The clinical value of KL-6 for predicting the occurrence and severity of connective tissue disease-associated interstitial lung disease is not affected by CTD type or treatment
- Published
- Accepted
- Received
- Academic Editor
- Ramcés Falfán-Valencia
- Subject Areas
- Immunology, Respiratory Medicine, Rheumatology
- Keywords
- Connective tissue disease associated interstitial lung disease, Krebs von den Lungen-6, Neutrophil to lymphocyte ratio, Systemic immune inflammation, Monocyte to lymphocyte ratio, Red blood cell distribution width
- Copyright
- © 2024 Xing and Liang
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. The clinical value of KL-6 for predicting the occurrence and severity of connective tissue disease-associated interstitial lung disease is not affected by CTD type or treatment. PeerJ 12:e17792 https://doi.org/10.7717/peerj.17792
Abstract
Objective
The aim of this study was to explore the potential values of Krebs von den Lungen-6 (KL-6), neutrophil to lymphocyte ratio (NLR), systemic immune inflammation (SII), platelet to lymphocyte ratio (PLR), monocyte to lymphocyte ratio (MLR) and red blood cell distribution width (RDW) in the diagnosis and evaluation of the severity of connective tissue disease-associated interstitial lung disease (CTD-ILD).
Methods
A total of 140 connective tissue disease (CTD) patients and 85 CTD-ILD patients were recruited for this study at Shanxi Provincial People’s Hospital from May 2022 to May 2023. Patients were divided into subgroups based on medication history and CTD subtypes to compare and analyze the clinical data and laboratory parameters of CTD-ILD patients and CTD patients. The receiver operating characteristic curve (ROC) was used to evaluate the diagnostic efficacy of KL-6, NLR, SII, PLR, MLR, and RDW in identifying CTD-ILD patients from CTD patients. A Spearman correlation analysis was conducted to elucidate the correlations between these markers and the lung function parameters of forced vital capacity (FVC, %), forced expired volume in one second (FEV1, %), and diffusing capacity of carbon monoxide (DLCO, %). Finally, binary logistic regression analysis was applied to discern the independent risk factors for CTD-ILD.
Results
NLR, SII, MLR, RDW, and KL-6 displayed significant statistical differences in the experimental groups. In both untreated and treated subgroups, KL-6 displayed higher values for CTD-ILD than CTD among all CTD subtypes. In untreated subgroups, there were significant differences in MLR levels between rheumatoid arthritis (RA) and RA-ILD patients and in NLR levels between Sjögren syndrome (SjS) and SjS-ILD patients. There were also significant differences in RDW-SD between the “other CTD” and “other CTD-ILD” groups. In treated subgroups, there were significant differences in both RDW-SD and RDW-CV between RA and RA-ILD patients and in NLR, SII, MLR, PLR, and RDW-SD between “other CTD” and “other CTD-ILD” groups. ROC revealed that KL-6 emerged as the most effective predictor for CTD-ILD in both treated and untreated groups. The multivariate logistic regression analysis results showed that both KL-6 and age were independent risk factors for CTD-ILD. NLR, SII, and PLR were negatively correlated with DLCO (%) in the untreated CTD-ILD group, and KL-6 was negatively correlated with various lung function parameters in both treated and untreated CTD-ILD groups.
Conclusion
KL-6 emerged as the most promising biomarker for diagnosing CTD-ILD and assessing its severity. The diagnostic value of KL-6 was unaffected by medication interference and surpassed the value of other parameters, such as NLR, SII, MLR, and RDW. The diagnostic value of RDW-SD was higher than that of RDW-CV in CTD-ILD patients. NLR, SII, MLR, and PLR have potential value in diagnosing the different types of CTD-ILD.
Introduction
Connective tissue disease (CTD) is a group of systemic autoimmune disorder diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (SSC) and so on (Cerro Chiang & Parimon, 2023; Pugashetti et al., 2023). The disease, orchestrated by the autoimmune system and its main pathological feature of CTD is chronic inflammation of blood vessels and connective tissues, which can affect any organ within the body. Lung tissue, which has an abundance of collagen and blood vessels, is particularly vulnerable to damage (Pugashetti et al., 2023; Shao et al., 2021). Interstitial lung disease (ILD) is a prevalent extra-articular complication of CTD, characterized by progressive pulmonary fibrosis and inflammation (Cerro Chiang & Parimon, 2023; Joy et al., 2023; Wu et al., 2023). The coexistence of CTD and ILD leads to a decline in quality of life and can even result in death (Wu et al., 2023). The exact pathogenic mechanism of connective tissue disease associated interstitial lung disease (CTD-ILD) is still unknown. It has been reported that CTD-ILD occurrence is related to alveolar epithelial cell injury, abnormal fibroproliferation, immune activation, excessive deposition of extracellular matrix (ECM), and vascular and endothelial cell damage (Jee et al., 2019). Some scholars propose that CTD-ILD may arise from damage to alveolar epithelial cells or the vascular system followed by abnormal activation of the immune system, leading to the infiltration of inflammatory cells into the lung interstitial and alveolar spaces, the activation of pulmonary fibroblasts producing ECM, especially the imbalance of collagen formation and degradation. This imbalance leads to the excessive deposition of collagen in the lung, causing chronic lung inflammation and pulmonary fibrosis (Shao et al., 2021; Wells & Denton, 2014). To date, there is still a lack of effective biomarkers for the diagnosis of CTD-ILD and the assessment of disease progression.
The subtypes of CTD are chronic inflammatory autoimmune diseases that exhibit distinct pathological features. For instance, RA is characterized by synovitis and vasculitis. SSc is characterized by inflammation and fibrosis in the skin and multiple internal organs. Polymyositis and dermatomyositis (PM/DM) is characterized by skeletal muscle inflammation and weakness. SjS primarily affects exocrine glands, such as tear and salivary glands. SLE involves tissue damage caused by acute immune complex deposition, affecting multiple systems throughout the body. ANCA-associated vasculitis(AAV) involves vasculitis (Wu et al., 2023). Undifferentiated connective tissue disease (UCTD) and mixed connective tissue disease (MCTD) may exhibit overlapping features CTD subtypes (Kondoh et al., 2021; Rubio & Kyttaris, 2023). The risk of progressive fibrosis phenotypes varies in different types of CTD-ILD (Yoo et al., 2022). Autoantibodies (anti-JO-1, anti-MDA5, et al.) may also impact ILD progression in CTD patients by inducing damage to lung epithelial cells (Richards et al., 2009).
High-resolution computed tomography (HRCT) and the pulmonary function test (PFT) are commonly used in clinical settings for the diagnosis and evaluation of CTD-ILD (Lee et al., 2019). However, these methods are not ideal. Because of the risk of radiation exposure when using HRCT, some patients are reluctant to be examined with repeated CT scans (Ahmed & Handa, 2022). Interpreting PFT results can be challenging in ILD patients who also have coexisting lung conditions such as bronchiectasis or diffuse alveolar hemorrhage (Wells, 2007). In clinical practice, some CTD patients have no symptoms or only mild respiratory symptoms but may have significant abnormal chest imaging and lung function impairment. As a result, the presence or severity of respiratory symptoms may not be completely consistent with diagnoses made using HRCT or PFT (Wang et al., 2021). There is a need for biomarkers that are easily obtainable, minimally invasive, and cost-effective for the diagnosis and assessment of CTD-ILD.
Krebs von den Lungen-6 (KL-6) a MUCIN 1 protein that is overexpressed when type II alveolar cells are damaged. KL-6 was initially used as a serum biomarker for diagnosing lung, breast, and pancreatic cancer, but its diagnostic accuracy was limited (Conticini et al., 2022; Jehn et al., 2023). Serum KL-6 has been proposed as a potentially effective biomarker for diagnosing and assessing the severity of CTD-ILD (Wang et al., 2023). KL-6 can differentiate between fibrotic lung diseases and non-fibrotic lung diseases (Oguz et al., 2016). However, there is still some controversy regarding the differential diagnosis of CTD-ILDs (Oguz et al., 2016; Zhou et al., 2023).
Red blood cell distribution width (RDW) is a key metric in routine blood tests, serving to gauge diversity in red blood cell sizes. RDW is a vital tool in the differential diagnosis of anemia because it is an accessible, low-cost metric (Song et al., 2023). A previous study showed that CTD-ILD pathogenesis is related to changes in red blood cell parameters, such as RDW, caused by oxidative stress and bone marrow hematopoietic function impairment, indicating that RDW elevation is associated with CTD-ILD pathogenesis (Shi et al., 2021).
It is well-established that innate and adaptive immunity play a key role in CTD-ILD pathogenesis. Macrophages (precursors of monocytes), neutrophils, and lymphocytes are capable of exerting pro-fibrotic effects on alveolar cell damage and inflammation activation in pulmonary fibrosis. The neutrophil to lymphocyte ratio (NLR), systemic immune inflammation (SII), monocytes to lymphocytes ratio (MLR), and platelet-lymphocyte ratio (PLR) are indicators of systemic inflammation and immune status (Lu et al., 2022; Fei et al., 2020). These indicators are elevated in CTD patients, especially in patients with CTD-ILD (Ruta et al., 2020). Therefore, NLR, SII, PLR, and MLR have potential value in the clinical diagnosis and prognosis of CTD-ILD.
This study analyzed serum KL-6, RDW, NLR, SII, MLR, and PLR, which can all be obtained through Serum sample measurements and routine blood tests (Ruta et al., 2020), aiming to evaluate their potential values in diagnosing and measuring severity in CTD-ILD patients.
Materials and Methods
Study population
This study recruited CTD patients (including all subtypes such as SLE, RA, SSC, SS, etc.) who were hospitalized in Shanxi Provincial People’s Hospital from May 2022 to May 2023. The included participants were divided into two groups: the CTD-ILD group and the CTD group. The participants were further divided into two subgroups: the untreated group, and the treated group, consisting of those who had undergone medication therapy for CTD at lesat within the past three months. Antifibrotic therapy was not administered to recruited patients. Participants were also divided into five groups based on CTD subtypes: RA, PM/DM, SSc, SjS, and “other CTD,” which included SLE, AAV, UCTD, and MCTD patients.
Inclusion and exclusion criteria
CTD patients were diagnosed based on the current standards (Aggarwal et al., 2017; Alarcon-Segovia & Cardiel, 1989; Aletaha et al., 2010; Aringer et al., 2019; Leavitt et al., 1990; Mosca, Neri & Bombardieri, 1999; Shiboski et al., 2012; van den Hoogen et al., 2013). ILD patients were diagnosed based on the 2013 American Thoracic Society (ATS)/European Respiratory Society (ERS) criteria for idiopathic interstitial pneumonia (IIP; Travis et al., 2013).
Subjects were excluded based on the following exclusion criteria: (a) presence of other lung diseases, such as COPD or asthma; (b) significant heart, liver, or kidney diseases; (c) coexistence of tuberculosis or malignant tumor; (d) hematological disorders; (e) presentation with multiple organ failure.
Ethics approval
This study adhered to the principles stated in the Declaration of Helsinki and received approval from the Medical Ethics Committee of Shanxi Provincial People’s Hospital (Ethical application Ref: 2023-229). All participants provided written informed consent prior to study participation.
Data collection and samples
All patients laboratory examination data was collected or examined prior to the current hospital admission. Some demographic characteristics were obtained from hospital records, such as age, gender, smoking history, hypertension history, diabetes history, and medication history, etc. In addition, a fasting 5 mL venous blood sample was collected from each patient at the elbow without any treatment. Blood samples were placed in a test tube containing ethylenediaminetetraacetic acid (EDTA) anticoagulant for routine blood examination and the NLR, SII, PLR, and MLR were calculated in each sample. The serum was then stored at −80 °C for further investigation.
Serum levels of KL-6
KL-6 was measured using a biochemical analyzer (Beckman Coulter AU5800) in conjunction with a kit provided by the manufacturer (Sekisui Medical Co., LTD, Tokyo, Japan) for the latex agglutination test.
Statistical methods
SPSS 26.0 software was used for the statistical analysis. Continuous variable data conforming to a normal distribution were expressed as mean ± standard deviation ( ± SD). The t test was employed for comparisons between two groups. The one-way analysis of variance (ANOVA) was used for comparisons between multiple groups. The Pearson correlation analysis was used to determine correlation between indicators. The non-normal distribution data were expressed as median and interquartile range, described with “M (P25, P75),” and compared using a non-parametric test. The Spearman correlation analysis was used to explore the correlation among parameters in the CTD-ILD groups. Categorical variables were expressed as frequency rates and percentages (%) and analyzed using an χ2 test.
Binary logistic regression analysis was applied to determine associations between laboratory indicators and CTD-ILD risk. The variables that were significant (P < 0.05) in the univariate analysis were included in the multivariate logistic regression analysis and were used to identify the independent risk factors of CTD-ILD. A receiver operating characteristic curve (ROC) analysis was conducted to evaluate the diagnostic efficacy of the indicators in predicting the incidence of CTD-ILD; the cut-off values were also calculated. An AUC of 1.0 denotes perfect diagnostic efficacy; an AUC > 0.9 indicates high diagnostic efficacy; an AUC ranging from 0.7 to 0.9 signifies moderate diagnostic efficacy; an AUC between 0.5 and 0.7 suggests low diagnostic efficacy; an AUC < 0.5 implies no diagnostic efficacy. P < 0.05 was considered statistically significant.
Results
General characteristics of the study population
The clinical characteristics of the enrolled patients are summarized in Table 1. The collected samples were screened according to the strict inclusion and exclusion criteria. There were 85 patients in the CTD-ILD group and 140 patients in the CTD group, respectively. Age, gender, BMI, smoking history, medication history, comorbidities, and CTD subtype were compared between these two groups. The CTD-ILD group consisted of 31 male and 54 female participants with an average age of 62.82 ± 12.81 years. The CTD group consisted of 27 male and 113 female individuals with an average age of 47.54 ± 17.35 years. There were significant differences in age and gender between the two groups, with a higher proportion of older and male participants in the CTD-ILD group (P < 0.05). Additionally, notable differences were observed in smoking, diabetes, dyspnea, cough, and CTD subtype between the two groups. However, there were no statistically significant differences between the CTD-ILD and CTD groups in BMI, medication history, hypertension, hospital stays, and CTD evolution time. There was also no statistically significant difference between the treated and untreated CTD-ILD groups in pulmonary function test parameters (Table S1).
| Parameters | CTD-ILD (n = 85) | CTD (n = 140) | P value |
|---|---|---|---|
| Age (years) | 62.82 ± 12.81 | 47.54 ± 17.35 | <0.001* |
| Sex, n (%) | 0.004* | ||
| Female | 54 (63.53%) | 113 (80.71%) | |
| Male | 31 (36.47%) | 27 (19.29%) | |
| BMI (kg/m2) | 23.05 (21.26, 25.95) | 22.88 (20.70, 25.47) | 0.220 |
| Smoking history, n (%) | 0.013* | ||
| Yes | 17 (20%) | 12 (8.57%) | |
| No | 68 (80%) | 128 (91.43%) | |
| Medication history, n (%) | 0.797 | ||
| Yes | 44 (51.76%) | 70 (50%) | |
| No | 41 (48.24%) | 70 (50%) | |
| Comorbidities, n (%) | |||
| Hypertension, n (%) | 28 (32.94%) | 39 (27.86%) | 0.419 |
| Diabetes, n (%) | 15 (17.65%) | 11 (7.86%) | 0.026* |
| Dyspnea, n (%) | 47 (55.29%) | 5 (3.57%) | <0.001* |
| Cough, n (%) | 41 (48.24%) | 11 (7.86%) | <0.001* |
| Hospital stays (day) | 11 (8, 14) | 9 (7, 14) | 0.379 |
| Specific CTDs | <0.001* | ||
| RA | 26 (30.59%) | 42 (30%) | |
| SLE | 4 (4.71%) | 31 (22.14%) | |
| PM/DM | 12 (14.12%) | 6 (4.29%) | |
| SSc | 8 (9.41%) | 0 (0%) | |
| SjS | 11 (12.94%) | 25 (17.86%) | |
| UCTD | 11 (12.94%) | 3 (2.14%) | |
| MCTD | 7 (8.24%) | 24 (17.14%) | |
| AAV | 6 (7.06%) | 9 (6.43%) | |
| Evolution time (year) | 3 (0.68, 5) | 3 (0.52, 8) | 0.478 |
| Medicine | |||
| GC, n (%) | 36 (81.82%) | 48 (68.57%) | |
| ISDS, n (%) | |||
| MTX | 7 (15.91%) | 11 (15.71%) | |
| MMF | 6 (13.64%) | 13 (18.57%) | |
| CYC | 8 (18.18%) | 9 (12.86%) | |
| HCQ | 8 (18.18%) | 20 (28.57%) | |
| LEF | 6 (13.64%) | 13 (18.57%) | |
| TWHF, n (%) | 10 (22.73%) | 8 (11.43%) | |
| NSAIDS, n (%) | 4 (9.10%) | 7 (10%) | |
| CNIS, n (%) | 1 (2.27%) | 4 (5.71%) | |
| Biological agents, n (%) | 1 (2.27%) | 4 (5.71%) |
Notes:
BMI, body mass index; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; PM/DM, polymyositis/dermatomyositis; SSc, systemic sclerosis; SjS, Sjögren syndrome; UCTD, unspecified connective tissue disease; MCTD, mixed connective tissue; AAV, ANCA-associated vasculitis. GC, Glucocorticoids; ISDS, Immunosuppressive drugs; MTX, Methotrexate; MMF, mycophenolate mofetil; CYC, Cyclophosphamide; HCQ, Hydroxychbroquine; LEF, Leflunomide; TWHF, Tripterygium Wilfordii Hook F; NSAIDS, Non-steroidal anti-inflammatory drugs; CNIS, Calcineurin inhibitors.
The levels of KL-6, NLR, SII, RDW, MLR, and PLR
KL-6, NLR, SII, RDW, MLR, and PLR levels are shown in Table 2. The enrolled patients were divided into CTD-ILD and CTD groups and various indicators were compared. The levels of white blood cells (WBC), neutrophils (Neu), monocytes (Mono), NLR, SII, MLR, RDW-SD, RDW-CV, and KL-6 in the CTD-ILD group were all higher than those in the CTD group, demonstrating significant differences between the two groups. Conversely, no significant differences were observed in lymphocytes (Lym), platelets (PLT), PLR, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin (Alb), D-dimer, and inflammatory markers (ESR, CRP) between the CTD-ILD and CTD groups (P > 0.05).
| Variables | CTD-ILD (n = 85) | CTD (n = 140) | P value |
|---|---|---|---|
| WBC (×109/L) | 7.34 (5.24, 9.26) | 5.10 (3.77, 7.26) | <0.001* |
| Neu (×109/L) | 4.67 (3.26, 6.92) | 3.11 (2.12, 4.82) | <0.001* |
| Lymph (×109/L) | 1.38 (0.93, 1.87) | 1.33 (1.04, 1.85) | 0.949 |
| Mono (×109/L) | 0.49 (0.35, 0.70) | 0.38 (0.27, 0.52) | <0.001* |
| PLT (×109/L) | 220 (162.50, 294.50) | 214 (171.25, 277.50) | 0.653 |
| NLR | 3.40 (2.09, 6.02) | 2.13 (1.46, 3.82) | <0.001* |
| SII (×109/L) | 684.68 (360.35, 1321.48) | 500.26 (282.75, 929.72) | 0.006* |
| MLR | 0.37 (0.23, 0.54) | 0.27 (0.21, 0.39) | 0.002* |
| PLR | 162.26 (101.87, 226.24) | 156.62 (120.03, 216.77) | 0.955 |
| RDW-SD (fL) | 48.40 (45, 51.40) | 44.40 (42, 48.10) | <0.001* |
| RDW-CV (%) | 14 (13.2, 15.15) | 13.40 (12.7, 14.68) | 0.003* |
| KL-6 (U/mL) | 877.47 (425.37, 1526.94) | 182.71 (137.80, 233.21) | <0.001* |
| AST | 18.23 (12.05, 28.06) | 18.37 (11.93, 32.57) | 0.868 |
| ALT | 23.18 (17.64, 31.12) | 21.09 (16.27, 32.88) | 0.293 |
| Alb | 33.67 (31.09, 38.03) | 35.70 (30.89, 38.59) | 0.339 |
| D-Dimer (ng/mL) | 349 (154.25, 893.25) | 255.50 (120, 633) | 0.191 |
| Inflammatory marker | |||
| CRP (mg/L) | 8.37 (3.34, 26.14) | 3.75 (3.30, 18.20) | 0.126 |
| ESR (mm/h) | 40 (16, 59.5) | 26 (13, 58.75) | 0.113 |
| Autoimmune antibody | NA | ||
| ANA (+) (n, %) | 57 (67.06%) | 100 (71.43%) | |
| dsDNA (+) | 2 (2.35%) | 22 (15.71%) | |
| SSA (+) | 15 (17.65%) | 52 (37.14%) | |
| SSB (+) | 4 (4.71%) | 21 (15.00%) | |
| Scl-70 (+) | 8 (9.41%) | 0 (0%) | |
| Ro52 (+) | 29 (34.12%) | 51 (36.43%) | |
| Jo.1 (+) | 5 (5.88%) | 1 (0.71%) | |
| CCP (+) | 28 (32.94%) | 46 (32.86%) | |
| RF (+) | 47 (55.29%) | 75 (53.57%) | |
| PFT index | |||
| FVC | 2.36 (1.96, 3.02) | – | |
| FVC% | 80 (67.5, 97) | – | |
| FEV1 | 2 ± 0.57 | – | |
| FEV1% | 83.26 ± 21.11 | – | |
| DLCO | 4.17 (3.26, 5.69) | – | |
| DLCO% | 56 (45.5, 72) | – |
Note:
WBC, white blood cell; Neu, neutrophil; Lym, lymphocyte; Mono, monocyte; PLT, platelet; NLR, Neutrophil to lymphocyte ratio; SII, systemic immune inflammation; MLR, Monocyte to lymphocyte ratio; PLR, Platelet to lymphocyte ratio; RDW-SD, Red blood cell distribution width standard deviation; RDW-CV, Red blood cell distribution width coefficient of variation; KL-6, Krebs von den Lungen-6; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; PFT, pulmonary function test; FVC, forced vital capacity; FEV1, forced expired volume in one second; DLCO, diffusing capacity of the lungs for carbon monoxide. *P < 0.05.
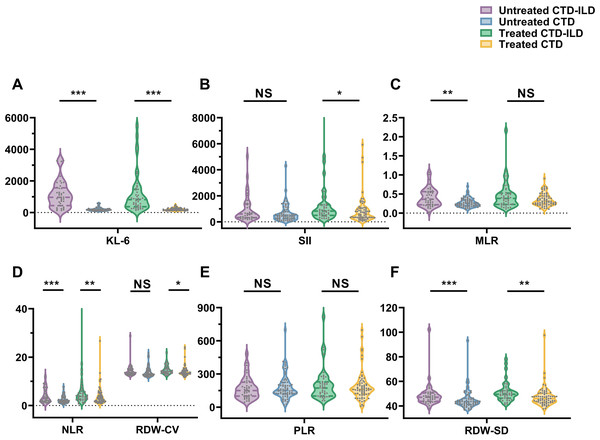
The clinical data was further analyzed by dividing the patients into treated and untreated subgroups based on medication history (Table 3 and Fig. 1). In both the treated and untreated subgroups, there were significant statistical differences in clinical data such as WBC, RDW-SD, NLR, and KL-6 between CTD-ILD and CTD patients, with higher values observed in the CTD-ILD patients. There were no significant differences observed for Lym, PLT, PLR, AST, ALT, Alb, D-Dimer, CRP, and ESR between CTD-ILD and CTD patients. Within the untreated group, CTD-ILD patients exhibited higher monocyte counts and MLR than CTD patients (P < 0.05). There were also significant differences in Neu, RDW-CV, and SII between CTD patients and CTD-ILD patients in the treated group.
| Variables | Untreated group | P value | Treated group | P value | ||
|---|---|---|---|---|---|---|
| CTD-ILD (n = 41) | CTD (n = 70) | CTD-ILD (n = 44) | CTD (n = 70) | |||
| WBC (×109/L) | 7.14 (5.41, 8.57) | 4.70 (3.48, 6.92) | <0.001* | 7.62 (5.13, 9.58) | 5.17 (4.02, 7.56) | 0.002* |
| Neu (×109/L) | 4.31 (3.19, 6.49) | 2.62 (1.90, 4.47) | 0.821 | 5.27 (3.46, 7.20) | 3.74 (2.23, 5.18) | 0.002* |
| Lymph (×109/L) | 1.55 (1.00, 1.86) | 1.49 ± 0.64 | 0.821 | 1.31 (0.84, 1.89) | 1.28 (1.01, 1.68) | 0.845 |
| Mono (×109/L) | 0.51 (0.33, 0.71) | 0.36 (0.25, 0.50) | 0.001* | 0.45 (0.36, 0.69) | 0.40 (0.29, 0.55) | 0.108 |
| PLT | 220 (147, 313) | 233.91 ± 108.84 | 0.966 | 229.07 ± 77.20 | 213 (172.75, 258.25) | 0.399 |
| NLR | 2.80 (1.70, 6.15) | 1.98 (1.34, 3.35) | 0.008* | 3.81 (2.60, 6.00) | 2.84 (1.57, 4.47) | 0.020* |
| SII (×109/L) | 601.17 (319.06, 1,355.71) | 470.75 (253.96, 797.44) | 0.088 | 826.92 (495.04, 1,307.04) | 578.30 (326.92, 1,024.17) | 0.033* |
| MLR | 0.36 (0.22, 0.56) | 0.25 (0.19, 0.34) | 0.002* | 0.38 (0.23, 0.52) | 0.31 (0.23, 0.45) | 0.198 |
| PLR | 150.83 (100.24, 225.95) | 151.27 (120.98, 222.60) | 0.691 | 173.06 (101.45, 228.44) | 160.86 (118.34, 212.41) | 0.594 |
| RDW-SD (fL) | 47.10 (43.75, 50.60) | 43.45 (41.28, 46.25) | <0.001* | 49.50 (46.30, 52.55) | 46.10 (43.08, 50.23) | 0.001* |
| RDW-CV (%) | 13.60 (13.05, 15.05) | 13.05 (12.50, 14.80) | 0.062 | 14.10 (13.43, 15.83) | 13.50 (13.08, 14.60) | 0.010* |
| KL-6 (U/mL) | 962.07 (438.99, 1,561.03) | 170.35 (126.93, 207.37) | <0.001* | 818.51 (370.05, 1,462.76) | 191.96 (142.46, 246.46) | <0.001* |
| AST | 17.72 (11.96, 29.94) | 18.98 (12.44, 35.98) | 0.730 | 19.24 (12.14, 25.61) | 15.39 (11.15, 30.17) | 0.552 |
| ALT | 24.31 (18.06, 32.50) | 21.13 (16.62, 35.07) | 0.604 | 22.43 (17.41, 29.39) | 21.08 (15.92, 31.43) | 0.337 |
| Alb | 33.84 ± 5.44 | 34.96 ± 6.47 | 0.358 | 34.28 ± 5.84 | 35.33 (31.63, 38.11) | 0.842 |
| D-Dimer (ng/mL) | 349 (163.25, 911.25) | 314.50 (91.75, 739) | 0.284 | 370 (101.25, 893.25) | 233.50 (122.75, 516.75) | 0.442 |
| Inflammatory marker | ||||||
| CRP (mg/L) | 5.81 (3.22, 14.36) | 3.46 (3.30, 13.99) | 0.492 | 14.50 (3.34, 49.20) | 5 (3.26, 24.50) | 0.133 |
| ESR (mm/h) | 38 (18,60) | 28 (14, 60) | 0.524 | 40 (15.75, 56.25) | 23 (10, 49) | 0.096 |
Notes:
WBC, white blood cell; Neu, neutrophil; Lym, lymphocyte; Mono, monocyte; PLT, platelet; NLR, Neutrophil to lymphocyte ratio; SII, Systemic immune inflammation; MLR, Monocyte to lymphocyte ratio; PLR, Platelet to lymphocyte ratio; RDW-SD, Red blood cell distribution width standard deviation; RDW-CV, Red blood cell distribution width coefficient of variation; KL-6, Krebs von den Lungen-6; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate.
Figure 1: Comparisons of (A) KL-6 (U/ml); (B) SII (×109/L); (C) MLR; (D) NLR; (D) RDW-CV (%); (E) PLR; and (F) RDW-SD (fL) in CTD-ILD and CTD patients based on medication history.
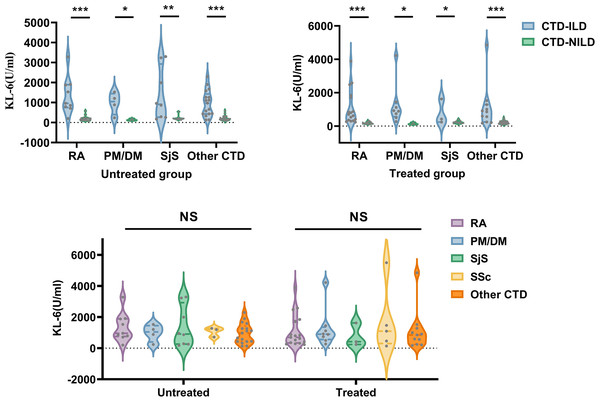
* P < 0.05, **P < 0.01, ***P < 0.001, NS, Not statistically significant. KL-6, Krebs von den Lungen-6; SII, Systemic immune inflammation; MLR, Monocyte to lymphocyte ratio; NLR, Neutrophil to lymphocyte ratio; RDW-CV, Red blood cell distribution width coefficient of variation; PLR, Platelet to lymphocyte ratio; RDW-SD, Red blood cell distribution width standard deviation.The results of the KL-6 analysis in the CTD and CTD-ILD subgroups are shown in Fig. 2. In both the untreated and treated subgroups, KL-6 was higher in CTD-ILD patients than in CTD patients among all CTD subtypes (P < 0.05). In untreated subgroups, there were significant differences in MLR levels between RA and RA-ILD patients, NLR levels between SjS and SjS-ILD patients, and RDW-SD levels between the “other CTD” and “other CTD-ILD” groups. In treated subgroups, there was a significant difference in RDW (RDW-SD and RDW-CV) levels between RA and RA-ILD patients. There were also significant differences between the “other CTD” and “other CTD-ILD” groups in NLR, SII, MLR, PLR, and RDW-SD levels (Table 4).
Figure 2: Comparison of KL-6 in CTD-ILD and CTD patients based on CTD subtypes and medication history.
KL-6, Krebs von den Lungen-6; RA, rheumatoid arthritis; PM/DM, polymyositis/dermatomyositis; SjS, Sjögren syndrome; Other CTD, systemic lupus erythematosus, unspecified connective tissue disease, mixed connective tissue and ANCA-associated vasculitis. *P < 0.05, **P < 0.01, ***P < 0.001, NS, Not statistically significant.| RA-ILD | RA | P | PM/DM-ILD | PM/DM | P | SjS-ILD | SjS | P | Other CTD-ILD | Other CTD | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Untreated group | ||||||||||||
| NLR | 3.35 (1.81, 6.61) |
2.10 (1.70, 3.73) |
0.304 | 4.70 ± 3.31 | 1.68 ± 0.48 | 0.165 | 3.44 ± 2.34 | 1.38 (1.01, 1.95) |
0.021* | 2.42 (1.44, 6.91) |
2.13 (1.26, 3.71) |
0.294 |
| SII | 724.03 (348.68, 1,440.84) |
644.87 (415.62, 1,149.08) |
0.572 | 926.37 ± 571.50 | 305.51 ± 221.45 | 0.140 | 547.94 (257.32, 1,687.77) |
272.75 (188.61, 387.96) |
0.054 | 379.75 (294.97, 662.12) |
470.75 (180.73, 779.97) |
0.721 |
| MLR | 0.39 ± 0.15 | 0.25 ± 0.08 | 0.023* | 0.55 ± 0.26 | 0.22 ± 0.05 | 0.082 | 0.36 ± 0.18 | 0.29 ± 0.08 | 0.296 | 0.29(0.17, 0.58) | 0.24(0.17, 0.36) | 0.350 |
| PLR | 183.96 (89.78, 216.23) |
160.90 (124.37, 221.57) |
0.850 | 194.57 ± 112.04 | 63.30 ± 42.03 | 0.117 | 138.83 (98.77, 216.21) |
131.37 (104.42, 208.63) |
0.877 | 132.08 (83.47, 185.44) |
166.28 (122.35, 240.68) |
0.223 |
| RDW-SD | 46.21 ± 4.14 | 43.6 (41.8, 45.2) |
0.216 | 45.15 (43.8, 47.55) |
43.90 ± 7.54 | 0.480 | 45.2 ± 2.87 | 44.28 ± 4.95 | 0.643 | 49 (44.75, 54.85) |
43.4 (40.43, 46.35) |
0.003* |
| RD-CV | 13.41 ± 0.73 | 13.1 (12.2, 14.2) |
0.769 | 13.25 (13.2, 14.125) |
13.43 ± 1.12 | 0.476 | 13.180.64 | 12.7 (12.025, 14.6) |
0.699 | 15 (13.05, 15.95) |
13.15 (12.7, 14.95) |
0.077 |
| Treated group | ||||||||||||
| NLR | 3.96 (2.42, 7.94) |
2.91 (1.59, 4.15) |
0.117 | 2.86 ± 1.27 | 2.51 ± 1.02 | 0.685 | 4.79 ± 1.19 | 1.99 (1.45, 5.09) |
0.201 | 5.31 (3.75, 10.53) |
2.92 (1.78, 5.13) |
0.021* |
| SII | 898.63 (557.84, 1,640.46) |
808.98 (363.17, 1,063.58) |
0.318 | 659.36 ± 328.85 | 788.27 ± 443.63 | 0.607 | 711.55 ± 117.13 | 330.45 (81.91, 760.80) |
0.158 | 1,336.29 (808.04, 2,412.34) |
558.19 (329.16, 1,049.35) |
0.032* |
| MLR | 0.4 (0.24, 0.52) |
0.34 ± 0.16 | 0.318 | 0.34 ± 0.20 | 0.28 ± 0.05 | 0.639 | 0.31 ± 0.13 | 0.28 (0.22, 0.51) |
0.840 | 0.59 ± 0.32 | 0.36 ± 0.15 | 0.035* |
| PLR | 175.42(97.71, 252.56) | 170.97 (129.82, 256.44) |
0.788 | 131.66 ± 57.28 | 155.59 ± 5.13 | 0.279 | 176.33 ± 68.44 | 152.15 (37.48, 210.54) |
0.459 | 257.97 (203.13, 313.70) |
160.90 (111.24, 201.77) |
0.021* |
| RDW-SD | 49.8 (46.6, 57.9) |
46.14 ± 4.17 | 0.015* | 48.95 ± 4.03 | 48.27 ± 0.90 | 0.784 | 57.8 ± 17.88 | 50.2 (44.5, 53.95) |
0.590 | 49.62 ± 4.17 | 44.8 (43, 47.8) |
0.015* |
| RD-CV | 15.21 ± 1.81 | 13.84 ± 1.23 | 0.012* | 13.94 ± 0.82 | 14 ± 0.60 | 0.908 | 16.1 ± 5.02 | 13.3 (13, 14.9) |
0.787 | 14.1 (13.6, 15.1) |
13.4 (13.1, 14.4) |
0.077 |
Notes:
NLR, Neutrophil to lymphocyte ratio; SII, Systemic immune inflammation; MLR, Monocyte to lymphocyte ratio; PLR, Platelet to lymphocyte ratio; RDW-SD, Red blood cell distribution width standard deviation; RDW-CV, Red blood cell distribution width coefficient of variation; RA, rheumatoid arthritis; PM/DM, polymyositis/dermatomyositis; SjS, Sjögren syndrome; Other CTD, systemic lupus erythematosus, unspecified connective tissue disease, mixed connective tissue and ANCA-associated vasculitis.
Analysis within the CTD-ILD subtype group is shown in Table S2. RDW-CV levels differed significantly among multiple untreated CTD-ILD subgroups, and NLR and PLR levels differed significantly among multiple subgroups. However, the Bonferroni correction analysis did not reveal significant differences in any indicator between any two groups (Table S2).
Correlation of these markers with inflammatory factors in CTD-ILD
Correlations between KL-6, NLR, SII, PLR, MLR, and RDW-SD and inflammatory factors (CRP and ESR) were analyzed in CTD-ILD and CTD patients (Table S3 and Fig. 3).
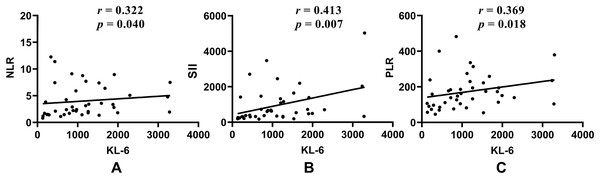
Figure 3: Correlations of KL-6 with (A) NLR; (B) SII; and (C) PLR in untreated CTD-ILD patients.
KL-6, Krebs von den Lungen-6; NLR, Neutrophil to lymphocyte ratio; SII, Systemic immune inflammation; PLR, Platelet to lymphocyte ratio.In the untreated subgroup, NLR, SII, and PLR were all positively correlated with CRP or ESR (P < 0.05) in both CTD-ILD and CTD patients. MLR was only positively correlated with CRP in CTD-ILD patients (r = 0.420, P = 0.006). KL-6 was only positively correlated with ESR in CTD patients (r = 0.344, P = 0.004), but KL-6 was positively correlated with NLR, SII, and PLR in untreated CTD-ILD patients (Fig. 3).
Within the treated subgroup, inflammatory factors were positively correlated with NLR, SII, and RDW-CV in the CTD-ILD group and with NLR, SII, and PLR in the CTD group. Notably, no correlation was observed between KL-6 and inflammatory factors in either the CTD-ILD or the CTD group (P > 0.05).
Binary logistic regression analysis for CTD-ILD
In the present study, the univariate logistic regression analysis identified age, gender, smoking, diabetes, dyspnea, cough, WBC, Mono, NLR, SII, MLR, RDW-SD, and KL-6 as risk factors for CTD-ILD (Table 5). After adjusting for age, sex, smoking history, diabetes, dyspnea, and cough, a multivariate logistic regression analysis indicated that both age (OR = 1.076, 95% CI [1.030–1.123], P = 0.001) and KL-6 (OR = 1.010, 95% CI [1.006–1.014], P < 0.001) were independent risk factors for CTD-ILD (Table 5).
| Parameters | β | OR [95% CI] | P value | Adjusted β | Adjusted OR [95% CI] | P value |
|---|---|---|---|---|---|---|
| Age | 0.065 | 1.067 [1.044–1.090] | <0.001 | 0.073 | 1.076 [1.030–1.123] | 0.001* |
| Gender (Female) | 0.877 | 2.403 [1.306–4.419] | 0.005 | 0.062 | 1.064 [0.228–4.972] | 0.937 |
| Smoking history | 0.996 | 2.706 [1.221–5.999] | 0.014 | −2.183 | 0.113 [0.011–1.156] | 0.066 |
| Diabetes | 0.921 | 2.513 [1.095–5.767] | 0.030 | 0.718 | 2.050 [0.437–9.627] | 0.363 |
| Dyspnea | 3.508 | 33.395 [12.412–89.849] | <0.001 | 1.910 | 6.751 [0.952–47.897] | 0.056 |
| Cough | 2.391 | 10.928 [5.170–23.095] | <0.001 | 1.406 | 4.078 [0.740–22.476] | 0.107 |
| WBC | 0.167 | 1.182 [1.078–1.295] | <0.001 | 0.235 | 1.265 [0.878–1.822] | 0.207 |
| Mono | 1.947 | 7.006 [2.346–20.916] | <0.001 | −0.616 | 0.540 [0.004–73.629] | 0.806 |
| NLR | 0.132 | 1.141 [1.036–1.256] | 0.007 | −0.028 | 0.972 [0.746–1.266] | 0.833 |
| SII | 0.000 | 1.000 [1.000–1.001] | 0.025 | 0.000 | 1.000 [0.999–1.001] | 0.638 |
| MLR | 2.868 | 17.600 [3.851–80.430] | <0.001 | 1.163 | 3.201 [0.115–89.190] | 0.493 |
| RDW-SD | 0.055 | 1.057 [1.016–1.099] | 0.005 | 0.027 | 1.028 [0.968–1.091] | 0.371 |
| KL-6 | 0.009 | 1.009 [1.006–1.013] | <0.001 | 0.010 | 1.010 [1.006–1.014] | <0.001* |
Notes:
WBC, white blood cell; Mono, monocyte; NLR, Neutrophil to lymphocyte ratio; SII, Systemic immune inflammation; MLR, Monocyte to lymphocyte ratio; PLR, Platelet to lymphocyte ratio; RDW-SD, Red blood cell distribution width standard deviation; KL-6, Krebs von den Lungen-6.
ROC curve analysis
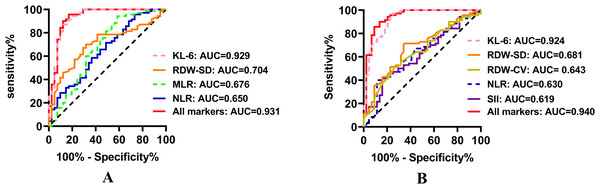
As delineated in Fig. 4 and Table S4, an ROC curve analysis was performed to assess the efficacy of NLR, SII, MLR, PLR, RDW-SD, RDW-CV, and KL-6 in predicting ILD occurrence among CTD patients in both the untreated and treated groups. KL-6 exhibited the highest predictive capability for CTD-ILD in both the untreated (AUC = 0.929, cut-off value = 255.88 U/mL) and treated group (AUC = 0.924, cut-off value = 401.41 U/mL); NLR and RDW-SD also demonstrated predictive efficacy in distinguishing CTD from CTD-ILD patients in both the untreated and treated groups.
Figure 4: Receiver operating characteristic curve of the efficacy of NLR, SII, MLR, PLR, RDW-SD, RDW-CV, and KL-6 in predicting ILD occurrence among CTD patients in both the (A) untreated and (B) treated groups.
KL-6, Krebs von den Lungen-6; RDW-SD, Red blood cell distribution width standard deviation; MLR, Monocyte to lymphocyte ratio; NLR, Neutrophil to lymphocyte ratio; RDW-CV, Red blood cell distribution width coefficient of variation; SII, Systemic immune inflammation.MLR exhibited predictive capability for CTD-ILD in the untreated group, while SII and RDW-CV showed predictive capability for CTD-ILD in the treated group. Notably, PLR demonstrated no ability to predict ILD in CTD patients (p > 0.05).
Moreover, the combination of these indicators displayed a higher AUC value than any single indicator in both groups (Table S4 and Fig. 4).
Disease severity in CTD-ILD
To study the potential of these markers in predicting disease severity in CTD-ILD, a Spearman correlation analysis was performed to assess the relationships of these parameters with lung functions (Table 6). In the untreated CTD-ILD subgroup, NLR, SII, and PLR were negatively correlated with both DLCO and DLCO%, and KL-6 was negatively correlated with FVC, FVC%, FEV1%, DLCO, and DLCO%.
| Parameters | FVC | FVC% | FEV1 | FEV1% | DLCO | DLCO% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Untreated group | ||||||||||||
| NLR | −0.144 | 0.369 | −0.277 | 0.079 | −0.080 | 0.618 | −0.216 | 0.175 | −0.326 | 0.046* | −0.349 | 0.032* |
| SII | −0.267 | 0.092 | −0.230 | 0.148 | −0.214 | 0.180 | −0.148 | 0.355 | −0.484 | 0.002* | −0.495 | 0.002* |
| MLR | 0.014 | 0.929 | −0.047 | 0.771 | −0.001 | 0.997 | −0.046 | 0.774 | −0.179 | 0.281 | −0.153 | 0.359 |
| PLR | −0.206 | 0.196 | −0.169 | 0.292 | −0.232 | 0.145 | −0.151 | 0.348 | −0.519 | 0.001* | −0.548 | <0.001* |
| RDW-SD | −0.055 | 0.735 | −0.011 | 0.945 | 0.027 | 0.869 | 0.021 | 0.897 | −0.052 | 0.756 | −0.032 | 0.850 |
| RDW-CV | −0.100 | 0.536 | −0.120 | 0.455 | 0.044 | 0.786 | −0.057 | 0.725 | −0.173 | 0.299 | −0.212 | 0.200 |
| KL-6 | −0.423 | 0.006* | −0.429 | 0.005* | −0.288 | 0.068 | −0.324 | 0.039* | −0.564 | <0.001* | −0.644 | <0.001* |
| Treated group | ||||||||||||
| NLR | 0.087 | 0.575 | −0.003 | 0.985 | 0.025 | 0.873 | 0.020 | 0.898 | −0.130 | 0.432 | −0.140 | 0.397 |
| SII | 0.033 | 0.829 | −0.066 | 0.672 | −0.022 | 0.886 | −0.053 | 0.735 | −0.092 | 0.579 | −0.219 | 0.181 |
| MLR | 0.161 | 0.297 | 0.066 | 0.668 | 0.045 | 0.773 | −0.001 | 0.996 | −0.053 | 0.748 | −0.078 | 0.636 |
| PLR | 0.150 | 0.332 | 0.138 | 0.371 | 0.042 | 0.788 | 0.121 | 0.435 | −0.207 | 0.206 | −0.252 | 0.122 |
| RDW-SD | 0.157 | 0.309 | 0.091 | 0.557 | 0.079 | 0.612 | 0.064 | 0.679 | 0.069 | 0.676 | 0.171 | 0.297 |
| RDW-CV | 0.070 | 0.652 | −0.016 | 0.920 | −0.004 | 0.979 | −0.019 | 0.903 | −0.152 | 0.357 | −0.079 | 0.633 |
| KL-6 | −0.491 | 0.001* | −0.663 | <0.001* | −0.444 | 0.003* | −0.633 | <0.001* | −0.395 | 0.013* | −0.582 | <0.001* |
Notes:
FVC, forced vital capacity; FEV1, forced expired volume in one second; DLCO, diffusing capacity of the lungs for carbon monoxide; NLR, Neutrophil to lymphocyte ratio; SII, Systemic immune inflammation; MLR, Monocyte to lymphocyte ratio; PLR, Platelet to lymphocyte ratio; RDW-SD, Red blood cell distribution width standard deviation; RDW-CV, Red blood cell distribution width coefficient of variation; KL-6, Krebs von den Lungen-6.
In the treated subgroup, only KL-6 was negatively correlated with lung function parameters including FVC, FVC%, FEV1, FEV1%, DLCO, and DLCO%. However, no correlation was observed between the other markers, such as NLR, SII, and PLR, and lung function parameters (P > 0.05).
Discussion
This study explored the potential predictive utility of KL-6, RDW, NLR, SII, MLR, and PLR for identifying ILD in CTD patients and for assessing the severity of CTD-ILD, with the aim of providing accessible, cost-effective, and efficient biomarkers for clinical applications.
It is well-established that KL-6 is associated with lung disease, promoting the fibrosis and anti-apoptotic effects on lung fibroblasts that lead to pulmonary fibrosis (Ahmed & Handa, 2022; Zuo et al., 2022). The present study showed that the elevated KL-6 levels observed in CTD-ILD patients were not related to their medication history during the disease course. In the CTD and CTD-ILD subtype analysis, KL-6 levels were high in all CTD-ILD patient subgroups regardless of treatment history, suggesting that KL-6 levels cannot distinguish CTD-ILD subgroups; this finding corresponds to the results of a previous study (Oguz et al., 2016). By contrast, a separate retrospective study showed that KL-6 levels in PM/DM-ILD patients were higher than in other CTD-ILD patients, indicating that PM/DM-ILD patients were more likely to have worse lung conditions (Zhou et al., 2023). This finding does not correspond with the results of the present study, but confounding factors (inflammation, tumors, tuberculosis, etc.,) in their study could have affected their results. Previous studies have reported a positive correlation between KL-6 and inflammatory factors (CRP or ESR) in RA-ILD patients (Merlino et al., 2004). The present study found a positve correlation between KL-6 and NLR, SII, and PLR in untreated CTD-ILD patients, indicating that KL-6 is probably not only involved in lung fibrosis but also in the lung inflammatory response in CTD-ILD patients. In an analysis of lung function, there were no significant differences between treated and untreated CTD-ILD patients. This finding indicates immunotherapy regulates the immune system and inflammatory response, but has little effect on KL-6 levels, indicating that KL-6 KL-6 is mainly more involved in the chronic pathological change of lung fibrosis. The lung function parameters of treated CTD-ILD patients and untreated CTD-ILD patients showed no significant differences (P > 0.05), indirectly confirming this conclusion.
Furthermore, the ROC analysis demonstrated that KL-6 exhibited the highest predictive capacity, with an AUC of 0.929 in untreated patients and 0.924 in treated patients. KL-6 was negatively correlated with pulmonary function parameters in a Spearman correlation analysis, unaffected by medication in CTD-ILD patients, indicating KL-6 is also able to predict disease severity in CTD-ILD. Indeed, it was also reproted that the posttreatment serum KL-6 was significantly higher than the pretreatment levels in interstitial pneumonia with autoimmune features (IPAF) patients with progressive disease. However, the patients showing improvement with medication treatement, the KL-6 levels were decreased significantly (Wang et al., 2020). Furthermore, serum KL-6 changes were significantly negatively correlated with lung function (FVC, DLCO and FEV1) changes. Idiopathic pulmonary fibrosis (IPF) patients monitored for 24 months with the anti-fibrotic agent Nintedanib showed persisted increased levels of KL-6 with a progressive decline of FVC percentages (d’Alessandro et al., 2021). Our study showed that the correlation between KL-6 and lung function correlated with the severity of the disease, while the medication did not affect the ability of KL-6 to respond to lung severity. Therefore, these outcomes suggested that KL-6 can monitor ILD progression and regular measurement of serum KL-6 to assess ILD progression and treatment response. A multivariate logistic regression analysis verified KL-6 as an independent risk factor for CTD-ILD. These findings are consistent with the findings of prior studies that serum KL-6 could reflect the severity of lung epithelial injury and disease and might be a potential biomarker for diagnosing and evaluating CTD-ILD (Rai et al., 2023; Zheng et al., 2021). The findings of the present study suggest that KL-6 is a viable biomarker to serve as a diagnostic tool and severity indicator for CTD-ILD, but its ability to differentiate between different types of CTD-ILD is limited.
Commonly used examination indicators for hospitalized patients, such as routine blood tests, can provide insights into the overall health status of the patients. Parameters such as RDW, neutrophils, lymphocytes, monocytes, and platelets are also involved in inflammation and/or immune responses (Chen et al., 2019; He et al., 2018; Kushwaha et al., 2023; Lippi et al., 2009). Indeed, neutrophils mediated by immune and inflammatory factors (proteases, oxidants, cytokines, chemokines, etc.,) contribute to interstitial fibrosis and abnormal repairment of lung tissue, leading to an increase of neutrophils and lymphocytes during the progression of CTD and CTD-ILD (d’Alessandro et al., 2022; Ruta et al., 2020). Considering that individual blood cell indicators (neutrophils, lymphocytes, and platelets) are influenced by multiple factors (hematopoietic system status, inflammatory status), a single indicator may not diagnose CTD-ILD effectively. Recent research indicates that the biomarkers derived from routine blood tests, such as NLR, PLR, and SII, have potential applications in the diagnosis of clinical diseases such as inflammatory disease or cancer (Asperges et al., 2023; Ouyang et al., 2023).
The present study found that CTD-ILD patients exhibited higher levels of NLR, SII, MLR, RDW-SD, and RDW-CV than CTD patients. Among these parameters, elevated RDW-SD in CTD-ILD patients was not related to medication. The AUC of RDW-SD was higher than the AUC of RDW-CV for diagnosing CTD-ILD (0.681 vs. 0.643). Similarly, a study of liver fibrosis in patients with chronic hepatitis B found that the AUC of RDW-SD in diagnosing signficant liver fibrosis was significantly higher than the AUC of RDW-CV (Yang et al., 2023). In another study of CTD-ILD patients, RDW-SD was significantly different between patients with CTD-ILD and those without, while there was no significant difference in RDW-CV in elderly and non-elderly individuals (Lu et al., 2022). RDW-SD is also better suited to represent red blood cell volume heterogeneity than RDW-CV (Hirahara et al., 2019). Bone marrow function and red blood cell maturation resulting from inflammatory and oxidative stress conditions lead to immature RBC release and a reduction in circulation half-life (Shi et al., 2021). RDW-SD differed significantly between CTD and CTD-ILD patients of both the treated and untreated groups in the present study because of RDW-SD’s higher sensitivity in reflecting red blood cell volume heterogeneity compared to RDW-CV. The statistical differences seen in RDW-CV between CTD and CTD-ILD patients in the treated group but not in the untreated group may be because medication suppresses bone marrow function and induces greater variability in RBC morphology, resulting in statistical differences of RDW-CV in treated groups. Because RDW-SD is not affected by medication, its diagnostic value surpasses RDW-CV in CTD-ILD patients.
The present study found that the NLR levels were higher in CTD-ILD patients than in CTD patients in both the untreated and treated groups, while MLR was only elevated in untreated CTD-ILD patients. Fibroblasts originate from monocyte cell lines in the bone marrow, indicating that monocytes are directly involved in the pathogenesis of pulmonary fibrosis (Guo et al., 2021). When alveolar cells are damaged, the inflammation response and monocyte-derived macrophages are activated to promote fibrosis. Macrophages can release reactive oxygen species, cytokines, and growth factors to enhance inflammation. Neutrophil extracellular traps (NETs) can induce the formation of autoantibodies, damaging pulmonary epithelial cells and possibly contributing to lung fibroblasts becoming myofibroblasts, the key cells for CTD-ILD pathogenesis (Cerro Chiang & Parimon, 2023). The elevated MLR seen in the present study in only untreated CTD-ILD patients may indicate that MLR is associated with different types of CTD and is influenced by medication. However, this finding could also be attributed to limitations in the sample size of this study, leading to biased results. A futher stratified analysis found different characteristics in different CTD subtypes, with MLR differing between RA and RA-ILD and NLR differing between SjS and SjS-ILD, findings that are partly consistent with the results of a previous study (Xu et al., 2022). The inflammatory cell infiltrates determine fibrotic activity, exhibiting a higher proportion of progressive fibrotic phenotypes in RA-ILD than in other CTD-ILDs (Yoo et al., 2022). Inflammatory cells and antibodies may also be involved in lung fibrosis. ILD patients with myeloperoxidases (MPO)-antineutrophil cytoplasmic antibodies (ANCA)-positive are common with the usual interstitial pneumoniaip pattern, exhibiting more pronounced infiltration of lymphatic follicles and mesenchymal monocytes compared with idiopathic pulmonary fibrosis (Kondoh et al., 2021). MLR is considered to be an independent risk factor for mortality in anti-MDA5+MA-ILD patients (Li et al., 2023). Changes in NLR, MLR, PLR, SII, and antibodies should be studied further in different subtypes of CTD-ILD.
NLR, SII, PLR, and MLR were positively correlated with inflammation factors in CTD-ILD and CTD patients in the present study, suggesting that the inflammatory response is heightened in both CTD-ILD and CTD, with ILD exhibiting a more severe inflammatory reaction. The observed negative correlations of NLR, SII, and PLR with pulmonary function parameters were no longer evident in CTD-ILD patients after medication, possibly due to a rapid decrease in the inflammatory response. Age was also found to be an independent risk factor for CTD-ILD, with older CTD patients more at risk for developing ILD, possibly because of age-related physical functioning (Fest et al., 2019). Clinicians should prioritize lung monitoring in older CTD patients to provide timely intervention.
This study conducted a comprehensive analysis of the NLR, SII, PLR, MLR, RDW, and KL-6 markers in CTD-ILD patients. However, this study also had certain limitations: (1) This study had a relatively small sample size, which may introduce bias into the results; future studies should increase the sample size for greater accuracy. (2) Some unknown confounding factors were not entirely excluded from the study; large-scale clinical trials and mechanism investigations are needed to validate the accuracy of these findings. (3) The presence of some missing data may have affected the current results. (4) This was a cross-sectional study with no patient follow-up. Future work should primarily focus on whether the dynamic changes in these indicators hold greater value for CTD-ILD patients than for CTD patients.
Conclusions
This study reaffirms that KL-6 is the most promising biomarker for diagnosing CTD-ILD and assessing its severity. The diagnostic value of RDW-SD surpasses that of RDW-CV for CTD-ILD. NLR, SII, MLR, and PLR have potential value in the diagnosis of different types of CTD-ILD. Moreover, the diagnostic value of KL-6 for CTD-ILD was unaffected by medication interference. Clinicians should monitor KL-6 levels in older CTD patients as they are at higher risk of ILD.