Bacterial co-infection in COVID-19: a call to stay vigilant
- Published
- Accepted
- Received
- Academic Editor
- Priyanka Banerjee
- Subject Areas
- Microbiology, Virology, Epidemiology, Infectious Diseases, COVID-19
- Keywords
- COVID-19, SARS-CoV-2, Co-infection, Antimicrobial resistance, Ventilator-associated pneumonia, Bloodstream infection
- Copyright
- © 2024 Liu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Bacterial co-infection in COVID-19: a call to stay vigilant. PeerJ 12:e18041 https://doi.org/10.7717/peerj.18041
Abstract
Co-infection with diverse bacteria is commonly seen in patients infected with the novel coronavirus, SARS-CoV-2. This type of co-infection significantly impacts the occurrence and development of novel coronavirus infection. Bacterial co-pathogens are typically identified in the respiratory system and blood culture, which complicates the diagnosis, treatment, and prognosis of COVID-19, and even exacerbates the severity of disease symptoms and increases mortality rates. However, the status and impact of bacterial co-infections during the COVID-19 pandemic have not been properly studied. Recently, the amount of literature on the co-infection of SARS-CoV-2 and bacteria has gradually increased, enabling a comprehensive discussion on this type of co-infection. In this study, we focus on bacterial infections in the respiratory system and blood of patients with COVID-19 because these infection types significantly affect the severity and mortality of COVID-19. Furthermore, the progression of COVID-19 has markedly elevated the antimicrobial resistance among specific bacteria, such as Klebsiella pneumoniae, in clinical settings including intensive care units (ICUs). Grasping these resistance patterns is pivotal for the optimal utilization and stewardship of antibiotics, including fluoroquinolones. Our study offers insights into these aspects and serves as a fundamental basis for devising effective therapeutic strategies. We primarily sourced our articles from PubMed, ScienceDirect, Scopus, and Google Scholar. We queried these databases using specific search terms related to COVID-19 and its co-infections with bacteria or fungi, and selectively chose relevant articles for inclusion in our review.
Introduction
Initially reported in Wuhan, China on December 8, 2019, COVID-19, caused by the novel coronavirus SARS-CoV-2, was declared a Public Health Emergency of International Concern (PHEIC) by the World Health Organization (WHO) in January 2020 (Huang et al., 2020; Lu, Stratton & Tang, 2020). Although the WHO de-escalated its status in May 2023, the potential threat of bacterial co-infections with COVID-19 remains a concern. Bacterial co-infection is a common complication of many respiratory viral infections, which leads to a significant increase in incidence and mortality rates (Bakaletz, 2017; Westblade, Simon & Satlin, 2021). During the 1918 influenza pandemic, bacterial co-infection was an important cause of most influenza-related deaths. Common respiratory tract bacteria, such as Streptococcus pneumoniae, β-Hemolytic Streptococcus, Haemophilus influenzae, and Staphylococcus aureus, were the most common pathogens (Morens, Taubenberger & Fauci, 2008; Chien, Klugman & Morens, 2009). During the 2009 influenza pandemic, 18–30% of all intensive care unit (ICU) patients with this disease developed bacterial co-infections (MacIntyre et al., 2018). Other respiratory viruses, such as seasonal/pandemic influenza, Middle East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-1, also exhibit varying levels of bacterial/fungal mixed infections (Zahariadis et al., 2006; Assiri et al., 2013; Joseph, Togawa & Shindo, 2013). In comparison, the infection and mortality rates of COVID-19 have far exceeded those of any other common influenza pandemics (Li et al., 2020b).
Clinical trials and metagenomic studies show that, in COVID-19 patients, other viruses, bacteria, and fungi, coexist with the novel coronavirus (Chen et al., 2020; Shen et al., 2020; Hoque et al., 2021b). About 50% of all patients who died of COVID-19 suffered from secondary bacterial infections (Chen et al., 2020; Zhou et al., 2020). Therefore, a bacterial co-infection may have additive effects on the pathophysiological progress of COVID-19. These additive effects may result in more difficult diagnosis and treatment, increased risk of shock and respiratory failure, prolonged hospitalization in an ICU, and even increased mortality rates (Ruuskanen et al., 2011; Li et al., 2020b; Zhou et al., 2020; Hoque et al., 2021b). In addition, evidence shows that, due to COVID-19, an increased number of hospitalized patients have received an empirical antimicrobial treatment, potentially increasing the number of drug-resistant infections worldwide (Chen et al., 2020; Zhou et al., 2020). A recent special report from the US Centers for Disease Control and Prevention found a 15% increase in drug-resistant microorganisms associated with the epidemic (US Centers for Disease Control and Prevention & National Center for Emerging and Zoonotic Infectious Diseases, 2022). Therefore, co-infection of drug-resistant bacteria not only brings difficulties in the treatment of COVID-19, but also challenges in the management of antibiotics. Unfortunately, during the pandemic, these issues did not receive proper research and discussion.
In this study, we collected and discussed research on bacterial co-infections published since the outbreak of COVID-19. We focused on bacterial co-infections of the respiratory system and bloodstream because they greatly aggravate the development of COVID-19 and increase the rates of incidence and mortality of patients. In addition, we also discuss the drug resistance of related bacteria. The purpose of this review is to emphasize that microbial co-infection is a factor that cannot be ignored in COVID-19. Furthermore, we provide valuable references for clinicians concerning the diagnosis or treatment of patients with COVID-19 and a bacterial co-infection.
Survey methodology
The search engines we employed included PubMed (https://pubmed.ncbi.nlm.nih.gov/), ScienceDirect (https://www.sciencedirect.com/), Scopus (http://www.scopus.com/), and Google Scholar (https://scholar.google.com/). We conducted keyword searches that centered around, but were not limited to, the terms “COVID-19”, “SARS-CoV-2”, “bacterial co-infection and COVID-19”, “risk factors for bacterial co-infection”, “pulmonary function and COVID-19”, “ventilator-associated pneumonia and COVID-19”, “bloodstream infections and COVID-19”, and “antibiotic resistance during COVID-19”.
To address potential bias or low-quality articles, we applied a rigorous screening process to the initial pool of 350 articles identified through our keyword searches. First, we conducted a title and abstract review, which led to the exclusion of 165 articles that were clearly irrelevant or did not meet our research criteria. This narrowed down our selection to 185 articles for the next stage. Next, we undertook a full-text review to assess the methodological quality and scientific validity of these remaining articles. During this phase, we excluded an additional 40 articles due to concerns over bias, low quality, or insufficiency in providing credible and unbiased evidence on our topic of interest. Ultimately, only 145 articles met our strict criteria: they provided credible and unbiased evidence on the topic, had a low risk of bias, and were of high quality according to our pre-specified standards. These articles were included in our comprehensive review. Additionally, to ensure the robustness of our findings, we performed sensitivity analyses to account for any potential bias introduced by the inclusion or exclusion of individual studies. This rigorous approach enabled us to present a high-quality and unbiased review of the available literature.
Early evidence on COVID-19 and bacterial infections
Although there are limited data on bacterial infections in COVID-19 patients in the early stages of the outbreak, potential links between the two infection types have been investigated. Guan et al. (2020) evaluated the medical records of 1,099 adult patients with laboratory-confirmed COVID-19 reported between December 11, 2019 and January 29, 2020. Many of these patients were admitted without sputum bacteriological or fungal assessments, possibly due to the overwhelmed state of many hospital resources at the time of the outbreak (Guan et al., 2020).
Across a large number of studies, reports of COVID-19 and bacterial infections have increased, and potential links have been discovered. A study in the United States of 12,075 subjects showed a high rate of coinfection among patients positive for SARS-CoV-2, with the most common coinfecting bacteria being Staphylococcus aureus (55.4%), Klebsiella pneumoniae (3.8%), and Mycoplasma catarrhalis (19.4%) (Massey, Jayathilake & Meltzer, 2020). Zhou et al. (2020) analyzed 191 adult patients hospitalized with COVID-19 in Wuhan and found that sepsis was the most prevalent complication, both among non-survivors and survivors. However, whether sepsis was caused by viral or bacterial infections in those patients still requires further study. A study conducted at Zhongnan Hospital in Wuhan revealed that among the 221 adult COVID-19 patients, the incidence of mixed bacterial infections was significantly higher in severe cases, such as those requiring ICU and mechanical ventilation, compared to non-severe cases (25.5% vs. 1.8%; p < 0.001) (Zhang et al., 2020). This difference suggests that bacterial infections may play a less relevant role in the early, non-severe stages of COVID-19. In fact, a study in the UK involving 836 patients with the novel coronavirus infection reported a relatively low frequency of bacterial co-infection during the early hospitalization for COVID-19 (3.2%, 0–5 days after admission) (Hughes et al., 2020). Similarly, Rawson et al. (2020) analyzed a survey from China and the United States and reported that 8% of 806 COVID-19 patients had a mixed bacterial or fungal infection. Of 712 hospitalized adult COVID-19 patients at the Valladolid football Club in Spain, 16% were reported to have a bacterial/fungal infection or co-infection (Nebreda-Mayoral et al., 2022). Another study reported that the rates of co-infection and secondary infection in patients with COVID-19 ranged from as low as 0.6% to as high as 45% (Lai, Wang & Hsueh, 2020). However, it should be emphasized that coinfection rates vary across different environments. In certain regions, the prevalence of coinfections may be elevated. Moreover, unpublished data may exist that indicates even higher rates of coinfection.
Altogether, these early reports suggest that bacterial infections are present in COVID-19 patients. Given the limited medical resources available in many hospitals in the early stages of the outbreak, information on the microbial causes and effects on COVID-19 patients has been limited. This limitation reinforces the need to further study the effects of bacterial infections on COVID-19 patients, which will be the focus of the following sections.
Main pathogenic microorganisms associated with co-infection in COVID-19 patients
Globally, the leading culprits in the cause of a hospital-acquired infections (HAIs) or community-acquired pneumonia (CAP) are the so-called ESKAPE pathogens: E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter species (Rice, 2008). A recent microbiological study on patients with COVID-19 showed that, in COVID-19 infected patients, specific genera including Staphylococcus, Nostoc, Anabaena, Mycobacterium, Cyanothece, Bradyrhizobium, Actinomyces, Pseudomonas, Propionibacterium, Corynebacterium, Rhodopseudomonas, Nodularia, Burkholderia, Micrococcus, Acinetobacter, Methylobacterium, Streptomyces, Rhodococcus, and Rhodobacter had higher relative abundances in comparison with the non-COVID samples (Hoque et al., 2021b). Specifically, among the bacterial species reported to co-infect with COVID-19, Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Acinetobacter baumannii, Legionella pneumophila, and Clamydia pneumoniae appear to be the most common (Khatiwada & Subedi, 2020; Peddu et al., 2020; Hoque et al., 2021a). The study by Sang et al. (2021) showed that, when patients with COVID-19 stayed in the ICU for more than 72 h, a secondary infection became very common (86.6%). The most common bacteria involved in these secondary infections were Klebsiella pneumoniae (24.5%), Acinetobacter baumannii (21.8%), Stenotrophomonas maltophilia (9.9%), Candida albicans (6.8%), and Pseudomonas spp. (4.8%) (Sang et al., 2021). In a retrospective, single-center study in Wuhan, among 99 cases of 2019 novel coronavirus pneumonia, the co-infected bacterial species included Acinetobacter baumannii and Klebsiella pneumoniae (Chen et al., 2020). In another retrospective cohort study, Garcia-Vidal et al. (2021) studied the community-acquired infection and HAIs of patients with COVID-19. In these community-acquired co-infections, 30 community-acquired bacterial co-infections were documented in 25 patients (2.5%). S. pneumoniae and S. aureus were the most common bacteria in this scenario. A total of 51 hospital-acquired infections were recorded among the 43 patients. Of these, 44 were bacterial and diagnosed in 38 patients (3.8%). The most commonly isolated microorganisms were P. aeruginosa (n = 8), E. coli (n = 6), Klebsiella spp. (n = 5), and S. aureus (n = 5) (Garcia-Vidal et al., 2021). In another study on the lung microbiome of 20 deceased COVID-19 patients using post-mortem biopsies, the most common bacterial genera identified were Acinetobacter, Chryseobacterium, Burkholderia, Brevundimonas, Sphingobium, and Enterobacteriaceae (Fan et al., 2020).
Concerning fungal infections, Aspergillus flavus, Candida glabrata, and Candida albicans were the most common co-infecting fungi (Bassetti, Kollef & Timsit, 2020; Sang et al., 2021). In patients with COVID-19, Zuo et al. (2020) observed remarkable enrichments of opportunistic fungal pathogens, specifically belonging to the Candida and Aspergillus lineages, during the course of the disease. Among them, C. albicans was represented excessively in COVID-19 patients (Zuo et al., 2020). In addition, a descriptive study by Chen et al. (2020) showed that Aspergillus flavus, Candida glabrata, and Candida albicans were the most common coinfecting fungi. Similarly, Salehi et al. (2020) analyzed 53 hospitalized COVID-19 patients with oropharyngeal candidiasis (OPC). These researchers found that C. albicans was the most prevalent pathogen, accounting for 70.7% of all infections, followed by C. glabrata (10.7%), C. dubliniensis (9.2%), C. parapsilosis sensu stricto (4.6%), C. tropicalis (3%), and C. krusei (1.5%) (Salehi et al., 2020).
There are a growing number of reports of bacterial infections in patients with COVID-19. Although the main types of bacterial infection in different regions of the world are different, it is clear that the rate of SARS-CoV-2 co-infection is in direct proportion to the severity of the disease (Denina et al., 2020). It is also clear that co-infection increases COVID-19 mortality rates (Bengoechea & Bamford, 2020). Therefore, carefully monitoring whether COVID-19 patients also have bacterial infections will help prevent and treat these patients.
Pathological alterations and resultant functional impairment in the lungs following co-infection with COVID-19 and bacterial pathogens
In recent years, a large body of evidence has indicated that the lungs are the organs most severely affected by COVID-19, as they undergo a series of pathophysiological events including diffuse alveolar epithelium destruction, hyaline membrane formation, capillary damage and bleeding, alveolar septal fibrous proliferation, and pulmonary consolidation (Mo et al., 2020; Shi et al., 2020; Torres-Castro et al., 2021; Steinbeis et al., 2022). An important characteristic of COVID-19 is the extensive injury of alveolar epithelial cells and endothelial cells with secondary fibroproliferation (Venkataraman & Frieman, 2017; Wendisch et al., 2021). This type of injury indicates a potential for chronic vascular and alveolar remodeling, which can lead to lung fibrosis and/or pulmonary hypertension (Frija-Masson et al., 2020; Wendisch et al., 2021). These changes in the lungs directly lead to the degradation of their function. According to statistics, 15–20% of ICU inpatients affected by COVID-19 received ventilation support (Karagiannidis et al., 2020; Grasselli et al., 2021a; Strålin et al., 2021). However, whether bacterial infection in COVID-19 played an important role in this process has not been discussed.
COVID-19 and ventilator-associated lower respiratory tract infections
Ventilator-associated pneumonia (VAP) is an important factor affecting lung respiratory function. VAP that complicates COVID-19 infections has often occurred in late stages of mechanical ventilation (Luyt et al., 2020; Blonz et al., 2021; Maes et al., 2021). Some studies have proved that the respiratory dysfunction of patients with COVID-19 has an important connection with bacterial ventilator-associated lower respiratory tract infections (VA-LRTIs) (Luyt et al., 2020; Blonz et al., 2021; COVID-ICU Group on Behalf of the REVA Network and the COVID-ICU Investigators, 2021; Rouzé et al., 2021; Razazi et al., 2023). Previous studies have found an increased risk of VAP and VA-LRTI in critically ill patients with COVID-19, with incidence rates ranging from 29% to 86% (Razazi et al., 2020; Blonz et al., 2021; Giacobbe et al., 2021; Maes et al., 2021). In a retrospective study involving 91 patients with COVID-19 respiratory failure (81 on a ventilator for >48 h), Maes et al. (2021) reported a hazard ratio of 2.1 compared to non-COVID-19 patients, and an incidence of VAP of 79%. In another study of 568 COVID-19 patients, 29% had VAP, which was a higher incidence than that observed in influenza pneumonia or non-viral pneumonia (Giacobbe et al., 2021). Additionally, Hedberg et al. (2022) compared the VA-LRTIs of the first and second waves of the epidemic. They reported that the duration of mechanical ventilation was shorter during the second wave (8 days; interquartile range (IQR): 5–16 days) compared with the first wave (11 days; IQR: 6–19 days). The mortality rate within 24 h after extubation in the second wave (32%, 30/93) was significantly higher than that in the first wave (20%, 76/381) (Hedberg et al., 2022). Bacteriological analysis of alveolar lavage fluid from VAP patients revealed that, at the time of ICU admission, the incidence of VA-LRTI was significantly higher in patients with COVID-19 pneumonia than in patients with influenza pneumonia or no viral infection. Gram-negative bacilli, primarily P. aeruginosa, Enterobacter spp., and Klebsiella spp., were the causative agents in the majority of the first VA-LRTI episodes, while gram-positive bacteria were mainly Staphylococcus aureus (Table 1) (Giacobbe et al., 2021; Grasselli et al., 2021b; Rouzé et al., 2021; Wicky, Niedermann & Timsit, 2021; Hedberg et al., 2022). Additionally, a considerably higher rate of extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-PE) compared to historical non-COVID-19 controls (72% vs. 47%) has been reported. Aspergillus appeared to be more common in COVID-19 respiratory failure patients than in other populations (Razazi et al., 2020).
| Bacterial classification | Bacterial species | References |
|---|---|---|
| Gram-negative bacteria | P. aeruginosa, Enterobacter spp., and Klebsiella spp., other ESBL-PE | Giacobbe et al. (2021), Grasselli et al. (2021b), Rouzé et al. (2021), Wicky, Niedermann & Timsit (2021), Hedberg et al. (2022) |
| Gram-positive bacteria | Staphylococcus aureus | Giacobbe et al. (2021), Grasselli et al. (2021b), Rouzé et al. (2021), Wicky, Niedermann & Timsit (2021), Hedberg et al. (2022) |
| fungi | Aspergillus | Razazi et al. (2020) |
The prevention of VAP is crucial in reducing patient morbidity and mortality. The VAP bundle represents a set of comprehensive intervention measures designed specifically for the prevention of VAP (Morris et al., 2011). Common practices within the bundles include elevation of the head of the bed, daily sedation vacations and assessments for extubation readiness, prophylaxis for peptic ulcer disease (PUD) and deep vein thrombosis (DVT), and oral hygiene maintenance (Resar et al., 2005; Mastrogianni et al., 2023). Studies have demonstrated that implementing the VAP bundles can effectively reduce the incidence of VAP, with reductions ranging from a low of 13% to a staggering 100%. Notably, studies that implemented the Institute for Healthcare Improvement (IHI) ventilator bundles, combined with appropriate endotracheal cuff pressure and subglottic suctioning, achieved significant decreases in VAP rates (Mastrogianni et al., 2023). A previous study indicated a remarkable improvement in compliance with VAP prevention measures from 2019 to 2020 (increasing from 93.25% to 99.85%) (AlAhdal et al., 2022). This enhancement could be attributed to the emphasis on infection control measures and educational training during the COVID-19 pandemic. However, despite improved compliance, the study did not reveal a statistically significant change in the incidence of VAP (AlAhdal et al., 2022). This could be influenced by multiple factors, including hand hygiene compliance, characteristics of infectious pathogens, and other unconsidered variables that clearly warrant further investigation.
Taken together, existing evidence suggests that COVID-19 is associated with exceptionally long durations of mechanical ventilation treatment and high VA-LRTI occurrence proportions. Compared to patients with influenza-induced pneumonia or those hospitalized in ICUs without viral infections, individuals infected with SARS-CoV-2 are significantly more likely to develop VA-LRTI. Therefore, understanding the pathophysiology of VA-LRTI in patients with COVID-19 infection is very important for epidemic prevention, as well as for the control and treatment of the disease.
Tuberculosis and COVID-19 co-infection: a double blow to lung function
In addition to the above-mentioned bacteria, Mycobacterium tuberculosis combined with COVID-19 infection also has an important impact on lung function. On the one hand, the lung is the main infection site of both COVID-19 and M. tuberculosis. As such, M. tuberculosis can also affect lung function during COVID-19 infection. On the other hand, lung damage caused by severe COVID-19 and the subsequent vulnerability to tuberculosis (TB) is also a significant concern (Mousquer, Peres & Fiegenbaum, 2021). The first cohort evaluating the association between TB and COVID-19 consisted of 49 cases of co-infection from eight different countries. This study identified a higher mortality rate among elderly people (12.3%) with a previous history of TB (Tadolini et al., 2020). Another study conducted in the Philippines provided further clarity on the detrimental impact of TB on COVID-19 and established a correlation between co-infection and a heightened risk of increased incidence and mortality (Sy, Haw & Uy, 2020). Furthermore, a meta-analysis of co-infection with SARS-CoV-2 and M. tuberculosis has shown a two-fold increase in mortality among co-infected patients, and multiple case-control studies have confirmed the changes in disease pathology associated with co-infection (Sarkar, Khanna & Singh, 2021).
M. tuberculosis seriously interferes with the pulmonary microenvironment. During a latent TB infection, the persistent presence of mycobacteria can induce chronic pro-inflammatory reactions in the lung parenchyma, leading to lung injury (Mousquer, Peres & Fiegenbaum, 2021; Shah et al., 2022). The results from previous studies showed that a large proportion of patients with COVID-19 further complicated by TB branch infection needed ventilation (18%, of which 7.4% needed intubation), and 32% of these patients needed supplemental oxygen with a significantly prolonged hospital stay. In addition to the time required for TB recovery, 61.7% of the patients needed an average of 14 days of hospital stay due to COVID-19 (Motta et al., 2020; Tadolini et al., 2020; Migliori et al., 2021; Visca et al., 2021). In a comprehensive view, the pulmonary cavitation caused by TB fundamentally alters the architecture of the lungs. The necrotic, thin-walled tissue is replaced by fibrous epithelial tissue, diminishing the available surface for gas exchange. Moreover, the bronchiectasis and bronchostenosis that were formed restrained the airflow, while obstructed capillaries compromised the lung fluid drainage (Dheda et al., 2005; Kılıç et al., 2022; Shah et al., 2022). Imaging findings reported in TB patients with COVID-19 revealed the development of a multiple and bilateral ground glass opacity, and consolidations with the air bronchogram (Lal et al., 2020; Motta et al., 2020; Tadolini et al., 2020; TB/COVID-19 Global Study Group, 2022). Unilateral pulmonary infiltrates were seen in 33% of patients with TB and COVID-19, while bilateral infiltrates were reported in 19% of the patients. Chest CT images suggesting the diagnosis of pulmonary TB have been obtained in cavitating lung lesions. Among TB patients, bilateral cavitary lesions have been reported more often (27%) than unilateral cavitary lesions (21%) (Motta et al., 2020; Tadolini et al., 2020; TB/COVID-19 Global Study Group, 2022).
In general, the macro-structural changes caused by TB damage the function and defense capacity of the lower respiratory tract. These consequences make the lungs more prone to severe complications, such as pneumonia and respiratory failure. In addition, M. tuberculosis infection is an inducer of the development of severe COVID-19. Lung injury caused by severe COVID-19, and the subsequent susceptibility to TB, is also a matter of concern. Understanding how pathogens affect lung function in the lung microenvironment, as well as clarifying the mechanisms of susceptibility and prognosis of the two infections, will be the basis for developing new strategies to prevent and treat the TB/COVID-19 dual infection.
COVID-19 complications in the presence of bloodstream co-infection
A bloodstream infection (BSI) refers to the presence of viable microorganisms in the bloodstream (Laupland & Church, 2014). BSI may lead to sepsis, which is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection (Singer et al., 2016). In patients receiving hemodialysis, BSI is an important cause of hospitalization, morbidity, and mortality. In all hospitalized patients, the BSI incidence rate is about 6%, and the associated mortality rate, which affects 10–20% of all intensive care patients, is about 15% and causes a three-fold increase in the mortality rate of this patient population (Rhee et al., 2017; Pijl et al., 2021). Although it has been observed that some COVID-19 patients have BSI (Giacobbe et al., 2020; He et al., 2020; Kokkoris et al., 2021; Patton et al., 2023), its co-occurrence has rarely been systematically discussed since the COVID-19 outbreak.
Incidence of BSI and pathogens
Current research has shown that BSI has a high mortality rate and is one of the main types of acquired infection in ICU patients with COVID-19 (Giacobbe et al., 2020; He et al., 2020; Grasselli et al., 2021b; Kokkoris et al., 2021). In a recent meta-analysis, BSI occurred in 7.3% of all hospitalized patients with COVID-19, and in 29.6% of those admitted to the ICU (Ippolito et al., 2021). It should be noted that the incidence rates of BSI vary among different regions, even exceeding 30% in some reports (Giacobbe et al., 2020; Grasselli et al., 2021b; Kokkoris et al., 2021). This variability may be related to the severity of the epidemic, timing of data statistics, and local medical conditions (Goyal et al., 2020; Langford et al., 2020; Kokkoris et al., 2021; Soriano et al., 2021). During the early phases of the pandemic, a study by Giacobbe et al. (2020) involving 78 critically ill COVID-19 patients concluded that 45 ICU-acquired BSI episodes were recorded in 31 patients, with an incidence of 47 episodes per 1,000 risky patients per day. This incidence rate is significantly higher than the five to 19 episodes per 1,000 patient-days registered in other heterogeneous, non-COVID-19 ICU patient populations (Giacobbe et al., 2020). Similarly, Grasselli et al. (2021b) observed that, out of the 774 patients included, 359 patients (46%) experienced 759 hospital infections, with BSIs accounting for 44% (including catheter-related BSIs). This BSI rate was significantly higher than that reported in the largest study published so far in the general population of ICU patients. It was even higher than that observed in patients undergoing extracorporeal membrane oxygenation for refractory respiratory failure (Vincent et al., 2009; Grasselli et al., 2017). Additionally, different types of COVID-19 may have different effects on the incidence of BSI; however, relevant literature is still limited in this field. Kang et al. (2023) reported that, during the surge of the Omicron variant, the incidence of central venous catheter-related BSI increased, which might be due to the increased workload of the attending nurses. Regarding the mortality rates of BSI, they greatly varied among COVID-19 patients in ICUs, with BSI rates ranging from 39% to 100% in different studies (Buetti et al., 2021; Grasselli et al., 2021b; Kokkoris et al., 2021; Massart et al., 2021; Kurt et al., 2022; Torrecillas et al., 2022; Strelkova et al., 2023). These relatively high mortality rates may be due to a larger median age of patients (in most studies, patients were 60–73 years old) (Buetti et al., 2021; Grasselli et al., 2021b; Kokkoris et al., 2021; Massart et al., 2021; Kurt et al., 2022; Torrecillas et al., 2022; Strelkova et al., 2023). In addition, infection with multidrug-resistant bacteria may be an important factor leading to high mortality rates in BSI patients. The study of Kurt et al. (2022) suggests that the high mortality rate of BSI (83%) may be related to the high comorbidity and incidence of multidrug-resistant Acinetobacter baumannii and carbapenem-resistant Klebsiella pneumoniae (60%). Similarly, the study by Palanisamy et al. (2021) found that Acinetobacter baumannii and Klebsiella pneumoniae dominated with a total mortality rate of 100%.
Regarding pathogens, most studies have shown that Gram-positive bacteria, mainly Enterococcus faecalis, coagulase-negative staphylococci, Staphylococcus aureus, and Candida spp., accounted for a large proportion (Table 2) (Giacobbe et al., 2020; He et al., 2020; Bonazzetti et al., 2021; Grasselli et al., 2021b; Kokkoris et al., 2021; Buetti et al., 2022; Gago et al., 2022; Mormeneo Bayo et al., 2022; Bonazzetti et al., 2023). Enterococci were more frequently isolated in the BSI of COVID-19 patients, ranging from 10% to 50% of all cases (Giacobbe et al., 2020; Bonazzetti et al., 2021; Grasselli et al., 2021b; Kokkoris et al., 2021; Buetti et al., 2022; Gago et al., 2022; Bonazzetti et al., 2023), which may be related to the imbalance of the intestinal microbiota and bacterial translocations into the blood during COVID-19 (Prasad et al., 2022; Bernard-Raichon et al., 2022). It should be noted that, although coagulase-negative Staphylococcus also accounts for a reasonable proportion of these infections, it needs to be ruled out when the infection is caused by blood culture contamination (Hughes et al., 2020; Mormeneo Bayo et al., 2022). Other studies have also shown that the main pathogens were Gram-negative bacteria (Kokkoris et al., 2021; Buetti et al., 2022; Andrianopoulos et al., 2023; Aslan et al., 2023; Strelkova et al., 2023), including Acinetobacter spp., Klebsiella spp., Escherichia coli, and Pseudomonas spp. which may be related to the use of immunosuppressants and bacterial resistance. It is worth noting that, although most studies have shown that Gram-negative bacteria were not the main isolates, they could lead to high mortality rates in ICU patients, which could be related to their elevated drug resistance (Palanisamy et al., 2021; Gago et al., 2022; Kurt et al., 2022; Aslan et al., 2023; Strelkova et al., 2023).
| Bacterial classification | Bacterial species | References |
|---|---|---|
| Gram-negative bacteria | Acinetobacter spp., Klebsiella spp., Escherichia coli, Pseudomonas spp. | Giacobbe et al. (2020), He et al. (2020), Bonazzetti et al. (2021), Grasselli et al. (2021b), Kokkoris et al. (2021), Buetti et al. (2022), Gago et al. (2022), Mormeneo Bayo et al. (2022), Bonazzetti et al. (2023) |
| Gram-positive bacteria | Enterococcus faecalis, coagulase-negative staphylococci, Staphylococcus aureus, Candida spp. | Giacobbe et al. (2020), He et al. (2020), Bonazzetti et al. (2021), Grasselli et al. (2021b), Kokkoris et al. (2021), Buetti et al. (2022), Gago et al. (2022), Mormeneo Bayo et al. (2022), Bonazzetti et al. (2023) |
| Drug-resistant bacteria | multidrug-resistant Acinetobacter baumannii, carbapenem-resistant Klebsiella pneumoniae | Kurt et al. (2022), Palanisamy et al. (2021) |
Risk factors of BSI
The number of studies on the risk factors of BSI in COVID-19 patients is limited but growing. According to existing reports, the incidence of BSI is mainly related to specific factors, such as the use of immunosuppressants (mainly corticosteroids and tocilizumab) (Kurt et al., 2022; Nakagawara et al., 2023) and antibiotics (Massart et al., 2021; Gago et al., 2022), invasive interventions (including mechanical ventilation, central venous catheter placement, and extracorporeal membrane oxygenation) (Gragueb-Chatti et al., 2021; Palanisamy et al., 2021; Kurt et al., 2022), comorbidities (such as hypertension, diabetes mellitus, chronic kidney disease, and malignancy) (Bonazzetti et al., 2023; Strelkova et al., 2023), the length of the ICU stay (Grasselli et al., 2021b; Khatri et al., 2021), and any anti-inflammatory effects (Giacobbe et al., 2020; Kurt et al., 2022). However, there is some controversy on the risk factors of BSI in COVID-19 patients, especially when concerning immunosuppressive agents. Some studies have shown that corticosteroids and interleukin inhibitors (mainly tocilizumab and anakinra) were associated with an increased probability of BSI development (Giacobbe et al., 2020; Buetti et al., 2021; Cona et al., 2021; Meynaar et al., 2022; Nakagawara et al., 2023), while others described that this increase only occurred in patients receiving combination therapy (Giacobbe et al., 2020; Khatri et al., 2021; Kurt et al., 2022; Meynaar et al., 2022). Moreover, several researchers found no connection between immunosuppressive therapy and BSI occurrence (Abelenda-Alonso et al., 2021; Kuwahara et al., 2022; Bonazzetti et al., 2023). In the study by Strelkova et al. (2023), immunosuppressive therapy during a previous hospitalization and a higher total dose of dexamethasone before transfer to the ICU were both risk factors for BSI. The patients included in this study received higher daily doses of dexamethasone, which were administered for longer time periods since the use of interleukin inhibitors is also very common (Strelkova et al., 2023). This finding indicates that the accumulation of high-dose corticosteroids with tocilizumab may lead to immune suppression and promote the occurrence of BSI (Kurt et al., 2022; Strelkova et al., 2023). Additionally, the presence of comorbidities also brings additional uncertainty factors to the therapeutic effects of immunosuppressants (Bonazzetti et al., 2023). On the one hand, different comorbidities may result in different immune environments in different patients, which may lead to different therapeutic effects of the immunosuppressants (Palanisamy et al., 2021; Kurt et al., 2022; Bonazzetti et al., 2023). On the other hand, the severity of the disease in different patients may require different invasive interventions, which further increase the complexity of the interplay between immunosuppressants and BSIs (Grasselli et al., 2021b; Kurt et al., 2022).
There are additional controversial aspects in this field. For example, most studies showed that the risk of BSI increased with the duration of hospitalization (Giacobbe et al., 2020; Kokkoris et al., 2021); however, other studies showed that a prolonged ICU hospitalization was an independent protective factor (Kurt et al., 2022) or had a negative correlation with the occurrence of SBI (Gago et al., 2022). Strelkova et al. (2023) found that stronger respiratory support during the first 48 h of ICU had a protective effect on BSIs. On the other hand, Cona et al. (2021) reported that both non-invasive mechanical ventilation (NIMV) and invasive mechanical ventilation (IMV) were independent risk factors for BSI. The reasons for these differences could be related to the timing of BSI occurrence, antibiotic use, underlying diseases, and the types of invasive interventions.
Taken together, the risk factors of BSI are diverse and complex. The prevalence of the local COVID-19 epidemic, medical conditions, health measures, and the comorbidities of the patients are factors that pose challenges to the co-infection assessment. Moreover, for the assessment of risk factors, more details may need to be considered, including the immortal time bias and classification of comorbidities (Abelenda-Alonso et al., 2021; Vail et al., 2021). These issues still require more extensive research for a systematic elucidation.
Antimicrobial resistance associated with COVID-19 infection
Growing evidence shows that antimicrobial resistance (AMR) may be increased in patients with COVID-19 under excessive use of antibiotics (Council of Canadian Academies, 2021; World Health Organization, 2021; Bengoechea & Bamford, 2020). The overuse of antibiotics can lead to antibiotic-resistant microorganisms that have a negative impact on humans. AMR is known as one of the top 10 threats to global public health. The frequent use of antibiotics in patients with COVID-19 may aggravate antibiotic resistance. A systematic analysis published in 2022 shows that between 900,000 and 1.7 million deaths in 2019 can be attributed to bacterial resistance, making it one of the main causes of global mortality (Antimicrobial Resistance Collaborators, 2022). Additionally, according to the previous analysis of COVID-19, it is estimated that, in the next 30 years, AMR will cause about 10 million deaths worldwide, which is higher than the predicted number of deaths caused by cancer (8.2 million) and diabetes (1.5 million) (Rizvi & Ahammad, 2022). The outbreak of COVID-19 has led to changes in the global antibiotic consumption patterns, and the number of people who die from AMR is expected to reach higher numbers in the future.
Recent evidence suggests that due to the COVID-19 pandemic, an increasing number of hospitalized patients have been receiving empirical antimicrobial treatments, which potentially increases the worldwide number of AMR infections (Nori et al., 2021; Rawson, Wilson & Holmes, 2021; Kariyawasam et al., 2022). Noteworthy data from a study shows that even without overlapping bacterial infections, the broad-spectrum antibiotic administration rates of patients with severe COVID-19 infections are of about 71.8% (Rawson et al., 2020; Goncalves Mendes Neto et al., 2021). A systematic review of 24 studies and 3,506 patients showed that 71.8% (95% confidence interval (CI) [56.1–87.7%]) of patients received antibiotic treatment at hospital admission (Langford et al., 2020). In Wuhan, China, Zhou et al. (2020) reported observations of 191 hospitalized patients and found that 95% of those patients received antibiotics. Overall, more than one half of COVID-19 patients received antibiotic treatment. Among the antibiotics used, the most common drugs were fluoroquinolones, cephalosporins, carbapenems, azithromycin, vancomycin, and linezolid (Langford et al., 2021; Antimicrobial Resistance Collaborators, 2022; Wu et al., 2022).
Notably, compared with pneumonia caused by other respiratory pathogens, pneumonia caused by SARS-CoV-2 usually leads to a longer course of the disease (Budinger et al., 2021). Patients with bacterial infections have longer hospital stays (Cheng et al., 2020; Baskaran et al., 2021). Therefore, long-term hospitalized patients have an increased risk of hospital-acquired AMR bacterial infections. As an aggravating factor, the widespread use of antibiotics during the epidemic has led to an increase in the prevalence of multidrug-resistant (MDR) bacteria (Hsu, 2020; Lai et al., 2021). A recent special report from the US Centers for Disease Control and Prevention demonstrates that the COVID-19 epidemic has led to an increase in the incidence of AMR. Moreover, the drug-resistant microorganisms associated with the COVID-19 epidemic have increased by 15% (US Centers for Disease Control and Prevention & National Center for Emerging and Zoonotic Infectious Diseases, 2022). This cascade amplification effect is worthy of our attention.
The data from two recent systematic reviews showed that, among patients with COVID-19 complicated with bacterial infections, the proportion of drug-resistant strains accounted for 29% and 37.5%, respectively (Kariyawasam et al., 2022; Langford et al., 2023). The proportion of patients infected with drug-resistant bacteria was as high as 60% (Langford et al., 2023). Most studies have shown that, among drug-resistant bacteria, the most common resistant Gram-negative bacteria were Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa (Li et al., 2020b; Tiri et al., 2020; Clancy et al., 2021; Gomez-Simmonds et al., 2021; Gysin et al., 2021; Kariyawasam et al., 2022; Pourajam et al., 2022; Wu et al., 2022). Among them, Acinetobacter baumannii and Klebsiella pneumoniae were one of the most common drug-resistant bacteria. Research has concluded that Acinetobacter baumannii had high resistance to carbapenems and quinolones, ranging from 90% to 100%, with carbapenem-resistant A. baumannii accounting for 91.7% (Li et al., 2020a; Rawson et al., 2020; Sharifipour et al., 2020; Bazaid et al., 2022; Sulayyim et al., 2022). Additionally, one study reported the highly increased resistance rates of A. baumannii to all tested antibiotics, except colistin (Sharifipour et al., 2020). Overall, according to current evidence, Acinetobacter baumannii exhibits high resistance rates to amikacin, cefepime, ceftazidime, gentamicin, meropenem, imipenem, ciprofloxacin, and piperacillin/tazobactam (Li et al., 2020b; Rawson et al., 2020; Sharifipour et al., 2020; Bazaid et al., 2022). Among drug-resistant bacteria, the proportion of carbapenem-resistant Klebsiella pneumoniae remains high, exceeding 70% (Li et al., 2020b; Tiri et al., 2020; Sang et al., 2021; Kariyawasam et al., 2022; Sulayyim et al., 2022). A systematic review by Sulayyim et al. (2022) showed that the resistance rates of Klebsiella pneumoniae to ampicillin, cefazolin, and ceftazidime were M 100% (IQR: 90.5–100%), M 93% (IQR: 78–95.5%), and M 93.5% (IQR: 83.7–97.9%), respectively, which are much higher than their corresponding values from studies published in and before 2019 (Azimi et al., 2019; Effah et al., 2020). It is worth noting that colistin, an important antibiotic for various types of Gram-negative bacteria, has also been observed in several studies to significantly increase the resistance of Klebsiella pneumoniae from 5% to 50% (Gaspar et al., 2021; de Carvalho Hessel Dias et al., 2022; Sulayyim et al., 2022).
Compared with Acinetobacter baumannii and Klebsiella pneumoniae, Pseudomonas aeruginosa is not the most detected drug-resistant bacterium, but its drug resistance rate is still higher than before the COVID-19 epidemic (Subedi, Vijay & Willcox, 2018; Kariyawasam et al., 2022; Wu et al., 2022; Langford et al., 2023). Research by Sulayyim et al. (2022) shows that their resistance to imipenem and ciprofloxacin has twice increased. In addition, the resistance rate of ESBL-producing Escherichia coli has also increased to 75% in comparison with the time period before the epidemic, (Azimi et al., 2019; Despotovic et al., 2021; Wardoyo et al., 2021; Kariyawasam et al., 2022). It shows high resistance to ampicillin, amoxicillin, clavulanic acid, levofloxacin, ceftriaxone, ciprofloxacin, and cefuroxime (Wardoyo et al., 2021; Sulayyim et al., 2022).
For Gram-positive bacteria, methicillin-resistant Staphylococcus aureus (MRSA), coagulase-negative Staphylococcus, and vancomycin-resistant Enterococcus genus are the most common resistant bacteria in current reports (Li et al., 2020b; Tiri et al., 2020; Caruso et al., 2021; Gysin et al., 2021; Saini et al., 2021; Jamnani et al., 2022; Pourajam et al., 2022). A study by Li et al. (2020b) found that 100% Staphylococcus aureus and coagulase-negative Staphylococcus had methicillin resistance; however, no vancomycin resistance was detected. This observation indicates that if a secondary infection occurs, vancomycin can be used as an empirical choice for Gram-positive bacteria. In addition, Staphylococcus aureus also has high resistance rates to oxacillin and clindamycin (Li et al., 2020b; Sulayyim et al., 2022). Enterococcus primarily includes Enterococcus faecalis and Enterococcus faecium, which are mainly resistant to ampicillin, erythromycin, ciprofloxacin, vancomycin, and tetracycline (Li et al., 2020b; Gisselø et al., 2022; Kariyawasam et al., 2022; Sulayyim et al., 2022). In addition, there are few reports on fungal resistance. In a systematic review by Kariyawasam et al. (2022), three types of fungal resistance were described: resistant Candida auricula, Candida albicans, and an unidentified Candida spp.
Collectively, the evidence shows that AMR is common and has significantly increased during the COVID-19 epidemic, which is related to the use of a large number of antibiotics. In current reports, the most commonly found drug resistance appears in Acinetobacter baumannii, Klebsiella pneumoniae, and MRSA. It is worth noting that there is significant heterogeneity among different reports concerning the type of pressure-resistant bacteria or the resistance rates. In general, difficulties arise whether general analysis applies on numerous study sites, due to: (1) heterogeneity being related to the local medical conditions and health management measures in different countries or regions; or (2) inherent difficulties in the statistical analysis of data, mainly due to the lack of relatively comprehensive implementation guidelines. From the literature we currently reviewed, less than 40% of all studies used clear explanatory standards and guidelines, such as the European Committee on Antimicrobial Susceptibility Testing (EUCAST) or the Clinical and Laboratory Standards Institute (CLSI). In addition, there is currently a lack of risk factor assessment for AMR, with only a few studies evaluating its risk factors, which include self-antibiotic medication, antibiotic prescription by general practitioners, and empirical antibiotic treatment before admission to the ICU (Caruso et al., 2021; Temperoni, Caiazzo & Barchiesi, 2021; Pourajam et al., 2022). Therefore, more scientific research guidelines and comprehensive antibiotic management plans are crucial in order to reduce the incidence of AMR.
Concluding remarks
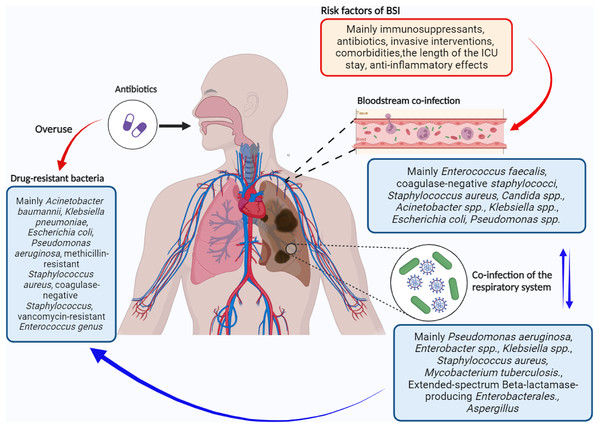
Mixed infection with various microorganisms is often found in patients infected with the novel coronavirus SARS-CoV-2. Here, we focused on infections in the respiratory system and bloodstream because they significantly affect the severity and mortality rates of COVID-19. However, our understanding of the co-infected microorganisms, as well as their interactions with other microorganisms and the host, is limited. Existing evidence shows that bacterial infections are common in patients with COVID-19, especially in ICU patients. Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae are the most common pathogens in mixed infections. Moreover, Acinetobacter baumannii, Klebsiella pneumoniae, and Staphylococcus aureus account for a large proportion of drug-resistant bacteria. In addition, Staphylococcus aureus and Enterococcus are the main Gram-positive bacteria that cause bloodstream infections (Fig. 1).
Figure 1: Simplistic schematic of bacterial co-infection in COVID-19.
Created using Biorender (https://app.biorender.com).It is important to note the significant heterogeneity among studies on bacterial mixed infections in COVID-19, possibly due to factors like disease severity, comorbidities, treatment variations, and environmental differences. This heterogeneity underscores the need for further research on the epidemiological, clinical, and laboratory traits of co-infected pathogens in diverse COVID-19 patient groups. Additionally, there’s a lack of comprehensive guidelines for data statistics, health management, and antibiotic use, emphasizing the necessity for judicious antibiotic administration to minimize resistance. Moreover, with the emergence of new coronavirus variants like Omicron (Viana et al., 2022), research on their co-infection with bacteria is urgently needed. Despite COVID-19 no longer being declared a public health emergency, weekly infections still exceed 300,000 globally, highlighting the persistent threat of COVID-19 and its bacterial co-infections. Therefore, we should not underestimate the serious threat of COVID-19 infection to human health and its associated global burden.