Toll-like receptor 4 damages the intestinal epithelial cells by activating endoplasmic reticulum stress in septic rats
- Published
- Accepted
- Received
- Academic Editor
- Jincheng Wang
- Subject Areas
- Biochemistry, Cell Biology, Microbiology, Histology
- Keywords
- Sepsis, TLR4, Lgr5+ cells, Goblet cells, Endoplasmic reticulum stress
- Copyright
- © 2024 Wu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Toll-like receptor 4 damages the intestinal epithelial cells by activating endoplasmic reticulum stress in septic rats. PeerJ 12:e18185 https://doi.org/10.7717/peerj.18185
Abstract
Background
The severity of acute gastrointestinal injury (AGI) is a critical determinant of survival in sepsis. However, there is no specifically interventional management for gastrointestinal dysfunction. Toll-like Receptor 4 (TLR4) is an important contributor to sepsis-induced multiple organ dysfunction syndrome. So, we investigated the effect of TLR4 on leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) + cells and goblet cells and its potential mechanism.
Methods
A cecal ligation and puncture (CLP) model reflecting the development of clinical sepsis was developed. Tak-242, a TLR4 inhibitor, was administered to septic rats at a dose of 3 mg/kg via intraperitoneal injection. Immunohistochemistry was performed to detect TLR4 and Lgr5+ cells. AB-PAS staining was performed to detect goblet cells. MUC1 and MUC2 secreted by goblet cells, biomarkers of endoplasmic reticulum (ER) stress and inflammatory cytokines in the intestine were detected by western blotting and real-time PCR.
Results
We found that the upregulation of the TLR4/NF-κB signaling pathway activated intestinal inflammatory response in sepsis. Meanwhile, the structure of intestinal mucosa was destroyed, Lgr5+ cells and goblet cells count were significantly reduced, and the secretory function of goblet cells also decreased. Further studies have found that TLR4 increased the levels of activating transcription factor-6 (ATF6), XBP1, ER chaperone (Bip) and CHOP, but did not activate the protein kinase RNA (PKR)-like ER kinase (P-PERK).
Conclusion
We concluded that the inhibition of TLR4/NF-κB signaling pathway can reduce intestinal inflammatory response, protect intestinal mucosa, protect Lgr5+ cells, goblet cells and relieve ER stress. Our findings suggest that Tak-242 protects Lgr5+ cells and goblet cells after sepsis, partly may be through the suppression of ER stress. Thus, inhibition of TLR4-mediated ER stress may be a promising therapy of septic AGI.
Introduction
Sepsis is a life-threatening organ dysfunction caused by dysregulated host response to infection (Evans et al., 2021). In 2017, there were an estimated 48.9 million cases of sepsis worldwide, and the global incidence of sepsis was 677.5 cases per 100,000. There were an estimated 11 million sepsis-related deaths, representing 19.7% of deaths that year (Rudd et al., 2020). However, in Chinese intensive care units, the 90-day mortality rate of sepsis is 35.5% (Xie et al., 2020). The severity of acute gastrointestinal injury (AGI) is a critical determinant of survival in critically ill patients (Hu et al., 2017). At present, the treatment of septic AGI mainly focus on early enteral nutrition and intestinal microbiota regulation (Haak, Prescott & Wiersinga, 2018; Kim et al., 2020). However, early enteral nutrition is limited by gastrointestinal bleeding, uncontrolled shock, bowel obstruction and feeding intolerance (Reintam Blaser et al., 2017). The ways to regulate intestinal microbiota include fecal microbiota transplant andprobiotics. However, patients in the setting of critical illness could suffer lethal complication of multiple drug resistant bacteremia associated with fecal microbiota transplant (DeFilipp et al., 2019; Keskey et al., 2020). The degree of gut colonization by probiotics considerably varies between individuals, which lead to different effects of probiotics (Suez et al., 2019). Therefore, we need to find a safe and effective treatment method urgently. The gastrointestinal microenvironment is destroyed during sepsis, which is comprised of cell layer epithelia, the microbiome, and local immune system (Fay, Ford & Coopersmith, 2017). Gastrointestinal dysfunction during sepsis is mainly due to the damage of intestinal epithelial cells. Sepsis induces a marked increase in apoptosis of gut epithelial cells, with a simultaneous decrease in crypt proliferation (Klingensmith & Coopersmith, 2016). Leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) is a receptor for R-spondin (Kim et al., 2005). Scientific evidence has demonstrated the remarkable long-term self-renewal capacity of the intestinal epithelium is achieved through Lgr5+ intestinal stem cells (Barker et al., 2007). Goblet cells and the mucus they secrete play a vital role in maintaining intestinal tract immunity and mucosal homeostasis (Nystrom et al., 2021; Yang & Yu, 2021).
Emerged evidence have found that the level of Toll-like Receptor 4 (TLR4) was increased in the intestinal tissues of septic mice (Krivan et al., 2019; Wang et al., 2022). However, the role and characteristic of TLR4 on th e Lgr5+ cells and goblet cells in septic rats remain unknown. TLR4, a member of the Toll-like receptor (TLR) family in the innate immune system, was identified as a receptor that responds to bacterial lipopolysaccharide (LPS) (Akira, Uematsu & Takeuchi, 2006). TLR4 activates the transcription factor nuclear factor-kappaB (NF-κB), which controls the expression of an array of inflammatory cytokine (Kawai & Akira, 2007). Excessive inflammatory response leads to organ dysfunction associated with sepsis (Singer et al., 2016). Evidence indicates that TLR4 could reduce Lgr5+ cell proliferation and increase apoptosis in inflammatory bowel diseases (Neal et al., 2012). A study on necrotizing enterocolitis found that TLR4 activation induces apoptosis of Lgr5+ cells in intestinal crypts (Afrazi et al., 2014). TLR4 signaling increase in the intestine and prevents goblet cells differentiation in mice (Sodhi et al., 2012). However, another study found that colonic goblet cells in tissues secrete more MUC2, the secretion of MUC2 depends on TLRs (Birchenough et al., 2016). Therefore, we hypothesis the Lgr5+ cells and goblet cells count and function was destroyed by TLR4. Meanwhile, the underlying mechanisms were explored.
Impairment of endoplasmic reticulum (ER) function in processing unfolded proteins is called ER stress (Ron & Walter, 2007). The unfolded protein response (UPR) is initiated by the unresolved ER stress, which is associated with cell differentiation and inflammatory responses (Grootjans et al., 2016). Under the condition of ER homeostasis, Bip (a ER chaperone) bind to UPR sensors: inositol-requiring protein-1 (IRE1), activating transcription factor-6 (ATF6), and protein kinase RNA (PKR)-like ER kinase (PERK) (Harding, Zhang & Ron, 1999). However, under condition of ER stress, Bip is released from the UPR transducers, leading to three UPR transcription activations (Pfaffenbach & Lee, 2011). IRE1 is a stress-activated endonuclease resident that functions by activating transcription factor XBP1 (Calfon, 2002). Activated ATF6 increase intestinal epithelial cells inflammatory signals (Stengel et al., 2020). PERK phosphorylates eIF2alpha on serine residue 51, which attenuates protein translation in response to ER stress (Harding, Zhang & Ron, 1999). TLR4 specifically activated IRE1α and its downstream target transcription factor XBP1; however, CHOP and ATF6α, two transcription factors induced or activated upon ER stress, were not detected in a study of TLR stimulation (Martinon et al., 2010). Another study in mice found that low-dose LPS suppressed CHOP expression and apoptosis in splenic macrophages, renal tubule cells, and hepatocytes and prevented renal dysfunction (Woo et al., 2009). However, prolonged TLR4 activation induces ER stress (Coope et al., 2012). Therefore, TLR4 responds differently to these three ER stress transducers in different cell types. In this study, we investigated whether ER stress is activated by TLR4 and influence Lgr5+ cells and goblet cells in sepsis.
Materials and Methods
Animals
Thirty male Wistar rats (7–8 weeks old and 250 ± 10 g weight) were purchased from Institute of Medical Biology, Chinese Academy of Medical Sciences (Kunming, China). Animals were fed in separate cages, maintained on stable conditions (light-dark cycle of 12:12 h, temperature:18 °C–28 °C) and provided with a basic diet for 1 week before the experiments. The Animal Care and Use Committee of Yunnan Luoyu Biotechnology approved all animals care and experimental protocols (No. PZ20211001, Date: October 9, 2021).
Experimental design
Experiments were performed from October 11, 2021 to December 31, 2021. Experimental abdominal sepsis was induced by cecal ligation and puncture (CLP) (Drechsler & Osuchowski, 2021). Intraperitoneal anesthesia (using 10% caldehyde at a dose of 300 g/ml) was administered before operation. The rats were divided into three groups via a table of random numbers: (1) the control group: n = 10; (2) the sepsis group: n = 10; (3) the treatment group: n = 10. The rats of control group received a sham operation (ventral midline incision, sterilized, and sutured, without cecal ligation and puncture). The sepsis rats and treatment rats underwent the CLP operation. Prewarmed Ringer (0.015 ml/g BW) solution was applied into the abdominal cavity after surgery in three groups. The treatment rats were administered with TLR4 inhibitor TAK-242 (3 mg/kg) by intraperitoneal injection (Wang et al., 2022), the control and the sepsis group rats were administered by an equal volume of 0.9% sterile saline via intraperitoneal injections once daily for seven days. If the following symptoms of systemic damage occured before the completion of the experiment, euthanasia was performed on the test rats by cervical dislocation method: (1) Animals bite each other, resulting in disability. (2) Weakness (unable to eat or drink): When the animal is unable to eat or drink without being anesthetized or sedated, and is unable to stand for up to 24 h or can only stand with great difficulty. (3) Dying/near death: When the animal shows mental depression accompanied by hypothermia (lower than 37 °C) without being anesthetized or sedated. All rats were euthanized in the conclusion of the experiment by the same method. The ileal tissue was obtained for analysis of histology, mRNA and protein.
Histology
The ileal tissue was fixed in 4% paraformaldehyde and embedded in paraffin. The paraffin blocks were cut into sections with a thickness of 5 µm. Tissue sections were subjected to hematoxylin and eosin staining for observation tissue injury under a light microscope.
Immunohistochemistry
The sections were deparaffinized with xylene, hydrated with ethanol. Endogenous peroxidase activity was eliminated in 3% hydrogen peroxide. After the sections were closed with 10% goat serum albumin, they were incubated with primary antibodies: anti-TLR4 antibody (1:200; Rabbit. no. PA5-23,124; Thermo Fisher Scientific, Waltham, MA, USA) and anti-lgr5 antibody (1:400; Rabbit. no. bs-20717R; Bioss, Inc.) overnight at 4 °C. The tissues were incubated with HRP rabbit secondary antibody. The number of goblet cells in each crypt was determined via the ImageJ software (version 1.8.0; National Institutes of Health) by analyzing eight fields per slide.
AB-PAS staining
The tissue sections were deparaffinized and hydrated, then placed in 1% Alcian blue solution for 15 min, followed by rinsing with running water. The sections were dipped into a 0.5% periodic acid reagent to stain for 15 min and then cleaned. Schiff reagent was poured kept for 30 min at room temperature without light. Tissue sections were rinsed with running water for 5 min and dehydrated with alcohol. In the end, we selected three high-magnification visual fields (200×) to observe and recorded the number of goblet cells per field.
Real-time PCR
Total RNA was isolated from rat ileal tissues using trizol lysate (15,596,026; Lifetech, Elmhurst, IL, USA) and cDNA was synthesized from total RNA using a FastKing RT Kit (with gDNase) FastKing cDNA (KR116 SYBR Green master mix: KAPA KK4601). qRT-PCR was performed using a LightCycler®480 Multiwell Plate 96 and primer sequences of TLR4, TNF-α, IL-1β, MUC1, MUC2, ATF6 and XBP1. The relative mRNA levels were calculated using the 2−ΔΔCt method. All primers were synthesized by Sangon (Invitrogen, Guangzhou, China). The following primers were used:
TLR4-forward: 5′-CAATCGCATAGAGACATC-3′,
TLR4-reverse: 5′-GTTCAACATTCACCAAGA-3′;
TNF-α-forward: 5′-ATGATCCGAGATGTGGAACTGG-3′
TNF-α-reverse: 5′-TGGGAACTTCTCCTCCTTGTTG-3′
IL-1β-forward: 5′-TGGAGTTTGAGTCTGCACAGTT-3′
IL-1β-reverse: 5′-TGGTGAAGTCAACTATGTCCCG-3′
MUC1- forward: 5′-CCTTCTTCTCGTTGTCTT-3′,
MUC1-reverse: 5′-TCTTCAGTTCTTGGTAGTAG-3′;
MUC2-forward: 5′-TGCTATGACTATGAGATACGA-3′,
MUC2-reverse: 5′-AGATGAGATTGGTGTTGAAG-3′;
ATF6-forward: 5′- TAATGGTGCTAAGTGAAGA-3′,
ATF6-reverse: 5′-ACTCTGTCGTGTTAATGA-3′;
XBP1-forward: 5′-TGTTGCCTCTTCAGATTC-3′,
XBP1-reverse: 5′-GAGTTCCTCCAGATTAGC-3′;
β-actin-forward: 5′-GCAGGAGTACGATGAGTCCG-3′,
β-actin-reverse: 5′-ACGCAGCTCAGTAACAGTCC-3′.
Nucleoplasmic protein isolation experiment
The ileum tissue was cut into very small fragments. The cytoplasmic protein lysis solution A and B (Biyuntian, Shanghai, China) were mixed at a ratio of 20:1, and after adding phenylmethanesulfonyl fluoride, a tissue homogenate was formed. The ileum tissue and tissue homogenizer were fully homogenized in a homogenizer at 4 °C and left for 15 min. The supernatant after centrifugation was aspirated to obtain cytoplasmic protein. After completely aspirating the supernatant, the nuclear protein lysis solution containing phenylmethanesulfonyl fluoride was added to the remaining precipitate, fully homogenized, and ice-bathed for 30 min. The supernatant after centrifugation was aspirated to obtain nuclear protein.
Western blot analysis
The cytoplasmic protein and nuclear protein extracted from rat ileal tissues were detected via the BCA assay (Biyuntian, Shanghai, China). Proteins were separated by SDS-PAGE and transferred to PVDF membranes (Millipore). The membranes were incubated with primary antibodies: anti-TLR4 (1:500, PA5-23,124; Thermo Fisher Scientific), anti-CHOP (1:1000, MA5-32,571; Thermo Fisher Scientific), anti-Bip (1:1000, Abcam ab21685), anti-P-PERK (1:1000, MA5-15033; Thermo Fisher Scientific) and anti-NF-κB-p65 (1:1000, AF5006; Affinity Biosciences), actin (1:2000, TA-09; Zhongshanjinqiao), actin (1:3000, AF7018; Affinity Biosciences) at 4 °C overnight. Then, the membranes were incubated with Horseradish peroxidase-conjugated secondary antibody. The protein bands were analyzed and quantified via ImageJ Software.
Statistical analysis
Data was presented as mean ± standard error of the mean and analyzed with SPSS 27.0 software. The normality was carried out by the Shapiro–Wilk (Royston) test. Differences between groups were analyzed by t-test and one-way analysis of variance (ANOVA) by the Bonferroni posttests (GraphPad Prism 9.0; GraphPad Software, La Jolla, CA, USA). Statistical significance was set at p < 0.05.
Results
Upregulation of TLR4/NF-κB pathway activated intestinal inflammatory response after CLP
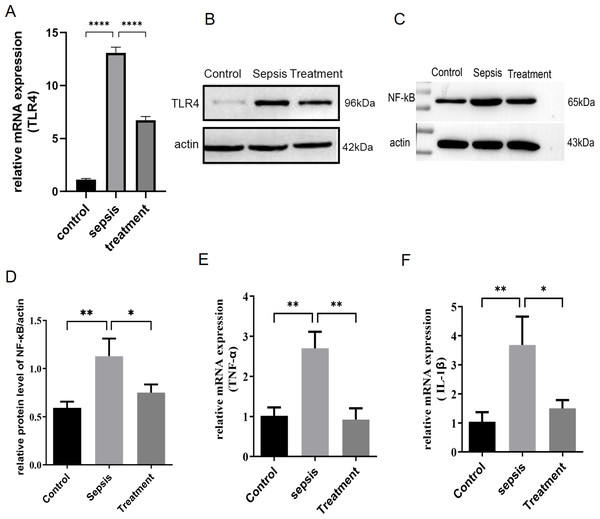
First, we measured TLR4 mRNA and protein levels in different groups by real-time PCR and western blotting. Compared with the control group, TLR4 mRNA and protein levels increased in sepsis group (P < 0.0001) (Figs. 1A–1B). Subsequently, this change was reversed in the treatment group (P < 0.0001) (Figs. 1A–1B). In sepsis group, NF-κB was activated with the upregulation of TLR4 (Figs. 1C–1D, p = 0.008). TLR4/NF-κB signaling was inhibited by treatment with Tak-242 (Figs. 1C–1D, p = 0.031). Further, the inflammatory cytokines in the intestine were detected. We found TNF-α (p = 0.003) and IL-1β (p = 0.002) levels increased in sepsis group (Figs. 1E–1F). Cytokines of TNF-α (p = 0.004) and IL-1β (p = 0.005) levels were reduced by inhibiting TLR4 signaling. In conclusion, upregulation of TLR4/NF-κB pathway activated intestinal inflammatory response.
Figure 1: TLR4 signal and intestinal inflammation activation in septic rats.
(A) TLR4 mRNA in different groups by qRT-PCR. (B) TLR4 protein levels in different groups by western blot. (C) The NF- κB level in intestinal tissue by western blot. (D) The relative protein level of NF-κB. The relative mRNA expression of TNF-α (E) and IL-1β (F) in small intestine. (n = 5), Data were based on three independent replications experiments. Statistical differences were determined by using the analysis of variance (ANOVA) with Bonferroni’s multiple comparison test. * p < 0.05, ** p < 0.01, **** p < 0.0001.Tak-242 improved feeding, body weight and prevention of intestinal mucosa in sepsis
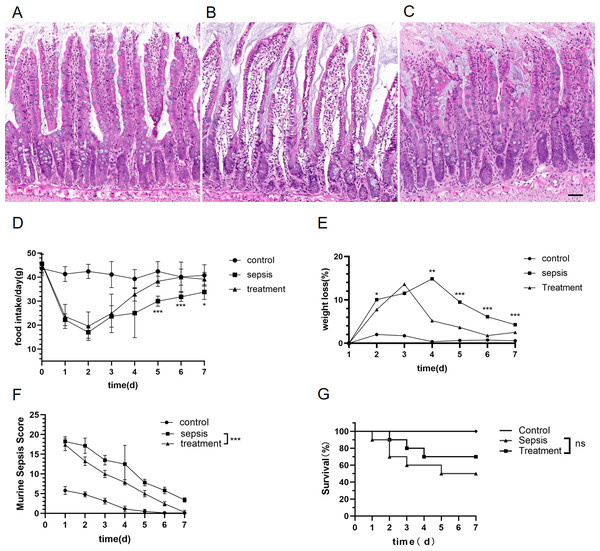
We explored whether morphological differences could be detected in rats with sepsis. Compared with the control group, the structure of the intestinal mucosa was destroyed in sepsis rats by hematoxylin eosin staining (HE) (Figs. 2A–2C). The structure of the intestinal mucosa was relatively intact in the treatment group (Figs. 2A–2C).
Figure 2: Tak-242 improved feeding, body weight and prevention of intestinal mucosa in septic rats.
(A–C) Structure of small intestinal mucosa in three groups by hematoxylin eosin staining. (Magnification × 20, n = 5 per group, Scale bars = 50 µm), (A) the control group; (B) the sepsis group; (C) the treatment group. (D) The rat’s food intake (n = 10 per group). (E) Weight loss (%) of rats daily (n = 10 per group). (F) Murine Sepsis Score (MSS) over time of rats (n = 10 per group). (G). Rat survival over time (n = 10 per group). Corresponding p values are reported in the figure with statistical analysis being performed using t-test, sepsis vs treatment, * p < 0.05, ** p < 0.01, *** p < 0.001.There was no significant difference in basal food intake among the three groups. Meanwhile, we found that food intake of rats began to decrease on the first day after CLP. From the fifth day, the food intake in the treatment group were higher than those in the sepsis group (Fig. 2D, D5: p < 0.001, D6: p < 0.001, D7: p = 0.012). Both sepsis and treatment group rats had initial loss of body weight after CLP when adjusted for starting body weight (Fig. 2E). The weight loss (%) in the treatment group was lower than that of the sepsis group (Fig. 2E, D2: p = 0.01, D4: p = 0.002, D5: p < 0.001, D6: p < 0.001, D7: p < 0.001). On the first day after CLP, the murine sepsis score (MSS) of sepsis group (p < 0.001) and treatment group (p < 0.001) was higher than the control group, and then gradually decreased. By the last day of the experiment, MSS in the treatment group had dropped to the level of the control group and was lower than the sepsis group (Fig. 2F, p < 0.001). Finally, we observed the mortality of the three groups. All the rats in the control group survived, and the survival rate of the sepsis rats was 50%, which was significantly higher than that of the control group. In the treatment group, the mortality of rats was 30%, but Tak-242 did not reduce the mortality of sepsis rats (Fig. 2G, p = 0.34). So, TAK-242 treatment can protect intestinal mucosa, increase daily food intake, and reduce the rate of weight loss in rats, but cannot improve survival of sepsis.
The upregulation of TLR4 lead to the reduction of Lgr5+ cells and goblet cells in small intestinal
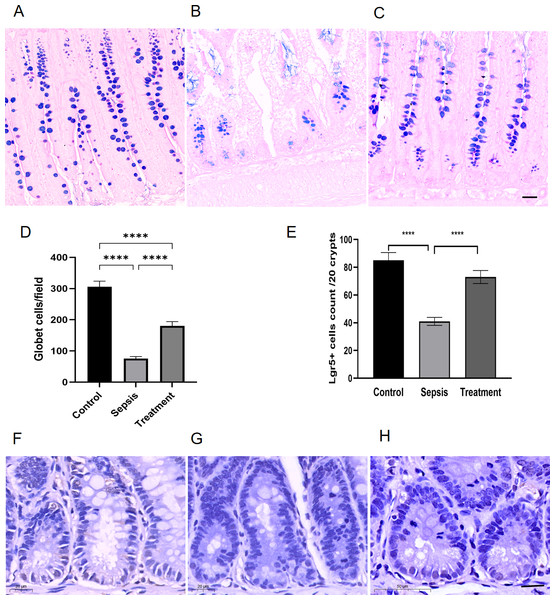
We observed goblet cells by using the goblet cells-specific marker AB-PAS. The mucins of the goblet cells were stained bluish-purple. Compared with the control group, the number of goblet cells decreased in sepsis group; After treatment with Tak-242, goblet cells count increased (Figs. 3A–3C). We selected three high-magnification visual fields to observe and record the number of goblet cells per field. By quantitative analysis, we found that the small intestinal goblet cells count decreased in sepsis group, and Tak-242 can protect goblet cells (Fig. 3D, p < 0.0001). Meanwhile, we found that the number of Lgr5+ cells decreased in the sepsis group compared with that in the control group by immunofluorescence staining (p < 0.0001) (Figs. 3E–3H). After treatment with Tak-242, Lgr5+ cells count increased too (p < 0.0001) (Figs. 3E–3H). The results showed that the upregulation of TLR4 damaged Lgr5+ cells and goblet cells in the ileal tissue of septic rats.
Figure 3: The number of Lgr5+ cells and goblet cells in small intestinal.
(A–C) AB-PAS staining of goblet cells. (Magnification × 20, n = 5 per group), (A) the control group; (B) the sepsis group; (C) the treatment group. (D) The number of goblet cells in each high-power field (n = 5 per group). (E) Semi-quantification of Lgr5+ cells by ImageJ software (n = 5 per group). (F–H) The Lgr5+ cells in different groups by immunohistochemical. (Magnification × 30, n = 5 per group), (F) the control group; (G) the sepsis group; (H) the treatment group. Statistical differences were determined by using ANOVA with Bonferroni’s multiple comparison test. Scale bar =20µm, **** p < 0.0001.The upregulation of TLR4 damaged the secretory function of goblet cells
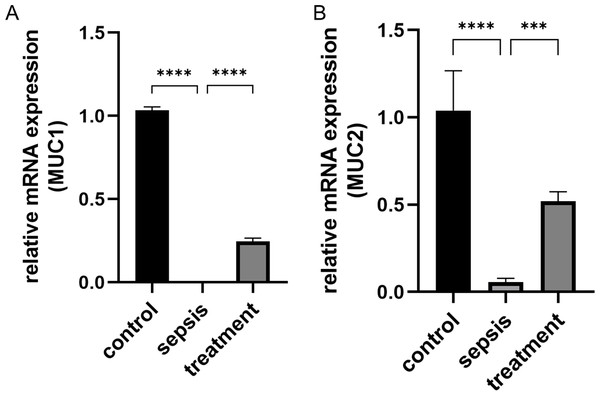
We measured the transcript levels of the small intestinal tissue in three groups, including MUC1 (p < 0.0001) and MUC2 (p < 0.0001) secreted by goblet cells, found the transcript levels in septic rats decreased (Figs. 4A–4B). However, the MUC1 mRNA (p < 0.0001) and MUC2 mRNA (p = 0.0002) after treatment with Tak-242 were significantly increased compared with those the sepsis group (Figs. 4A–4B). These results indicate that the inhibition of TLR4 upregulation in sepsis by Tak-242 can improve the secretory function of goblet cells.
Figure 4: The change of MUC1 and MUC2 mRNA.
(A) The relative mRNA expression of MUC1 among groups. (B) The relative mRNA expression of MUC2 in three groups. Statistical differences were determined by using ANOVA with Bonferroni’s multiple comparison test. (n = 5 per group). Data were based on three independent replications experiments. *** p < 0.001, **** p < 0.0001.TLR4 activate ER stress in ileal of septic rats
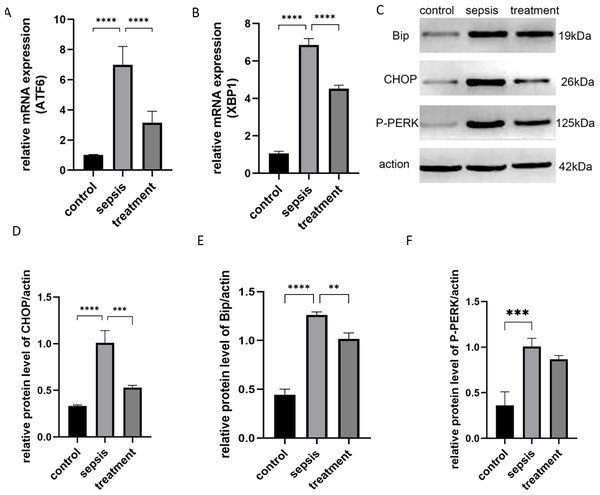
Next, we tested whether TLR4 could activate the ER stress response. We stimulated rats with CLP and analyzed the activation of XBP1, P-PERK and ATF6. ER stress-induced mRNAs, including ATF6 (p < 0.0001) and XBP1 (p < 0.0001), were activated after CLP compared with the sham group (Figs. 5A–5B). However, ATF6 mRNA (P < 0.0001) and XBP1 mRNA (P < 0.0001) were significantly decreased in the treatment group (Figs. 5A–5B). We found that CLP induced ER stress response measured by the expression of CHOP, Bip and P-PERK. Compared with the control group, CHOP (P < 0.0001), Bip (P < 0.0001) and P-PERK (P = 0.0006) protein levels increased in the sepsis group (Figs. 5C–5F). Secondly, we explored whether Tak-242 could inhibit ER stress. Treatment with Tak-242 downregulated the expression levels of these proteins. However, only the expression of CHOP (P = 0.007) and Bip (P = 0.0027) were statistically significant (Figs. 5D–5E). These findings revealed that TLR4 contributes to the upregulation of ATF6, XBP1, CHOP and Bip, but not to P-PERK.
Figure 5: The ER stress response by TLR4.
(A) The relative mRNA expression of ATF6 among groups. (B) The relative mRNA expression of XBP1 in three groups. (C) The Bip, CHOP and P-PERK protein levels in different groups by western blot. (D–F) The relative protein level of CHOP/actin, Bip/actin and P-PERK/actin (n = 5 per group). Data were based on three independent replications experiments. Statistical differences were determined by using the Analysis of Variance (ANOVA) with Bonferroni’s multiple comparison test. ** p < 0.01, *** p < 0.001, **** p < 0.0001.Discussion
The uncontrolled inflammatory response contributes to sepsis, which is associated with TLR activity, so the use of TLRs antagonists can be effective for controlling immune overactivity (Flórez-Álvarez et al., 2020). TLR4 is a potential target for inhibiting inflammatory response (Ding & Liu, 2019). Intestinal epithelium barrier dysfunction plays a major role in intestinal inflammatory pathology in septic-AGI. The rapid self-renewal of intestinal epithelial is orchestrated by Lgr5+ intestinal stem cells that can multiply and differentiate into other intestinal epithelial cells, which are important for maintaining the integrity of the intestinal mucosa (Vereecke, Beyaert & Van Loo, 2011). With the use of Tak-242 (a TLR4 antagonist), we found that TLR4 plays an important suppressive role in Lgr5+ cells and goblet cells in septic rats. Meanwhile, we found TLR4 contributes to the upregulation of ATF6, XBP1, CHOP and Bip. These findings promote our understanding of the biological function of TLR4 in the intestinal epithelium and highlight the crucial role of TLR4-mediated ER stress in the pathogenesis of septic-AGI.
The interaction between intestinal epithelial cells and commensal bacteria, both maintain a dynamic balance in physiological state, is regulated by pattern recognition receptors. However, when specific bacterial and viral components are recognized, different pattern recognition receptors (TLRs or NOD-like receptors) activate the NF-κB signaling (Vereecke, Beyaert & Van Loo, 2011). In the current study, we detected TLR4, NF-κB, and inflammatory cytokines (TNF-α, IL-1β) in the intestine. The results show that upregulation of TLR4/NF-κB signaling pathway activated intestinal inflammatory response after CLP. Meanwhile, intestinal mucosal structure was damaged. We found that inhibition of TLR4/NF-κB signaling can alleviate intestinal inflammatory response, protect intestinal mucosa, increase food intake, reduce weight loss (%) and MSS, but cannot improve the survival of sepsis. As reported, the LPS receptor TLR4 can active the MyD88- and TRIF-dependent signal pathways and induce excessive inflammatory response (Ding & Liu, 2019). Studies have shown that Tak-242 can inhibit inflammatory response and improve organ function (Ono et al., 2020; Xia et al., 2024) in sepsis. Although animal model studies have shown Tak-242 improve the survival rate (Takashima et al., 2009), but clinical trials suggest that it does not improve prognosis (Rice et al., 2010). First of all, inflammatory factors in the blood were not measured in our experiment, and the effect of Tak-242 on the systemic inflammatory response is uncertain. Secondly, no antibiotic treatment was used in our experimental process. The control of infection and inflammation is crucial for the survival of sepsis (Evans et al., 2021). Previous studies have shown that treatment with TAK-242 in combination with imipenem after CLP increased the survival rates of mice (Sha, Iizawa & Ii, 2011). Therefore, it seems reasonable that the TLR4 signal transduction inhibitor did not improve the survival of sepsis. In the present study, we focused on the effects of TLR4/NF-κB signaling blockade to intestinal epithelial cells in sepsis, which cannot be examined in the clinical trials.
Evidence have shown that TLR4 was present on the intestinal epithelium (Neal et al., 2006). At the same time, intestinal TLR4 signaling has been shown to be markedly elevated, which is consistent with other animal model (Krivan et al., 2019; Yu et al., 2007). Human autopsy studies and animal models of sepsis both found there were increased gut epithelial apoptosis (Hotchkiss et al., 1999; Mittal & Coopersmith, 2014). Inhibiting gut epithelial apoptosis was associated with a survival advantage in septic murine (Coopersmith et al., 2002). However, the underlying mechanisms remain unclear. Our results showed that TLR4 damages the Lgr5+ cells and goblet cells, Tak-242 prevents TLR4-induced inhibition of Lgr5+ cells and goblet cells count and function.
Lgr5+ cells are important epithelial cells that can multiply and differentiate into other intestinal epithelial cells, which is important for maintaining the integrity of intestinal mucosa. Feng et al. (2022) addressed that targeted regulation of the TLR4 signaling pathway can promote the proliferation and regeneration of intestinal stem cells (ISCs) in severe ionizing radiation mice. As reported, TLR4 lead to apoptosis of intestinal stem cells and inhibited proliferation through the up-regulation of PUMA (p53 upregulated modulator of apoptosis) in necrotizing enterocolitis (Neal et al., 2012). Leibowitz et al. (2018) demonstrated that p53-dependent PUMA induction mediates chemotherapy-induced loss of Lgr5+ stem cells. Endogenous hyaluronic acid increases Lgr5+ cells proliferation through CD44 and TLR4 (Riehl et al., 2015). Our results are consistent with those of previous studies. In conclusion, TLR4 take part in the update of Lgr5+ cells in these diseases with intestinal damage.
The small intestinal goblet cells have emerged as a tower of strength owing to their functions in producing the mucus layer, sensing changes in the local environment, and shaping gut immunity (Gustafsson & Johansson, 2022). The major function of goblet cells is to secrete mucus, which forms a protective layer that protects the intestinal mucosa against bacterial invasion. Mucus secretion is regulated by inflammasome (Hooper, 2015). McGuckin & Hasnain (2017) showed that the mucus layer often diminishes during infections and chronic inflammation. Birchenough et al. (2016) addressed that activated TLR signaling induced MUC2 secretion from the upper crypt goblet cells and stimulated an increase in mucus thickness in colon, while ileal goblet cells were insensitive to it. Thus, TLR take part in the protective function of colonic goblet cells to colonic crypt. However, our data showed that TLR4 restrained the secretion of MUC1 and MUC2 in sepsis, Tak-242 promoted more goblet cells and mucus in small intestinal. It is possible that the slight activation of TLR4 is beneficial to body defense, but excessive upregulation damages the goblet cells and mucus. Previous study shows that TLR4 lead to apoptosis of Lgr5+ cells and inhibit proliferation (Neal et al., 2012). Epithelial differentiation into goblet cells was prevented by upregulating TLR4 signaling (Sodhi et al., 2012). In conclusion, TLR4 signaling damages goblet cells may be through Lgr5+ cells partly.
Goblet cells and Paneth cells are highly sensitive to ER stress, and the functional defect of goblet cells could be associated with inflammatory pathology (Vereecke, Beyaert & Van Loo, 2011). The production of large quantities of mucins is challenging for goblet cells, which require ER stress with a high capacity for protein folding and modification. ER stress affects the survival of goblet cells (Vereecke, Beyaert & Van Loo, 2011). To investigate whether TLR4 could regulate Lgr5+ cells and goblet cells levels by ER stress, we performed an intensive investigation.
In this study, we found that three UPR branches (IRE1, PERK and ATF6) of ER Stress were activated in sepsis. In addition, we found that Tak-242 decreased the expression of CHOP and Bip , protected ATF6 and XBP1 mRNA compared with the sepsis group. However, there was no difference in P-PERK expression after treatment with Tak-242. Cell fate (survival or apoptosis) depends on the duration of individual UPR branch activation (Lin et al., 2007). It is known that, high-dose LPS lead to prolonged ER stress. Lin et al. found that the activities of Bip, IRE1, and ATF6 were attenuated by persistent ER stress in human cells, and PERK signaling was maintained (Lin et al., 2007). Our results are in agreement with the finding that TLR4 increases CHOP expression, but does not influence PERK or eIF-2α (Woo et al., 2009). ER stress-mediated cell death modulators include the Bcl-2 family (Bcl-2, Bcl-xL, Bax, Bak, and Bik), CHOP/Gadd153, valosin-containing proteins, and apoptosis-linked gene-2 (Rao, Ellerby & Bredesen, 2004). Prolonged CHOP expression is cytotoxic. Afrazi et al. (2014) reported that TLR4-induced ER stress leads to ISCs apoptosis via PERK/CHOP signaling, but not ATF6 and IRE1. These results indicate that upregulation of TLR4 damaged the Lgr5+ cells and goblet cells count and function after sepsis, partly may be through CHOP suppression. However, we did not explore the direct effects of CHOP on Lgr5+ cells and goblet cells. Future research will confirm the role of CHOP on intestinal epithelial cells.
We selected the healthy rats with the same sex, age, and weight, which limited the baseline variability. Meanwhile, CLP is the “gold standard” model, because it closely resembles the progression and characteristics in human polymicrobial abdominal sepsis (Dejager et al., 2011). We performed randomization by a table of random numbers and used blinding in the process of experimental design, implementation and data analysis to minimize bias. However, human patients are variable in ages, sex, weight, and causes of sepsis. We did not perform antibiotics and source control, which did not simulate the clinical process exactly. Therefore, the translation of these results to septic patients is still limited. We did not explore that the effects of TLR4/NF-κB signaling on apoptosis, proliferation, differentiation and dedifferentiation of intestinal epithelial cells. Additionally, goblet cells are differentiated from Lgr5+ cells. It is not clear whether activated TLR4/NF-κB signaling damages goblet cells directly or affects goblet cells through Lgr5+ cells. In the further, we prepare to perform cell experiments to verified the positive results.
Conclusion
In summary, we concluded that TLR4/NF-κB signaling pathway was upregulated after CLP in rat ileal tissues, which destroyed the structure of the intestinal mucosa and activated ATF6, XBP1, CHOP, and Bip along with the damage of Lgr5+ cells and goblet cells count and function. Our findings suggest that Tak-242 protects Lgr5+ cells and goblet cells after sepsis, may be through the suppression of ER stress. TLR4 contributes to sepsis-induced gastrointestinal dysfunction greatly. Thus, the inhibition of TLR4-mediated ER stress may be a promising therapy of septic AGI.
Supplemental Information
The small intestinal mucosa structure in three groups by Hematoxylin Eosin staining
C31-C35: the controp group; S31-S35:the sepsis group; T31-T35: the treatment group.(Magnification × 10).
The lgr5+cell by immunohistochemistry
C31-C35: the control group; S31-S35: the sepsis group; T31-T35: the treatment group (Magnification × 2).
The Goblet cell numbers by immunohistochemistry using the goblet cell-specific marker AB-PAS
C31-C35: the controp group; S31-S35: the sepsis group; T31-T35: the treatment group (Magnification × 20).
The mRNA expression
The raw data of mRNA (TLR4, XBP1, ATF6, MUC1, MUC2).
The raw data of NF-κB
The level in intestinal tissue by western blot and the relative protein level of NF-κB.
The raw data of TNF-α and IL-1β
The mRNA expression of TNF-α and IL-1β in small intestine.