Radical chemoradiotherapy for superficial esophageal cancer complicated with liver cirrhosis
- Published
- Accepted
- Received
- Academic Editor
- Xin Zhang
- Subject Areas
- Oncology, Radiology and Medical Imaging
- Keywords
- Radical chemoradiotherapy, Superficial esophageal cancer, Liver cirrhosis, Retrospective study
- Copyright
- © 2024 Bao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Radical chemoradiotherapy for superficial esophageal cancer complicated with liver cirrhosis. PeerJ 12:e18065 https://doi.org/10.7717/peerj.18065
Abstract
Background
Although chemoradiotherapy is an effective treatment for esophageal cancer, its feasibility in esophageal cancer with cirrhosis remains largely unclear.
Methods
We retrospectively studied 11 patients with superficial esophageal cancer with liver cirrhosis (Child-Pugh score ≤8) who underwent radical chemoradiotherapy from four centers, and the overall survival rate, local control rate and adverse events at 1 and 3 years were explored.
Results
The median age of the included patients was 67 years (Inter-Quartile Range 60–75 years). Complete response was observed in most patients (n = 10, 90.9%), and the remaining patient was unevaluable. The 1- and 3-year overall survival and local control rates were 90.9% and 90.9%, and 72.7% and 63.6%, respectively. Hematotoxicity was a common adverse reaction, and seven patients developed radiation esophagitis, with grade 3–4 observed in two cases. All cases of radiation dermatitis (n = 4) and radiation pneumonia (n = 2) were grade 1–2. Gastrointestinal bleeding occurred in two patients, including one with grade 1–2 bleeding, and one died.
Conclusion
Radical chemoradiotherapy is a potential treatment option for patients with superficial esophageal cancer complicated with cirrhosis. However, it can increase the risk of bleeding, which warrants prompt recognition and intervention.
Introduction
Esophageal cancer (EC) is a common malignant digestive tract tumor with a poor prognosis and high mortality (Rogers et al., 2022; Li et al., 2023). Notwithstanding that the past decade has witnessed unprecedented medical advances, including combination strategies, the 5-year survival rates for patients with esophageal cancer remain relatively low (Waters & Reznik, 2022; Xu et al., 2024; Dai et al., 2024). It is well-established that Asia has a high incidence of liver cirrhosis, and since liver cirrhosis and esophageal cancer have common risk factors, such as alcohol and smoking, the incidence of esophageal malignancies in patients with cirrhosis is high. In this respect, studies have shown that esophageal cancer and liver cirrhosis coexist in approximately 7.0% of patients (Bassegoda et al., 2022; Asti et al., 2018; Fujisaki et al., 2022).
T1a and T1b esophageal squamous cell carcinomas (ESCCs) are designated superficial esophageal neoplasms (SENs) regardless of lymph node or distant organ metastasis (Noh et al., 2020). Endoscopic submucosal dissection (ESD) has gained broad recognition as a treatment for SEN to avoid esophagectomy (Kawachi et al., 2022; Chen, 2022). However, due to the technical complexity of esophageal ESD, there is a significant risk of bleeding (2.1%) and perforation (5%) (Ishihara, 2022; Kim et al., 2023). Severe delayed bleeding may also occur after ESD (Lin, Lin & Gong, 2021). The use of ESD in patients with cirrhosis has been associated with a higher risk of complications due to coagulopathy, decreased platelet count, and/or co-presence of esophageal varices (Kolb et al., 2021). Accordingly, some endoscopists consider the coexistence of cirrhosis and SEN a contraindication for ESD treatment. The incidence of lymph node metastasis (including vascular invasion) after surgical resection in T1b-SM1 ESCC patients ranged from 8.3% to 53.1% (Akutsu et al., 2013; Li et al., 2013). Several studies have reported on adjuvant chemotherapy and postoperative adjuvant radiotherapy in pT1a-MM/pT1b-SM1 ESCC patients after ESD, demonstrating the significant role of chemoradiotherapy in the treatment of early esophageal cancer (Koterazawa et al., 2018; Yoshimizu et al., 2018; Nishibuchi et al., 2020; Zhang et al., 2020; Lu et al., 2024).
Esophagectomy is usually recommended when esophageal tumors involve the submucosa (Min et al., 2018). However, cirrhosis patients are at high risk of perioperative complications and death due to impaired liver function, with mortality rates reaching 45% for esophageal surgery and other extrahepatic surgery (Yamada et al., 2006). Alternatively, some patients receive chemoradiotherapy, depending on patient comorbidities, tumor location, and metastasis (Emi et al., 2022). Several cases of cT1bN0M0 esophageal cancer patients that underwent RT have recently been reported (Kawamoto et al., 2022; Kodaira et al., 2010). Chemoradiotherapy (CRT) outcomes revealed a non-inferiority trend in overall survival (OS) of cT1bN0M0 EC patients compared to surgery (Lyu et al., 2022; Suzuki et al., 2022).
Although the life expectancy of patients with liver cirrhosis combined with esophageal varices is shortened, with the development of esophageal cancer treatment methods, it is recommended to intervene in such patients, especially for those with well-compensated cirrhosis and those who may choose liver transplantation (Tapper & Parikh, 2023). For patients with esophageal cancer who cannot undergo endoscopic or surgical treatment, radiotherapy can also achieve a high disease control rate and is an alternative treatment option (Ai et al., 2024; Jiang et al., 2024; McPhail et al., 2024). To our knowledge, there are still few literatures on local treatment methods, drug treatment methods and outcomes of patients with esophageal cancer combined with cirrhosis. There is no report on the feasibility of chemoradiotherapy for esophageal cancer in the context of cirrhosis. To assist clinicians in treating this challenging patient group, we conducted a retrospective analysis of the outcomes of chemoradiotherapy for esophageal cancer in the context of cirrhosis, aiming to evaluate the efficacy and safety of chemoradiotherapy in patients with compensated cirrhosis and esophageal varices.
Methods
Patient selection
We reviewed the medical records, radiotherapy plans and diagnostic images of EC patients who underwent radiotherapy/chemoradiotherapy (RT/CCRT) at Guangzhou Panyu Central Hospital, Chongqing University Three Gorges Hospital, Southern Medical University Nanfang Hospital, and Sun Yat-Sen University Cancer Center from January 2014 to December 2021. The inclusion criteria were as follows: (I) esophageal squamous cell carcinoma confirmed by pathology; (II) The Eastern Cooperative Oncology Group (ECOG) performance score was 0–2; (III) cT1N0M0 Cancer based on the Eighth Edition of the Tumour, Node, Metastasis (TNM) classification of the International Union Against Cancer (UICC); (IV) Compensatory cirrhosis (Child-Pugh score ≤8). All patients were pathologically diagnosed by biopsy, and staging was confirmed by computed tomography (CT) scan or positron emission tomography/computed tomography, esophagogastroduodenoscopy and Lugol staining, and endoscopic ultrasound (EUS). Exclusion criteria include other concurrent malignancies, difficult-to-treat infections and serious comorbidities such as diabetes. The present study was approved by the ethics committees of the Ethics Committee of Panyu Central Hospital (PYRC-2023-188). Patients provided informed written consent at the time of data collection.
Chemoradiotherapy
All enrolled patients underwent CT imaging for tumor localization. In cases that were difficult to identify with CT imaging, a titanium clip was placed during gastroscopy to facilitate tumor localization (Case 3-Figs. 1A–AH). The patient was placed in the supine position with arms hanging down on both sides of the body. The thermoplastic film was fixed in place, and the slice thickness was 5 mm for contrast-enhanced CT. CT was conducted to identify the upper and lower boundaries of esophageal tumors or the titanium clips in cases where the lesion could not be observed. According to the International Committee on Radiometric Unit Points, selective lymph node irradiation (ENI) was used to cover bilateral supraclavicular and mediastinal lymph node areas, or affected field irradiation (IFI), including primary tumors with margins of 2 to 4 cm. The linear accelerator was used to deliver 6 or 10 MV-X external irradiation, and IMRT radiotherapy was administered to all patients. The normal tissue tolerance dose limits were set as follows: the maximum dose to the spinal cord was less than 38 Gy, heart V30 <20%, V5 <40%, V20 <20%, and V30 <10% for both lungs. The subsequent treatment plan was developed by physicists in accordance with the prescribed dose requirements. The daily dose of RT was 1.8–2.0 Gy, 5 days a week, and the total dose was 50.4 to 60 Gy.
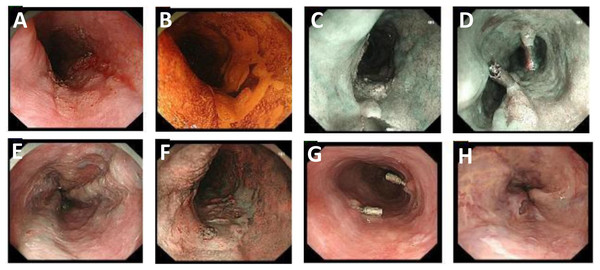
Figure 1: Gastroscopic images of Case 1 before and during treatment.
(A and B) During gastroscopy, a large erosion area, partially coarse and covered with white keratinized tissue, was observed in the esophagus 25–30 cm away from the incisors. (C and D) During gastroscopy, esophageal masses were labeled with titanium clips that were inserted at the upper and lower margins of the lesions. (E and F) Several linear and nodular varicose veins can be seen from the middle and lower esophagus to the cardia surface with negative red-wale signs. (G and H) Repeat endoscopy after radiotherapy showed that titanium clips were inserted in the original esophageal lesion with rough localized mucosa, and no ulcers or masses were observed.All patients received chemotherapy combined with radiotherapy except those in poor general condition (ECOG = 2). The chemotherapy regimen consisted of S-1, 70 mg/m2 daily, taken orally on days 1 to 14 and 29 to 42; 5-fluorouracil 700 mg/m2 on days 1–4, every 4 weeks, plus cisplatin 70 mg/m2 on day 1, every 4 weeks. Alternatively, paclitaxel 50 mg/m2 and carboplatin (target area under curve 2 mg/ml/min) were administered on the first day, four times per week.
This study used the Monaco treatment planning system to characterize patient images and outline the gross tumor volume (GTV) and clinical target volume (CTV). The thickness of CT sections was 5 mm, and the skin, esophageal varices, and liver of patients were delineated. After the outline of the organs was delineated, the GTV, CTV, esophageal varices volume (EVV) and liver volume were directly calculated using the volume estimation function of the software. A dose-volume histogram (DVH) of the measurement curve was generated to record V20, V30 and V40 of esophageal varices.
Evaluation and statistical analysis
In the present study, complete response (CR) was defined as the absence of the primary tumor and irregular erosive, ulcerative, or significantly elevated lesions observed during endoscopy and/or the absence of malignant cells in the biopsy specimen (Kim et al., 2015). Local control rates and the time from the start of radiotherapy until the patient died from any cause were assessed. Adverse events were classified according to the National Cancer Institute Standard for Common Terminology for Adverse Events (NCI CTCAE, Version 4.0). Kaplan-Meier survival analysis was used to calculate the 1- and 3-year overall survival and local control rates.
Results
Clinical and therapeutic characteristics of patients
Table 1 shows the clinical and therapeutic characteristics of the included patients (n = 11). The median age of patients included was 67 years (range 60–75 years), with male predominance (n = 8, 72.7%) and most patients older than 65 years (n = 7, 63.6%) and having hepatitis B or C-related cirrhosis (n = 8, 72.7%). Seven patients (63.6%) reported regular alcohol consumption. The ECOG score was 0–1 in most cases (n = 9, 81.8%). The median albumin, total bilirubin, international normalized ratio (INR) value and platelet count were 36.1 g/L (IQR, 33.5–39.3), 1 mg/dL (IQR, 0.8–1.35), 1.01 (IQR, 0.94–1.15), and 120 × 109/L (IQR, 90–181), respectively. The included patients presented with stages T1a (n = 6, 54.5%) and T1b (n = 5, 45.5%). The tumors were located in the upper (n = 2, 18.2%), middle (n = 6, 54.5%) and lower (n = 3, 27.3%) thorax. Synchronous chemotherapy included S-1 (n = 4, 36.4%), cisplatin plus 5-FU (PF) (n = 1, 9.1%), and taxanes and cisplatin (TP) (n = 4, 36.4%). The radiotherapy dose range was 50.4–60 Gy. Five (45.5%) patients were treated with ENI, and six (54.5%) with IFI.
| Liver cirrhosis (n = 11) | |
|---|---|
| Age, years | |
| ≥65 yr | 7 (63.6%) |
| <65 yr | 4 (36.4%) |
| Sex | |
| Female | 3 (27.3%) |
| Male | 8 (72.7%) |
| Drinker | 7 (63.6%) |
| ECOG score | |
| 0-1 | 9 (81.8%) |
| 2 | 2 (18.2%) |
| Laboratory tests, mean level of | |
| Albumin, g/L (IQRrange) | 36.1 (33.5–39.3) |
| Total bilirubin, mg/dL (IQRrange) | 1 (0.8–1.35) |
| INR (IQRrange) | 1.01 (0.94–1.15) |
| Platelet count × 109/L (IQRrange) | 120 (90–181) |
| T stage | |
| T1a | 6 (54.5%) |
| T1b | 5 (45.5%) |
| Tumor location | |
| Upper thoracic | 2 (18.2%) |
| Middle thoracic | 6 (54.5%) |
| Lower thoracic | 3 (27.3%) |
| Concurrent chemotherapy | |
| None | 2 (18.2%) |
| S-1 | 4 (36.4%) |
| PF | 1 (9.1%) |
| TP | 4 (36.4%) |
| Total radiation dose | |
| 50.4 Gy | 5 (45.5%) |
| 60 Gy | 6 (54.5%) |
| Radiation field | |
| ENI | 5 (45.5%) |
| IFI | 6 (54.5%) |
Note:
Data are n (%). ECOG, Eastern Cooperative Oncology Group; IQR, Inter-Quartile Range; INR, international normalized ratio; T, tumor; ER, endoscopic resection; RT, additional radiotherapy; CCRT, concurrent chemoradiotherapy; S-1, tegafur, 5-chloro-2,4-dihydroxypyridine, and potassium oxonate; PF, 5-fluorouracil and cisplatin; TP, paclitaxel with carboplatin; ENI, elective nodal irradiation; IFI, involved-field irradiation.
Treatment efficacy in patients
The median GTV volume was 28.7 cm3 (IQR, 22.45–32.2 cm3), the median CTV volume was 148 cm3 (IQR, 135.8–156.45 cm3), the median EVV volume was 11 cm3 (IQR, 9.1–12.85 cm3), and the median liver volume was 1,050 cm3 (IQR, 988.95–1,101.6 cm3). Most patients presented with EVV V20 at 0–50% (n = 7), followed by EVV V20 at 50–75% (n = 3) and >75% (n = 1). Consistently, most patients presented with EVV V30 of 0–50% (n = 8), followed by EVV V30 at 50–75% (n = 3) and >75% (n = 1). Finally, most patients exhibited EVV V40 at 0–50% (n = 8), followed by EVV V40 at 50–75% (n = 3) and >75% (n = 1) (Table 2). In Figs. 2A–2F, Case 1 is depicted with a tumor in the mid-thoracic segment and varicose veins partially overlapping the target area. Case 2, whose tumor was in the lower thoracic segment and whose varicose veins extended beyond the target range, is depicted in Figs. 3A–3F. Figures 4A–4F shows Case 3, whose tumor was in the upper thoracic segment, with minimal overlap between varicose veins and the target area. Figures S1A, S1B illustrates the dose-volume histogram of Cases 1 and 2.
| Patients | GTV, cm3 | CTV, cm3 | EVV, cm3 | Cause of liver cirrhosis | Child-Pugh class(score) | The stage of esophageal varices | Liver volume, cm3 | EVV, V20 | EVV, V30 | EVV, V40 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29.7 | 154.3 | 8.3 | Hepatitis B | B (7) | GII | 1,240.0 | 41.5% | 37.98% | 37.8% |
| 2 | 33.0 | 158.8 | 19.7 | Hepatitis B | A (5) | GIII | 1,192.0 | 93.14% | 91.53% | 91.53% |
| 3 | 19.6 | 123.4 | 7.1 | Alcoholic Hepatitis | A (6) | GI | 1,071.5 | 21.02% | 20.76% | 20.37% |
| 4 | 28.7 | 142.3 | 12.3 | Alcoholic Hepatitis | B (7) | GI | 1,091.2 | 46.7% | 45.53% | 45.27% |
| 5 | 31.6 | 157.7 | 9.2 | Hepatitis B | A (5) | GII | 977.6 | 72.16% | 71.64% | 71.23% |
| 6 | 20.5 | 148.0 | 9.0 | Hepatitis B | A (6) | GI | 886.3 | 40.89% | 38.76% | 37.98% |
| 7 | 21.0 | 132.3 | 15.2 | Hepatitis C | B (7) | GII | 987.8 | 65.22% | 63.87% | 63.1% |
| 8 | 35.0 | 160.5 | 11.2 | Hepatitis B | A (5) | GI | 1,112.0 | 50.12% | 47.56% | 47.11% |
| 9 | 23.9 | 135.6 | 9.5 | Alcoholic Hepatitis | A (5) | GI | 1,002.6 | 39.8% | 37.55% | 36.67% |
| 10 | 28.0 | 136.0 | 11.0 | Hepatitis B | A (6) | GI | 1,050.0 | 31.22% | 30.62% | 30.23% |
| 11 | 32.8 | 155.2 | 13.4 | Hepatitis B | B (7) | GII | 990.1 | 27.3% | 26.57% | 26.2% |
Note:
Data are n (%). GTV, Gross tumorvolume; CTV, Clinical target volume; EVV, esophageal varices volume.
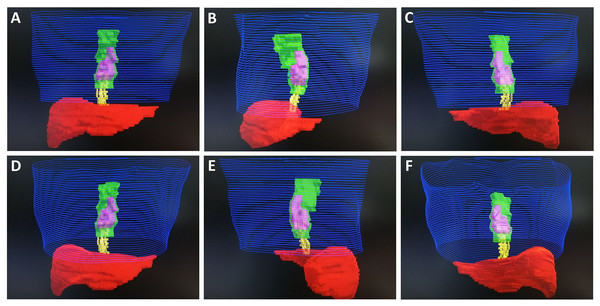
Figure 2: Monaco system target image of Case 1.
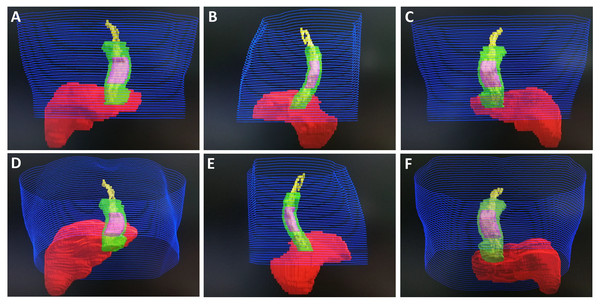
Blue represents skin, green represents GTV, pink represents CTV, red represents liver, and yellow represents EVV. (A) Front view. (B) Left lateral view. (C) Posterior view. (D) Front and inferior view. (E) Right lateral view. (F) Posterior and inferior view.Figure 3: Monaco system target image of Case 2.
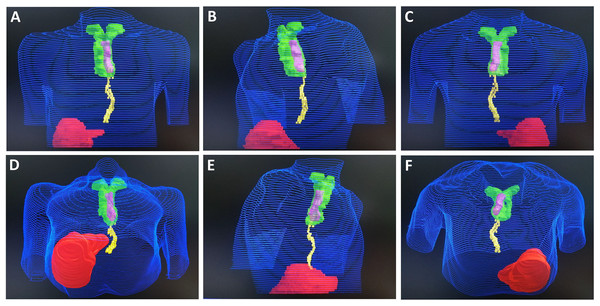
(A) Front view. (B) Left lateral view. (C) Posterior view. (D) Front and inferior view. (E) Right lateral view. (F) Posterior and inferior view.Figure 4: Monaco system target image of Case 3.
(A) Front view. (B) Left lateral view. (C) Posterior view. (D) Front and inferior view. (E) Right lateral view. (F) Posterior and inferior view.The median follow-up period for the entire cohort was 36 months (IQR, 24–43.5 months). Most patients achieved CR (n = 10), and one patient died from massive variceal bleeding. The 1- and 3-year overall survival and local control rates were 90.9% (95% CI [59–100%]) and 90.9% (95% CI [59–100%]), and 72.7% (95% CI [48–98%]) and 63.6% (95% CI [39–94%]), respectively (Fig. S2).
Treatment-related adverse events
Hematotoxicity was a common adverse reaction, which could be mainly characterized as a decrease in leukocytes and platelets (grade 1–2, n = 4), a decrease in neutrophils (grade 1–2, n = 5), a decrease in lymphocytes (grade 1–2, n = 6), as well as a severe decrease in lymphocytes (grade 3–4, n = 1). Different degrees of radiation esophagitis (n = 7) were observed, with only two cases with grade 3–4. There were four cases of radiation dermatitis and two cases of radiation pneumonia, all of which were grade 1–2.
There were two patients with gastrointestinal bleeding: one experienced grade 1–2 bleeding, and the other died due to bleeding (Case 2) (Table 3). Case 2 was a 64-year-old man who developed gastrointestinal bleeding half a month after the end of radiotherapy and passed away after ineffective rescue. Case 7 also experienced gastrointestinal bleeding after radiotherapy. After endoscopic and drug treatment, hemostasis was achieved, and the patient attained CR (Figs. S3A–S3D).
| Treatment-related adverse events | Grade 1–2 | Grade 3–4 | Grade 5 |
|---|---|---|---|
| Fatigue | 2 (18.2%) | ||
| Nausea | 3 (27.3%) | ||
| Vomiting | 3 (27.3%) | ||
| Anorexia | 2 (18.2%) | ||
| Diarrhea | 3 (27.3%) | ||
| Anemia | 3 (27.3%) | ||
| White blood cell decreased | 4 (36.4%) | ||
| Neutrophil count decreased | 5 (45.5%) | ||
| Lymphocyte count decreased | 6 (54.5%) | 1 (9.1%) | |
| Platelet count decreased | 4 (36.4%) | ||
| Esophageal stricture | 2 (18.2%) | ||
| Hemorrhage | 1 (9.1%) | 1 (9.1%) | |
| Radiation esophagitis | 5 (45.5%) | 1 (9.1%) | |
| Radiation pneumonitis | 1 (9.1%) | ||
| Radiodermatitis | 3 (27.3%) |
Note:
Data are n (%).
Discussion
It is well-established that there is a risk of subclinical lymph node metastasis in esophageal tumors involving musculo-mucosal (m3) or submucosal (sm) disease (Mönig et al., 2018). Radical esophagectomy combined with prolonged lymph node dissection remains the standard of care treatment for pT1b disease, even if the lymph nodes are clinically negative (Fedorova & Watson, 2021). In this regard, a single-center study Valmasoni et al. (2017) reviewing patients with esophageal cancer and cirrhosis (n = 73) who underwent surgery found a higher 90-day complication rate and mortality than patients without cirrhosis (n = 146). Patients with cirrhosis also experienced a significantly higher incidence of respiratory events (p = 0.013), infections (p = 0.005), and severe anastomotic complications (p = 0.046), with no difference in 5-year survival. Another meta-analysis (Schizas et al., 2020) included 12 observational studies reporting 1,938 patients who underwent surgery for esophageal cancer and found that patients with cirrhosis were more likely to develop postoperative pulmonary complications, ascites, and anastomotic fistula within 30 days after esophageal cancer surgery. Although patients with cirrhosis had a higher 30-day mortality rate (OR: 3.04; 95% CI [1.71–5.39]), mortality at 90 days or late mortality was unaffected.
While surgical treatment does not seem to impact long-term survival, the high complication (83–87%) and mortality (17–30%) rates in cirrhotic patients undergoing esophageal resection suggest ESD combined with CCRT represents a viable alternative for those with esophageal submucosal lesions and cirrhosis (Suzuki et al., 2022). Radical chemoradiotherapy is an alternative early superficial esophageal cancer treatment option (Nemoto et al., 2006), with a reported overall survival rate comparable to radical surgery due to the advantages of organ preservation. However, the local failure rate (up to 30%) and morbidity associated with dose escalation are major limitations of this strategy (Emi et al., 2022). Therefore, ER combined with CRT holds much promise for the conservative management of esophageal lesions involving pT1a m3 or pT1b. ER ensures local control and confirms the depth of invasion, while auxiliary CRT consolidates local control in the presence of positive margins or deep lesions and improves progression-free survival by treating lymph nodes in areas at risk of tumor invasion.
It is widely acknowledged that endoscopic mucosal resection (EMR) or ESD can achieve a good local tumor control rate in cases of early esophageal cancer. A retrospective study Tsou et al. (2016) evaluated 40 patients with SENs treated with ESD. The non-cirrhotic group included 32 patients, and the cirrhotic group included eight patients, of whom four patients had esophageal varices. R0 removal rates were 77.8% and 94.3% in the cirrhotic and non-cirrhotic groups, respectively (p = 0. 16). Intraoperative bleeding was more common in patients with cirrhosis than those without cirrhosis (18.2% vs. 0%, p = 0.045). None of the patients experienced esophageal perforation, postoperative bleeding, or ESD-related death. Another study Choi et al. (2022) reviewed 437 patients, including 15 with cirrhosis, and showed no difference in overall (88.2% vs. 97.0%) and radical (64.7% vs. 78.9%) excision rates between cirrhosis and non-cirrhosis groups (p = 0.105 and p = 0.224, respectively). Bleeding was more common in patients with cirrhosis (p = 0.054) and was successfully controlled by endoscopy in all cases. Endoscopic submucosal resection of early esophageal squamous cell carcinoma in cirrhotic patients has rarely been reported in the literature (Wang et al., 2022).
At present, few cases of the application of chemoradiotherapy in early esophageal malignancies complicated with cirrhosis have been reported in the literature (Kam et al., 2018). The severity of esophageal varices in cirrhosis is mainly determined by endoscopic visualization. Significant inroads in technology have been achieved in recent years. The radiotherapy target delineation system enables the evaluation of the organ volume and the dose of organs at risk. Multiple superficial esophageal cancers were found by endoscopy in a 55-year-old cirrhotic patient (Maruyama et al., 2003). Due to the patient’s pancytopenia, radiotherapy was performed. After 16 radiotherapy sessions, the patient was hospitalized due to hematemesis. Finally, the patient completed radiotherapy with a total dose of 66 Gy and attained CR. Eight months after treatment, the patient showed no signs of relapse. An additional case (Katano, Yamashita & Nakagawa, 2019) was reported in a 63-year-old Japanese male with alcoholic cirrhosis and superficial esophageal squamous cell carcinoma, who underwent endoscopic submucosal dissection with pathological stage pT1bN0M0. The patient also received chemoradiotherapy and was followed up for 30 months without recurrence.
As for radiotherapy for early esophageal cancer, a study Koide et al. (2017) included 20 patients with stage T1aN0M0 who received radical radiotherapy or chemoradiotherapy due to contraindications for endoscopic treatment, and all patients achieved CR. The 1-, 3- and 5-year overall and disease-specific survival rates were 100% and 100%, 83% and 100%, and 67% and 100%, respectively. Relapse occurred in eight patients, but no recurrence was observed after salvage therapy. The patients with early esophageal cancer in this study included patients with T1b disease complicated with cirrhosis, accounting for the lower overall survival rate compared to the above studies. Patients with esophageal cancer and cirrhosis pose unique challenges in terms of treatment options due to limitations in surgical and endoscopic techniques. Therefore, radical chemoradiotherapy has emerged as a viable alternative treatment option.
In our case series, the incidence of radiation esophagitis was relatively high (mostly grade I-II), which improved after timely drug treatment. Similar outcomes were observed for radiation dermatitis and radiation pneumonia. No perforation or cicatricial stenosis was found in the 11 patients. There were two cases of gastrointestinal bleeding after radiotherapy, of which one case had a good prognosis. After timely endoscopic hemostasis and drug therapy, the tumor was no longer visible upon endoscopy during follow-up. However, the other patient died due to variceal bleeding. The patient had post-hepatitis B cirrhosis in the past and underwent a splenectomy due to uncontrollable portal hypertension. The cirrhosis was well controlled in the past 5 years, and early esophageal cancer was later found. EVVV20, V30 and V40 were all over 90%. Half a month after the completion of radiotherapy, the patient had black stools. However, the patient did not pay attention and seek medical treatment in time. When the patient had hematemesis and was sent to the emergency department, the patient had already suffered from hemorrhagic shock and eventually died despite rescue efforts.
Similarly, the risk of bleeding also increases with other treatment strategies. The risk of bleeding in ESD for treating precancerous lesions of the upper gastrointestinal tract in patients with cirrhosis is 13.1–50%, requiring endoscopic hemostasis (Tan et al., 2023; Repici et al., 2012). The incidence of bleeding in this study was 18.2%, which did not exceed the incidence of delayed bleeding in ESD-treated patients with cirrhosis. However, one patient died, which may be related to the patient’s failure to seek medical treatment in time. Based on the analysis of the causes of increased bleeding risk in the cases reported in this study, it was found that the relative positional overlap between the tumor and varicose veins was large in the two bleeding patients, which seemed to increase their bleeding risk. However, there was no previous literature reporting the causal relationship between the two. The overlap of the radiation field and the position of varicose veins as a radiotherapy contraindication also lacks evidence. Overall, the impact of the relationship between the radiation field and the position of varicose veins on the bleeding risk awaits further studies with larger sample sizes for analysis. For dose limitations of organs at risk, especially EV V20-V40, the results indicated that it was relatively safe below 50%, while treatment should be cautious when V20-V40 exceeded 50%.
Other treatment options for esophageal cancer with cirrhosis include radiofrequency ablation (RFA). Interestingly, a study Wang et al. (2017) investigated the efficacy and safety of RFA in early ESCNs with cirrhosis and esophageal varices. Six of the eight patients achieved CR, while the two remaining cases had residual squamous epithelioma. After additional focal RFA treatment, all patients achieved CR at 12 months. No tumor progression or recurrence occurred during a median follow-up period of 21.6 months (13–42 months), but the incidence of adverse events was significantly higher than in patients without cirrhosis. At present, no consensus has been reached on the treatment and follow-up methods for this special patient population. Based on the literature, various approaches have been employed, encompassing surgical operation, endoscopic resection, radical chemoradiotherapy, RFA (Wang et al., 2017; Cheng, Shiu & Ko, 2024) alone or combined therapy.
During the follow-up, local recurrence occurred in three cases in our study. Subsequently, some patients received surgery and some received radiotherapy again. It has been reported in the literature that although salvage surgery has the potential for cure, the high mortality rate, anastomotic fistula and pulmonary complications limit the number of candidate patients for salvage surgery (Markar et al., 2015). Endoscopic resection, if complete resection of locally residual/recurrent cancer can be achieved, is a minimally invasive and effective alternative to esophageal resection (Tani et al., 2023; Ego et al., 2021). It has been reported that chemotherapy, radiotherapy or combined methods can provide survival benefits for salvage treatment of recurrent esophageal cancer, but there is still no consensus at present (Xiang et al., 2023; Xu et al., 2019). Simple chemotherapy is the first choice for systemic treatment of patients with metastatic disease or multiple-site recurrence. However, salvage systemic chemotherapy after local recurrence and metastasis is not very satisfactory, with an estimated median overall survival of only 5 months (Sudo et al., 2013).
However, our study still has some limitations. This is a retrospective study with a relatively small number of cases and a short follow-up time. This is due to the small number of patients with liver cirrhosis combined with early esophageal cancer and even fewer patients receiving CCRT treatment. In addition, the application of radiation doses in clinical practice, different tumor locations, etc. also affect the results. The differences in chemotherapy regimens of patients also affect the results. However, this study first reports the value of CCRT treatment in early esophageal malignancies combined with cirrhosis. This study collected data from multiple centers, and the patients were from areas with high incidences of esophageal cancer and cirrhosis, which has certain advantages.
Conclusions
In summary, we retrospectively analyzed the efficacy and safety of radiotherapy and chemotherapy in early esophageal malignancies complicated with cirrhosis from four centers. We also explored strategies to prevent adverse events, especially variceal bleeding. Importantly, the organ dose at risk for esophageal varices was investigated. In conclusion, radical chemoradiotherapy is feasible for patients with esophageal cancer complicated with cirrhosis.
Supplemental Information
Kaplan-Meier survival analysis showed the 1-year and 3-year overall survival and local control rates.
Endoscopic treatment of gastrointestinal bleeding in Patient No. 7.
Figure S3.
(A-B) Bleeding from varicose veins in the fundus of the stomach and cardia was observed under electronic gastroscopy. 10ml hardener and 1.5ml tissue glue (Goruba 2) were injected in the 2 o’clock direction of the bleeding vein. (C-D) Local hemostasis at the injection site was achieved after endoscopic compression.