Passive smoking and risk of pancreatic cancer: an updated systematic review and meta-analysis
- Published
- Accepted
- Received
- Academic Editor
- Bijaya Padhi
- Subject Areas
- Global Health, Internal Medicine
- Keywords
- Passive smoking, Pancreatic cancer, Systemic review, Meta-analysis
- Copyright
- © 2024 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Passive smoking and risk of pancreatic cancer: an updated systematic review and meta-analysis. PeerJ 12:e18017 https://doi.org/10.7717/peerj.18017
Abstract
Background
Previous meta-analysis has demonstrated that no association was validated between passive smoking and pancreatic cancer. However, there is growing evidence on this issue recently. This study aimed to confirm this association.
Methods
PubMed, Embase, Web of Science, and Cochrane Library databases were searched up to April 2024 for retrieval of full articles. Studies with the exposure of passive smoking and outcome of pancreatic cancer were eligible for the analysis. We generated pooled relative risks (RRs) and 95% confidence intervals (CIs) using DerSimonian–Laird random-effects models. Quality of evidence was assessed using the GRADE system.
Results
Fourteen studies were included, with 5,560 pancreatic cancer patients. Passive smoking was associated with a moderate increased risk of pancreatic cancer (RR = 1.20, 95% CI: 1.11–1.30, p < 0.001). The results were consistent in both case-control (p=0.013) and cohort studies (p < 0.001) and in studies with high (p = 0.007) and moderate quality (p < 0.001). In subgroup analysis, the risk was significant for both current (RR=1.91, 95% CI: 1.45-2.51, p < 0.001) and non-current smokers (RR = 1.17, 95% CI: 1.01-1.36, p = 0.037), for exposure both in adulthood (RR = 1.18, 95% CI: 1.06-1.31, p = 0.002) and childhood (RR = 1.20, 95% CI: 1.08-1.34, p = 0.001). However, only regular or daily exposure (RR=1.28, 95% CI: 1.08-1.50, p = 0.003), rather than exposing occasionally, seldom or few times per week (p = 0.421), to passive smoking could increase the risk of pancreatic cancer.
Conclusion
Passive smoking exposure confers a significant increased risk for pancreatic cancer. The risk was valid in both case-control and cohort, high and moderate quality studies, in current and non-current smokers, and for both childhood and adulthood exposure. Regular or daily exposure rather than exposing occasionally, seldom or few times per week could exert a detrimental effect on pancreatic cancer.
Introduction
Pancreatic cancer, a common digestive system malignant tumor, is characterized by aggressive clinical behaviors and low survival rate of approximately 9% (Siegel, Miller & Jemal, 2020). It imposes a great burden on human health, with increasing incidence and mortality (Huang et al., 2021), as well as vague and nonspecific symptoms before the tumor is locally unresectable with metastasis (Park, Chawla & O’Reilly, 2021). The risk factors for pancreatic cancer include individual characteristics such as sex (Pijnappel et al., 2022), race, age, ABO blood group (Risch et al., 2013; Wolpin et al., 2010), family history (Hamada et al., 2019), and genetic mutations (Yakar, Bozkirli & Ceyhan, 2022), as well as lifestyle and environment factors such as smoking (Lugo et al., 2018), trace element exposure (Amaral et al., 2012; Molina-Montes et al., 2012), dietary habits (Larsson, Bergkvist & Wolk, 2006; Petrick et al., 2020), alcohol consumption (Jayasekara et al., 2019), obesity (Zohar et al., 2019), etc.
The process of passive smoking, also known as environmental tobacco smoking (ETS) or second-hand smoking, still releases several procarcinogenic elements. Apart from the established association between active smoking and pancreatic cancer, the role of passive smoking still remains controversial. Several studies reported a detrimental effect on pancreatic cancer (Andersson et al., 2016; Bao et al., 2009; Lo et al., 2007; Vedie et al., 2023) and others proved a neutral effect (Chuang et al., 2011; Ding et al., 2015; Gallicchio et al., 2006; Hassan et al., 2007; Heinen et al., 2010; Molina-Montes et al., 2020; Nishino et al., 2001; Tranah et al., 2011; Villeneuve et al., 2004). Previous meta-analysis has proved a non-significant association between exposure to ETS and risk of pancreatic cancer(Zhou, Wellenius & Michaud, 2012), which included limited studies. Besides, the reference groups among studies were slightly different, causing possible bias in the final analysis. Recently, more population-based studies were published (Andersson et al., 2016; Ding et al., 2015; Molina-Montes et al., 2020; Vedie et al., 2023), adding more solid evidence to the issue. Thus, we conducted an updated systematic review and meta-analysis to explore the association between passive smoking and pancreatic cancer.
Materials & Methods
Study selection
This study was registered on PROSPERO (ID: CRD42024528620). PubMed, Embase, Web of Science, and Cochrane Library databases were searched from 1971 up to April 2024 for retrieval of published articles in peer-reviewed journals in English investigating the association between passive smoking and pancreatic cancer. The search strategy included the following terms: (“tobacco smoke pollution” OR (“tobacco” AND “smoke” AND “pollution”) OR “tobacco smoke pollution” OR (“passive” AND “smoking”) OR “passive smoking”) OR “second hand smoking” AND (“pancreatic neoplasms” OR (“pancreatic” AND “neoplasms”) OR “pancreatic neoplasms” OR (“pancreatic” AND “cancer”) OR “pancreatic cancer”). The review protocol was not published or submitted online.
Inclusion and exclusion criteria
The inclusion criteria were displayed in PECO format: (a) the population (P) of interest were mainly from volunteer participants or community inhabitants; (b) the exposure (E) was passive smoking; (c) the comparison (C) was between participants exposed to passive smoking and not; and (d) outcome (O) of interest was the development of pancreatic cancer. The exclusion criteria were as follows: (a) case reports/series, letters, reviews, guidelines, protocols, replies, and conference abstracts; (b) studies that did not precisely report original data or whose data were not calculable for the outcome; (c) records not in English; and (d) basic science or experimental studies.
Data extraction and quality assessment
Two researchers independently performed the data extraction (XD W and XJ W). All potential studies were comprehensively reviewed by both reviewers. Relevant information, including author and publication year, study design, origin, exposure, follow-up end/period, sample size, number of pancreatic cancer cases, effect size (Odds ratio (OR), hazard ratio (HR), relative risk (RR), or other calculable data from each study) and adjusted confounding factors were extracted for each study and summarized in one table by XD W. Those above basic characteristics were then cross-checked by ZH W. The discrepancies were resolved by the senior author (XJ W). The Newcastle-Ottawa Scale (NOS) was used to assess the study quality. Studies with a score of 7 to 9, 4 to 6, and 0 to 3 was considered as high, moderate, and low quality.
GRADE assesement
The certainty of the evidence for each outcome was verified in accordance with the GRADE system on the online GRADEpro software (https://www.gradepro.org/) (Atkins et al., 2004). The following dimensions were taken into consideration, including study design limitations, risk of bias, inconsistency between studies, indirectness, imprecision, and other considerations (Guyatt et al., 2008), which generated five levels of evidence for each outcome: high, moderate, low, very low quality of evidence and no evidence.
Statistical analysis
We used STATA 17.0 (StataCorp LLC, College Station, TX, USA) and Rstudio software to conduct all the analyses. ORs, HRs, RRs, other calculable data and the corresponding 95% confidence intervals (CIs) were pooled using DerSimonian–Laird random-effects models. Subgroup analyses were performed based on study type, quality of study, geographic region, smoking status of participants, exposing time and frequency of passive smoking. Heterogeneity was assessed using the I 2 statistic. Publication bias was verified by funnel plot. Sensitivity analysis was performed to find the potential source of heterogeneity.
Results
Literature search and description of included studies
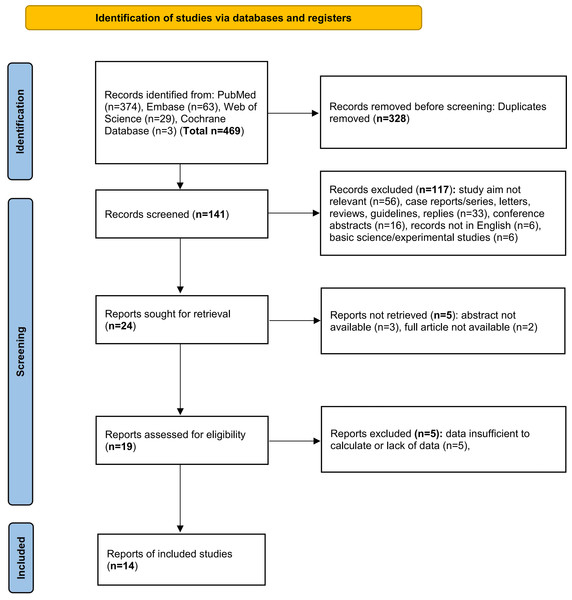
A total of 469 articles were initially searched, of which 328 duplicates were excluded. After records screening, 117 items were excluded due to the following reasons: study aim not relevant (n = 56), case reports/series, letters, reviews, guidelines, replies (n = 33), conference abstracts (n = 16), records not in English (n = 6), basic science/experimental studies (n = 6), which left 24 records for retrieval. After excluding 5 records whose abstracts or full articles were not available, 19 items were assessed for eligibility. Additionally, articles whose data were missing or insufficient to calculate were excluded. Finally, a total of 14 studies (Andersson et al., 2016; Bao et al., 2009; Chuang et al., 2011; Ding et al., 2015; Gallicchio et al., 2006; Hassan et al., 2007; Heinen et al., 2010; Lo et al., 2007; Molina-Montes et al., 2020; Nishino et al., 2001; Tranah et al., 2011; Vedie et al., 2023; Villeneuve et al., 2004; Vrieling et al., 2010) met the inclusion criteria and the flow diagram for study selection was displayed in Fig. 1. The basic characteristics were indicated in Table 1. The type of passive smoking mainly included ETS, childhood and adulthood passive smoking from relatives. The follow-up time in cohort studies ranged from 8.9 to 24 years, which was sufficient to observe the outcome. The diagnosis of pancreatic cancer was mainly verified based on medical records. The results of data extraction and NOS scoring were also displayed in Table 1. The mean NOS score of the 14 studies was 6.1. Of all the included studies, five studies showed high quality and nine studies showed moderate quality.
Figure 1: PRISMA flow diagram showing the study selection process.
| Study | Study design | Origin | Exposure | Follow-up period/end | Sample size | No. PC cases | Effect size (95%CI) | Adjusted confounding factors | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Nishino et al. (2001) | Prospective cohort study | Asia | Husband’s smoking | 9 years | 9,675 | 19 | RR 1.2 (0.45–3.1) | None | 5 |
| Villeneuve et al. (2004) (child only) | Case-control study | America | Environmental tobacco smoke | Not specified | 5,396 | 105 | OR 1.37 (0.46–4.07) | Age, BMI, income adequacy and province of residence | 6 |
| Villeneuve et al. (2004) (adult only) | OR 1.01 (0.41–2.50) | ||||||||
| Villeneuve et al. (2004) (child and adult) | OR 1.21 (0.60–2.44) | ||||||||
| Gallicchio et al. (2006) (1963 cohort) | Retrospective cohort study | America | Household passive smoking | 15 years | 45,749 | 8 | RR 1.1 (0.4–2.8) | Age, education, and marital status | 8 |
| Gallicchio et al. (2006) (1975 cohort) | 19 years | 48,172 | 7 | RR 0.9 (0.4–2.3) | |||||
| (Lo et al., 2007) | Case-control study | Africa | Passive smoking | – | 388 | 21 | OR 6.0 (2.4–14.8) | Age, sex, and residence | 4 |
| Hassan et al. (2007) | Case-control study | America | Passive smoking | – | 1,616 | 735 | OR 1.3 (0.9–1.7) | Age, sex, race/ethnicity, cigarette smoking, history of diabetes, alcohol consumption, educational level, state of residency, and marital status | 4 |
| Bao et al. (2009) (from mother) | Prospective cohort study | America | Passive smoking | 24 years | 86,673 | 93 | RR 1.42 (1.07–1.89) | Age, height, smoking, diabetes, and BMI | 8 |
| Bao et al. (2009) (from father) | 211 | RR 0.97 (0.77–1.21) | |||||||
| Bao et al. (2009) (from unknown person) | 33 | RR 1.00 (0.68–1.48) | |||||||
| Heinen et al. (2010) | Retrospective cohort study | Europe | Passive smoking | 16.3 years | 120,852 | 520 | HR 0.90 (0.54–1.50) | Age, BMI, and level of education | 8 |
| Vrieling et al. (2010) (during childhood) | Prospective cohort study | Europe | Environmental tobacco smoke | 8.9 years | 465,910 | 524 | HR 1.33 (0.86–2.07) | Weight, height, and history of diabetes mellitus | 8 |
| Vrieling et al. (2010) (at home and/or at work) | HR 1.54 (1.00–2.39) | ||||||||
| Chuang et al. (2011) | Prospective cohort study | Europe | Childhood environmental tobacco smoke | until cancer development, death, emigration, or the end of the follow-up period | 112,430 | 121 | HR 1.32 (0.85–2.04) | Age, sex, and study center, education, baseline alcohol drinking, BMI, physical activity, vegetable intake, fruit intake, non-alcoholic energy intake, and adulthood passive smoking, and self-reported diabetes status | 5 |
| Tranah et al. (2011) (Childhood household exposure) | Case-control study | America | Passive smoking | - | 2,233 | 532 | OR 0.99 (0.80–1.2) | Age, education, race, smoking status, ethnicity, diabetes, pancreatitis, gallbladder disease, alcohol intake and BMI | 6 |
| Tranah et al. (2011) (Adulthood household exposure) | OR 1.2 (0.96–1.5) | ||||||||
| Tranah et al. (2011) (Adulthood workplace exposure) | OR 1.1 (0.86–1.3) | ||||||||
| Ding et al. (2015) | Case-control study | Asia | Passive smoking (from parents) | – | 1,076 | 113 | RR 0.97 (0.83–1.26) | Age, height, smoking status | 4 |
| Andersson et al. (2016) (For <10 years) | Prospective cohort study | Europe | Environmental tobacco smoke at work | until December 31, 2013 | 28,098 | 163 | HR 1.44 (0.86–2.41) | Age and sex | 6 |
| Andersson et al. (2016) (For 10–20 years) | HR 1.40 (0.84–2.34) | ||||||||
| Andersson et al. (2016) (For >20 years) | HR 2.03 (1.37–3.02) | ||||||||
| Molina-Montes et al. (2020) | Case-control study | Europe | Childhood environmental tobacco smoke | – | 3,541 | 2009 | OR 1.07 (0.81–1.42) | Age, gender, and country | 5 |
| Vedie et al. (2023)a | Prospective cohort study | Europe | Passive smoking | 24 years | 96,594 | 346 | HR 1.47 (1.08–2.00) | Age, stratified by birth generation, active smoking status, BMI, history of diabetes, education level, physical activity | 8 |
| Vedie et al. (2023)b | HR 1.16 (0.91–1.47) |
Passive smoking and pancreatic cancer
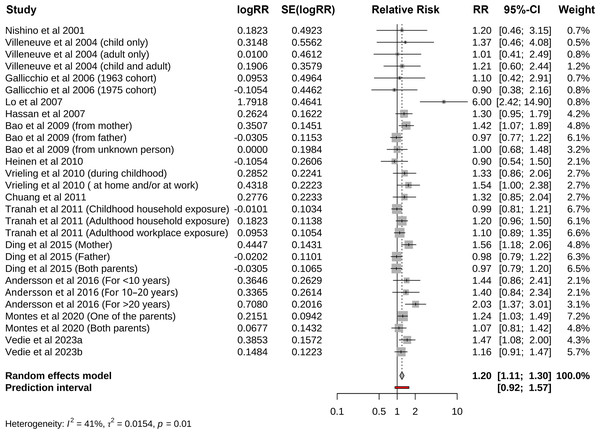
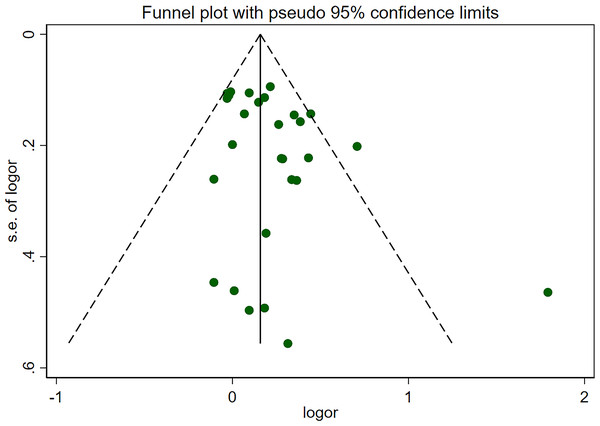
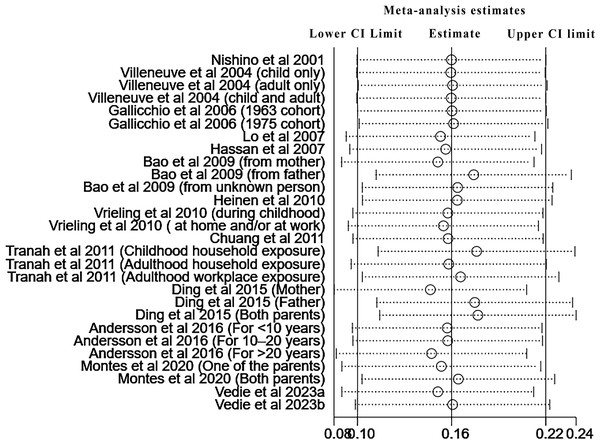
Pooled risk of pancreatic cancer for all kinds of passive smoking was 1.20 (95% CI [1.11–1.30], p<0.001, I 2 =41.0%), with moderate heterogeneity, as shown in Fig. 2. The funnel plot, displayed in Fig. 3, did not suggest that there was publication bias. Sensitivity analysis (Fig. 4) indicated that the results were stable and credible.
Figure 2: Forest plot for passive smoking and risk of pancreatic cancer.
Figure 3: Funnel plot for publication bias.
Figure 4: Sensitivity analysis of all included studies.
Subgroup analyses
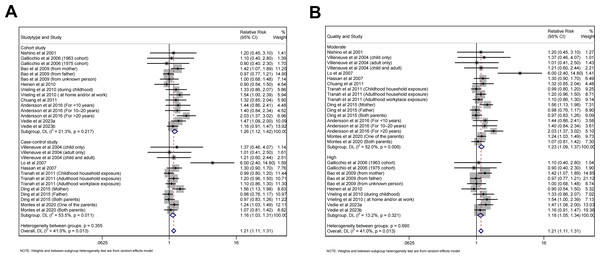
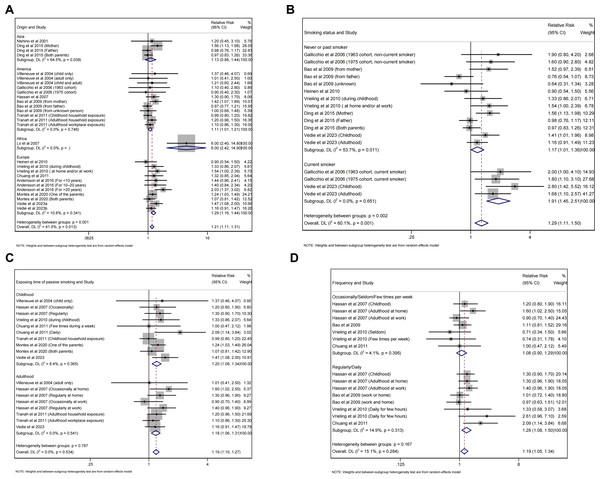
Subgroup analyses were performed based on study type, quality of study, geographic region, smoking status of participants, exposing time and frequency of passive smoking (Table 2). The results were consistent with the general finding both for case-control (RR =1.16, 95% CI [1.03–1.31], p = 0.013, I 2 =53.5%, Fig. 5A) and cohort studies (RR =1.26, 95% CI [1.12–1.42], p<0.001, I 2 =21.3%, Fig. 5A), as well as for high (RR =1.18, 95% CI [1.05–1.34], p = 0.007, I 2 =13.2%, Fig. 5B) and moderate quality studies (RR =1.23, 95% CI [1.09–1.37], p<0.001, I 2 =52.0%, Fig. 5B). Besides, the risk was significant for European (RR =1.29, 95% CI [1.16–1.44], p<0.001, I 2 =10.8%, Fig. 6A) and American (RR =1.11, 95% CI [1.01–1.21], p = 0.026, I 2 =0.0%, Fig. 6A) population, but not for Asian population with few studies included. As for smoking status of participants, both non-current (RR =1.17, 95% CI [1.01–1.36], p = 0.037, I 2 =53.7%, Fig. 6B) and current smokers (RR =1.91, 95% CI [1.45–2.51], p<0.001, I 2 =0.0%, Fig. 6B) showed increased risk of pancreatic cancer. Childhood (RR =1.20, 95% CI [1.08–1.34], p = 0.001, I 2 =8.4%, Fig. 6C) and adulthood exposure (RR =1.18, 95% CI [1.06–1.31], p = 0.002, I 2 =0.0%, Fig. 6C) both can increase the risk according to exposing time of passive smoking. For the frequency of exposure, regular or daily exposure (RR =1.28, 95% CI [1.08–1.50], p = 0.003, I 2 =14.9%, Fig. 6D), rather than exposing occasionally, seldom or few times per week, displayed increased risk of pancreatic cancer.
| Group | Subgroup | RR (95% CI) | Test for overall effect (P value) | HeterogeneityI2, % |
|---|---|---|---|---|
| Study type | Case-control studies | 1.16 (1.03-1.31) | 0.013 | 53.5% |
| Cohort studies | 1.26 (1.12-1.42) | <0.001 | 21.3% | |
| Quality of study | High | 1.18 (1.05-1.34) | 0.007 | 13.2% |
| Moderate | 1.23 (1.09-1.37) | <0.001 | 52.0% | |
| Geographic region | Europe | 1.29 (1.16-1.44) | <0.001 | 10.8% |
| America | 1.11 (1.01-1.21) | 0.026 | 0.0% | |
| Asia | 1.13 (0.88–1.44) | 0.350 | 64.5% | |
| Africa | – | – | – | |
| Smoking status of participants | Non-current smoker | 1.17 (1.01-1.36) | 0.037 | 53.7% |
| Current smoker | 1.91 (1.45-2.51) | <0.001 | 0.0% | |
| Exposing time of passive smoking | Childhood exposure | 1.20 (1.08-1.34) | 0.001 | 8.4% |
| Adulthood exposure | 1.18 (1.06-1.31) | 0.002 | 0.0% | |
| Frequency of passive smoking | Occasionally/Seldom/Few times per week | 1.08 (0.90–1.29) | 0.421 | 4.1% |
| Regularly/Daily | 1.28 (1.08-1.50) | 0.003 | 14.9% |
Figure 5: Forest plot of relative risk between passive smoking and pancreatic cancer in subgroup analysis.
(A) Study type. (B) Quality of study.Figure 6: Forest plot of relative risk between passive smoking and pancreatic cancer in subgroup analysis.
(A) Geographic region. (B) Smoking status of participants. (C) Exposing time of passive smoking. (D) Frequency of passive smoking.Quality of evidence
For the outcome of pancreatic cancer with both case-control studies, the quality of the evidence is “very low” (Table 3). No high-quality evidence was validated. The main reason for the downgrade of evidence was the limitations due to non-randomized controlled trials, significant heterogeneity in the overall analysis, and publication bias.
Discussion
In this comprehensive meta-analysis we found a positive association between passive smoking and risk of pancreatic cancer. The risk was valid in both case-control and cohort, high and moderate quality studies, in current and non-current smokers, and for both childhood and adulthood exposure. The current evidence supported that only regular or daily exposure, but not exposing occasionally, seldom or few times per week could increase the risk.
Previous meta-analysis conducted by Zhou, Wellenius & Michaud (2012) firstly concluded that exposure to ETS did not increase the risk of pancreatic cancer. With more population-based studies in our study, we demonstrated that the association was significant, as well as in subgroup analysis. The deleterious effect of current smoking on pancreatic cancer was well established (Klein, 2021; Momi et al., 2012; Weissman et al., 2020). However, the underlying mechanisms of passive smoking have not been fully clarified. In the case of passive smoking, concentrations of several procarcinogenic chemicals, such as 4-Aminobiphenyl, benzene, nickel compounds were much higher in sidestream smoke than mainstream smoke (Tredaniel et al., 1993; Woodward & McMichael, 1991). These chemicals above were known as important carcinogens in several types of cancers, such as bladder cancer (Van Hemelrijck et al., 2009), lung cancer (Sciannameo et al., 2019; Warden et al., 2018), leukemia (Lu, Shahbaz & Winn, 2020), non-Hodgkin lymphoma (Rana et al., 2021), etc. Besides, in animal studies, these chemicals have been proved to be associated with onset of pancreatic cancer (Antwi et al., 2015; Ogawa et al., 1998; Shirai et al., 1989).
| Certainty assessment | Effect | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Relative (95% CI) | ||
| Pancreatic cancer (case-control studies) | |||||||||
| 6 | Non-randomised studies | Serious | Not serious | Not serious | Not serious | Publication bias strongly suspected | OR 1.16 (1.03 to 1.31) | ⨁ Very low |
CRITICAL |
| Pancreatic cancer (cohort studies) (follow-up: range 8.9 years to 24 years) | |||||||||
| 8 | non-randomised studies | serious | Not serious | Not serious | Not serious | Publication bias strongly suspected | RR 1.26 (1.12 to 1.42) | ⨁ Very low |
CRITICAL |
Notes:
- CI
-
confidence interval
- OR
-
odds ratio
- RR
-
risk ratio
Apart from the findings that current and passive smoking contributed to the development of several types of cancer, smoking cessation or reduction may also attenuate the risks of cancer (Choi et al., 2018; Saito et al., 2017), even for heavy smokers (Saito et al., 2017). As for lung cancer, Faers et al. highlighted the beneficial effects for long durations of smoking cessation (particularly for those with quitting time of more than 5 years before cancer diagnosis) (Fares et al., 2023). They also concluded that the benefits were valid at any time of abstinence (Fares et al., 2023). Evidence from other research also showed beneficial results of smoking quitting or reduction for lung cancer (Chang et al., 2021; Choi et al., 2018). Besides, smoking cessation was also associated with decreased risk of other types of cancers, including esophageal squamous cell carcinoma (Wang et al., 2017; Xie et al., 2017), head and neck cancer (Marron et al., 2010; Wellmann, 1964), pancreatic cancer (Bosetti et al., 2012; Lynch et al., 2009), bladder cancer (Freedman et al., 2011; Jiang et al., 2012), etc, and help reduce cancer-related mortality (Lee et al., 2023), improve prognosis and quality of life (Gallaway et al., 2019; Von Kroge et al., 2020). Since our study proved that passive smoking contributed risk of pancreatic cancer, this meta-analysis also shed some light on possible prevention of pancreatic cancer following passive smoking cesstion, which needed further investigation.
Subgroup analysis further confirmed the association. As for the type and quality of included studies, results of both case-control and cohort studies, as well as high and moderate quality studies were consistent, indicating that the study type and quality did not affect the overall findings, which further confirmed the validity of the conclusion. For both current and non-current smokers, childhood and adulthood exposure, passive smoking displayed increased risk of pancreatic cancer. Besides, current smokers had 1.6-fold risk than non-current smokers. Another important finding was that only regular or daily exposure could induce the risk, but not for exposing occasionally, seldom, or few times per week. This trend was in accordance with the situation for current smoking, indicating that high frequency and long-time smoking could induce higher odds ratio (Bosetti et al., 2012). This conclusion did not definitely mean that occasional smoking may not cause detrimental effect on risk of pancreatic cancer. Only 4 studies were included in this subgroup analysis and a uniform conclusion should be drawn with more population-based studies included.
Our study has several limitations. Although we adopted available adjusted estimates in all included studies, unmeasured confounding factors and bias still remained, such as genetic differences, lifestyle-related factors, exclusion of non-English articles, etc. Besides, the certainty of the evidence was very low according to GRADE assessment, mainly due to non-randomised design and possible publication bias. In some subgroup analysis, the number of included studies was still limited, such as in workplace and home, exposure from one relative and two or more relatives. Therefore, more population-based studies were needed to verify these associations.
Conclusions
In conclusion, we found a significant association between passive smoking and risk of pancreatic cancer in both case-control and cohort studies. We also demonstrated that the risk was significant for both current and non-current smokers, for exposure both in adulthood and childhood. Current evidence indicated that only regular or daily exposure to passive smoking could increase the risk of pancreatic cancer. Our meta-analysis provides further concerns for prevention and surveillance strategies of pancreatic cancer.