of three independent trials were expressed as mean ±standard deviation. ∗∗P < 0.01, ∗∗∗P < 0.001.Positive correlation between Pdk3 and relevant signaling pathways in RANKL-induced osteoclastogenesis in vitro
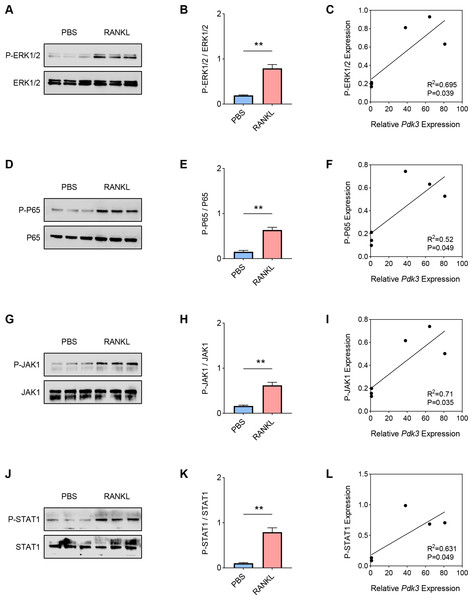
Hereafter, the involvement of signaling pathways relevant to osteoclastogenesis like ERK, P65, and JAK/STAT was determined by western blot. Increased level of phosphorylated-ERK1/2 was seen following RANKL intervention (Figs. 2A–2B, P < 0.01), which was positively correlated with Pdk3 (Fig. 2C, R2 = 0.695, P = 0.039). Further, RANKL exposure also resulted in significant upregulation of phosphorylation of P65 (Figs. 2D–2E, P < 0.01), JAK1 (Figs. 2G–2H, P < 0.01) and STAT1 (Figs. 2J–2K, P < 0.01), which were all positively correlated with Pdk3 (Figs. 2F, 2I, 2L).
Figure 2: Positive correlation between Pdk3 and relevant signaling pathways in RANKL-induced osteoclastogenesis in vitro.
(A–B) Phosphorylation level of ERK1/2 in RANKL-induced osteoclastogenesis in vitro. (C) Correlation between phosphorylation level of ERK1/2 and Pdk3. (D–E) Phosphorylation level of P65 in RANKL-induced osteoclastogenesis in vitro. (F) Correlation between phosphorylation level of P65 and Pdk3. (G–H) Phosphorylation level of JAK1 in RANKL-induced osteoclastogenesis in vitro. (I) Correlation between phosphorylation level of JAK1 and Pdk3. (J–K) Phosphorylation level of STAT1 in RANKL-induced osteoclastogenesis in vitro. (L) Correlation between phosphorylation level of STAT1 and Pdk3. All experimental data of three independent trials were expressed as mean ±standard deviation. ∗∗P < 0.01.Pdk3 knockdown led to the inactivation of relevant signaling pathways in RANKL-induced osteoclastogenesis in vitro
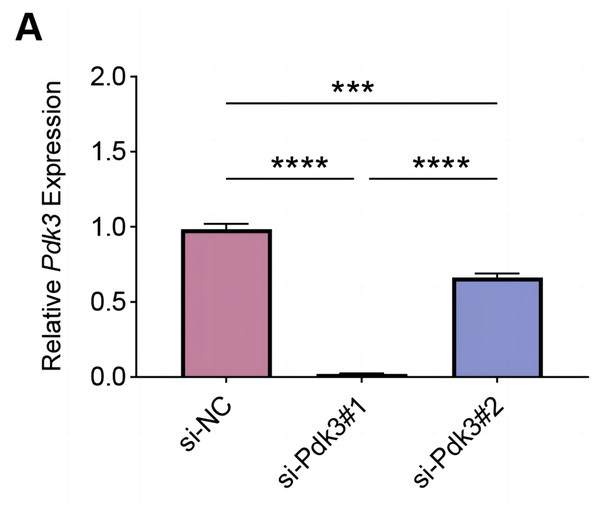
To explore the specific role of Pdk3 in RANKL-induced osteoclastogenesis in vitro, we first verified that transfection of Pdk3 in this cell was successful (Fig. 3A, P < 0.001).
Figure 3: Knockdown efficiency validation.
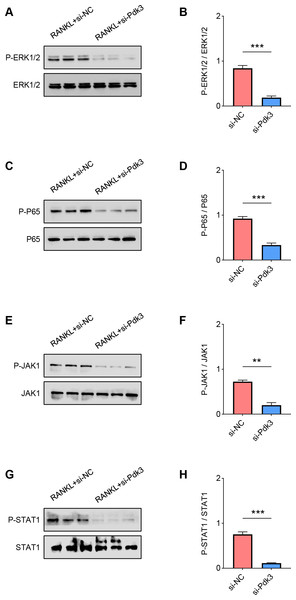
(A) Pdk3-specific small interfering RNAs were applied for the transfection and the knockdown efficiency was tested. All experimental data of three independent trials were expressed as mean ±standard deviation. ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.The levels of proteins related to the signaling pathways were measured again with the presence of Pdk3-specific siRNA. Accordingly, the results of western blot have manifested the phosphorylation levels of all these proteins were significantly down-regulated compared to the control, including ERK1/2 (Figs. 4A–4B, P < 0.001), P65 (Figs. 4C–4D, P < 0.001), JAK1 (Figs. 4E–4F, P < 0.01) and STAT1 (Figs. 4G–4H, P < 0.001).
Figure 4: Pdk3 knockdown led to the inactivation of relevant signaling pathways in RANKL-induced osteoclastogenesis in vitro.
(A–B) Following the silence of Pdk3, the quantified phosphorylation level of ERK1/2 in RANKL-induced osteoclastogenesis in vitro. (C–D) Phosphorylation level of P65 after the knockdown of Pdk3 in RANKL-induced osteoclastogenesis in vitro. (E–F) After the transfection of Pdk3-specific siRNA, the level of JAK1 phosphorylation in RANKL-induced osteoclastogenesis in vitro. (G-H) Phosphorylation level of STAT1 in RANKL-induced osteoclastogenesis in vitro following the silencing of Pdk3. All experimental data of three independent trials were expressed as mean ±standard deviation. ∗∗P < 0.01, ∗∗∗P < 0.001.Effects of Pdk3 silencing on RANKL-induced osteoclastogenesis in vitro
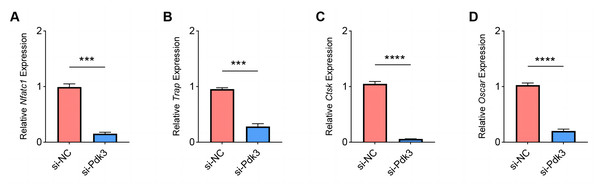
The expressions of osteoclastogenesis feature genes were then quantified to further examine the effects of Pdk3 silencing on RANKL-induced osteoclastogenesis in vitro. According to the relevant results of qPCR, we observed that Pdk3 silencing significantly downregulated the levels of genes characteristic of osteoclastogenesis relative to controls, including Nfatc1 (Fig. 5A, P < 0.001), Trap (Fig. 5B, P < 0.001), Ctsk (Fig. 5C, P < 0.0001), Oscar (Fig. 5D, P < 0.0001).
Figure 5: Effects of Pdk3 silencing on RANKL-induced osteoclastogenesis in vitro.
(A–D) Expression levels of osteoclastogenesis feature genes Nfatc1 (A), Trap (B), Ctsk (C), Oscar (D) following the silencing of Pdk3. All experimental data of three independent trials were expressed as mean ±standard deviation. ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.Effects of Pdk3 silencing on RANKL-induced apoptosis and inflammation in vitro
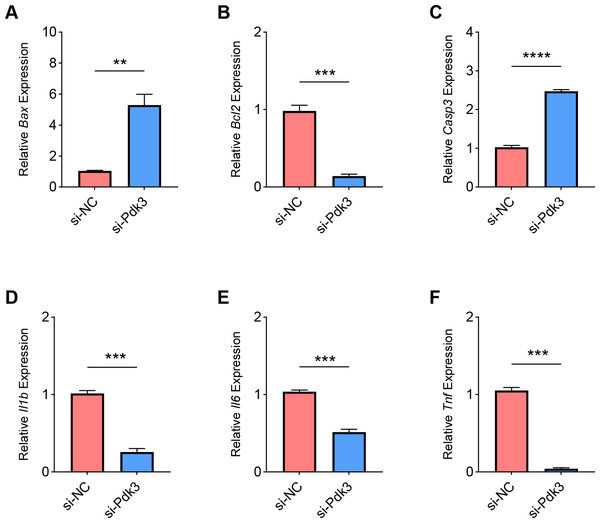
Finally, we determined the effects of Pdk3 silencing on RANKL-induced apoptosis and inflammation in vitro. It was clearly seen that Pdk3 silencing led to the elevation of Bax and Casp3 expression yet suppressed that of Bcl2 (Figs. 6A–6C, P < 0.01). Relevant results on the inflammatory cytokines expressions have additionally proven that Pdk3 silencing led to the suppression on all the inflammatory cytokines including Il1b, Il6 and Tnf (Figs. 6D–6F, P < 0.001).
Figure 6: Effects of Pdk3 silencing on RANKL-induced inflammation and apoptosis in vitro.
(A–C) Expression levels of apoptosis-related proteins Bax (A), Bcl2 (B) and Casp3 (C) in response of Pdk3 knockdown. (D–F) Levels of inflammatory cytokines Il-1 β (D), Il-6 (E) and Tnf (F) following the knockdown of Pdk3. All experimental data of three independent trials were expressed as mean ±standard deviation. ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.Discussion
The specific effects and mechanism of Pdk3 regulation in osteoclastogenesis have not been systematically interpreted, which thus provides us an opportunity to commence this research to fill the blank. In our current study, we first found genetic evidence in RANKL-induced BMMs that Pdk3 gene plays a crucial role in osteoclastogenesis. In other words, Pdk3 expression was proven to be elevated in response to RANKL exposure, and a positive correlation was seen in Pdk3 and osteoclastogenesis feature genes Nfatc1 (a master transcription factor required for osteoclast differentiation Kang et al., 2020), Trap (a gene critical to osteoclast activation Takegahara, Kim & Choi, 2024), Ctsk (a member of the papain family of cysteine proteases highly expressed by activated osteoclasts Costa et al., 2011), and Oscar (a regulator of osteoclast differentiation Nedeva et al., 2021). The subsequent assay results have confirmed that the silencing of Pdk3 could repress the levels of these feature genes and attenuate the inflammation yet promote the apoptosis of BMMs-derived osteoclasts. Thus, our findings provide new insights into understanding the role of Pdk3 in osteoclasts in OP and provide a theoretical basis for therapeutic strategies for OP patients.
Osteoclasts, multinucleated cells deriving from monocyte/macrophage-lineage cells and resorbing bone, have been documented to continuously destroy the bone in order to maintain the bone volume and calcium homeostasis throughout the lifespan of vertebrates (Udagawa et al., 2021). RANKL is the membrane-bound factor expressed by osteoclastogenesis-supporting cells like osteoblasts and osteocytes and critically involved in pathologic bone disorders (Takayanagi, 2021; Sigl & Penninger, 2014). Osteoclast precursors can express RANK (a known RANKL receptor), recognize RANKL expressed by the osteoblasts via cell–cell communication and differentiate into osteoclasts in the presence of M-CSF (Udagawa et al., 2021). RANKL/RANK pathway has been underlined to control osteoclasts activity and formation, which therefore has been identified as a key factor on bone turnover in diverse pathological conditions (Amin et al., 2020). Existing studies of studying osteoclast in vitro have extensively applied the technique of isolating osteoclast from primary BMMs or culturing the RAW264.7 cell lines, all of which have been widely engaged in bone homeostasis research (Song et al., 2019). The former technique of isolation was applied in our current study, and the isolated BMMs were treated with M-CSF and RANKL to induce an osteoclast-like cells so as to examine the effects of Pdk3 on RANKL-induced osteoclastogenesis in OP in vitro. The study of Lee et al. (2021) has demonstrated that the deficiency of PDK2, another member of the PDK family, can prevent the ovariectomy-induced bone loss in mice via regulating the RANKL-NFATc1 pathway during osteoclastogenesis. Our current study, likewise, proved the involvement of Pdk3 in RANKL-treated BMMs-derived osteoclasts. Specifically, following the confirmation that Pdk3 was highly expressed in RANKL-treated BMMs-derived osteoclasts, the additional investigation has suggested that Pdk3 knockdown could diminish the expression of osteoclastogenesis feature genes in vitro, which hinted the potential of Pdk3 on osteoclastogenesis.
Osteoclastogenesis has been defined as an ongoing rigorous course including osteoclast precursors fusion and bone resorption executed by the degradative enzymes, which is also underscored to be controlled by and relevant to some processes like inflammation (Tong et al., 2022). Meanwhile, both survival and apoptosis are of major importance in the life cycle of osteoclasts, and the regulation of osteoclast apoptosis has been recognized as a critical factor in bone remodeling, where Bcl2 family member proteins and caspases have been shown to take part (Soysa & Alles, 2019; Ke et al., 2019; Song et al., 2020). In addition to the known endocrine, metabolic and mechanical factors, emerging evidences have further pointed out that inflammation also exerts significant influence on bone turnover, thus inducing OP (Ginaldi, Di Benedetto & De Martinis, 2005). Certain pro-inflammatory cytokines play possible critical roles both in normal bone remodeling process and in the pathogenesis of OP (Ginaldi, Di Benedetto & De Martinis, 2005). IL-6, for instance, promotes osteoclast differentiation and activation, while IL-1 is another potent bone resorption stimulator linked to accelerated bone loss (Manolagas, 2000; Wei et al., 2005). Further, TNF is proven to play a pivotal role in osteoclast maturation (Epsley et al., 2020). Besides, TNF has been underscored to signal via NF- κB and the MAPKs, and Il6 via the JAK-STAT pathway (Osta, Benedetti & Miossec, 2014). In RANKL-induced osteoblasts, the phosphorylation levels of JAK1 and STAT1 were significantly increased, suggesting that this signaling pathway was activated during osteoblast activation. Knockdown of the Pdk3 gene significantly inhibited the phosphorylation of JAK1 and STAT1, further suggesting that Pdk3 may affect the differentiation and activity of osteoblasts through the regulation of the JAK1-STAT1 signaling pathway. All these pathways have been revealed to be involved in OP, according to some relevant studies (Li et al., 2022; Yang et al., 2023; Xu et al., 2018). While trying to link the association between PDKs and these pathways, Tnf can promote the degradation of PDK4 in endothelial cells to support pro-inflammatory cytokines in a NF- κB-dependent manner (Boutagy et al., 2023). In the meantime, another study on bladder cancer has stressed the anti-metastatic effects of PDK4 via the ERK and JNK pathways in bladder cancer cells (Lee et al., 2022). In our current study, we firstly proved the modulation of Pdk3 on these signaling pathways in RANKL-induced BMMs-derived osteoclasts, as supported by the fact that Pdk3 silencing diminished the phosphorylation of P65, ERK1/2 and JAK/STAT.
Nonetheless, it should be noted that there are some limitations to our study. First, all experiments in this study were performed in an in vitro model, and in the future by constructing Pdk3 knockout or overexpression mouse models to be able to more validate its specific role in OP. In addition, we did not address other functional phenotypes of osteoclasts (e.g., bone resorption capacity, cell migration and proliferation capacity, etc). Therefore, it is important to further expand the scope of the experiments to incorporate functional phenotyping experiments to be able to comprehensively assess the effects of Pdk3 on osteoclast function. Finally, although Pdk3, as a member of the pyruvate dehydrogenase kinase family, may play an important role in cellular metabolism, its metabolic regulatory role in osteoclasts or osteoblasts was not explored in depth in this study. Future studies should explore the specific role of Pdk3 in cellular metabolism by combining metabolic analysis techniques, such as glycolytic flux assay and mitochondrial function assay. This will help to reveal the broader biological functions of Pdk3 in osteoporosis.
So far as we are concerned, is the first to interpret the effect of Pdk3 on OP via modulating the osteoclastogenesis using RANKL-induced BMMs. The relevant mechanisms of Pdk3 were preliminarily explored to be related to the suppression of the phosphorylation of ERK, P65 and JAK/STAT, the reduced expressions of osteoclastogenesis feature genes, the attenuated inflammation-associated cytokines, and regulated the expression of apoptosis-related proteins. This study indeed opens up a novel avenue for OP prevention and provides a rationale for the development of therapies targeting Pdk3.