The role of protein post-translational modifications in prostate cancer
- Published
- Accepted
- Received
- Academic Editor
- Gwyn Gould
- Subject Areas
- Biochemistry, Cell Biology, Molecular Biology, Oncology, Urology
- Keywords
- Protein post-translational modification, Prostate cancer, Diagnosis, Treatment, Prognosis
- Copyright
- © 2024 Hao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. The role of protein post-translational modifications in prostate cancer. PeerJ 12:e17768 https://doi.org/10.7717/peerj.17768
Abstract
Involving addition of chemical groups or protein units to specific residues of the target protein, post-translational modifications (PTMs) alter the charge, hydrophobicity, and conformation of a protein, which in turn influences protein function, protein–protein interaction, and protein aggregation. These alterations, which include phosphorylation, glycosylation, ubiquitination, methylation, acetylation, lipidation, and lactylation, are significant biological events in the development of cancer, and play vital roles in numerous biological processes. The processes behind essential functions, the screening of clinical illness signs, and the identification of therapeutic targets all depend heavily on further research into the PTMs. This review outlines the influence of several PTM types on prostate cancer (PCa) diagnosis, therapy, and prognosis in an effort to shed fresh light on the molecular causes and progression of the disease.
Introduction
Protein composition is limited by the genetic code and consists of relatively few natural amino acids. However, proteins perform a wide variety of functions and have shown a remarkable capacity for regulation and immediate response to both intracellular and extracellular stimuli (Kokkinidis, Glykos & Fadouloglou, 2020). To some extent, the wide variability in protein function can be attributed to the post-translational modifications (PTMs) that almost all proteins undergo, such as phosphorylation, methylation, acetylation, glycosylation, and ubiquitination (Goldtzvik et al., 2023). Multiple PTMs can act in concert, or compete for the same sites to drive opposite outputs (Vu, Gevaert & De Smet, 2018; Antfolk et al., 2019). As a result, incorrect regulation of PTM’s influence the charge, conformation, and stability of proteins, which in turn affects numerous biological processes (Table S1) and incorrect regulation of PTM’s is linked to a number of human disorders (Liu et al., 2022), including cancer, heart failure, autoimmune diseases, and neurodegenerative diseases (Ramazi, Allahverdi & Zahiri, 2020). For example, the tumor-derived ubiquitin protein ligase E3 module N-recognition 5 (UBR5) regulates tumor sphere formation in ovarian cancer through the p53-β-catenin pathway and then enhances immunosuppression by recruiting tumor-associated macrophages (Song et al., 2020).
Prostate cancer (PCa) is the second most common tumor in men, mostly in middle-aged and elderly men (Shah, Ioffe & Chang, 2022), with nearly 400,000 deaths per year (Pejčić et al., 2023). It has high morbidity, mortality and biochemical recurrence rate (BCR), poor prognosis, and significant heterogeneity in immunological features, genome and molecules (Jairath et al., 2020). Due to the relatively insidious onset and slow growth of prostate cancer (Raina et al., 2022), the symptoms in most patients are not obvious, which is likely to lead to misdiagnosis or missed diagnosis (Mottet et al., 2021; Grozescu & Popa, 2017; Halsey-Nichols & McCoin, 2021). Currently, the diagnosis of prostate cancer is mainly based on pathological prostate biopsy (Bhanji, Allaway & Gorin, 2021). With the improvement of risk stratification and advances in magnetic resonance and functional imaging, as well as the emergence of biomarkers, the identification and characterization of the disease is becoming more accurate (Litwin & Tan, 2017). Non-surgical treatments for prostate cancer include androgen deprivation therapy (ADT) for high-risk local or systemic advanced disease ineligible for radical surgery, radiation therapy (RT), salvage ablative therapy for moderate- to low-risk disease or for clinically localized disease that has failed radiotherapy, chemotherapy currently used to treat androgen-dependent disease, and emerging immunotherapy (Evans, 2018). This article examines the impact of PTMs such as protein phosphorylation, glycosylation, ubiquitination, acetylation, methylation, succinylation, and lipidation on the diagnosis, treatment, and prognosis of PCa, to provide a new understanding of the molecular mechanism of its formation and development of prostate cancer.
Survey Methodology
The PubMed database was utilized to search related literature using the keyword “prostate cancer,” “cancer,” “protein phosphorylation,” “protein glycosylation,” “protein ubiquitination,” “protein acetylation,” “protein methylation,” “protein succinylation,” and “protein lipidation.”
Rationale
This study explores the influence of PTMs on the diagnosis, treatment and prognosis of prostate cancer. It aims to provide a new perspective on the molecular mechanism underlying the occurrence and development of prostate cancer, provide new targets and screening methods for drug research and development, and promote the discovery and development of new drugs.
Audience
This review describes the post-translational modifications of proteins related to prostate cancer in recent years, which is conducive to readers’ understanding of the development mechanism, treatment and prognosis of prostate cancer, and also opens up ideas for the treatment of prostate cancer. Therefore, it is considered appropriate for the journal’s diverse readership.
Classification and Clinical Application of the PTMs Types in Prostate Cancer
Phosphorylation
Phosphorylation influences the molecular mechanism of prostate cancer
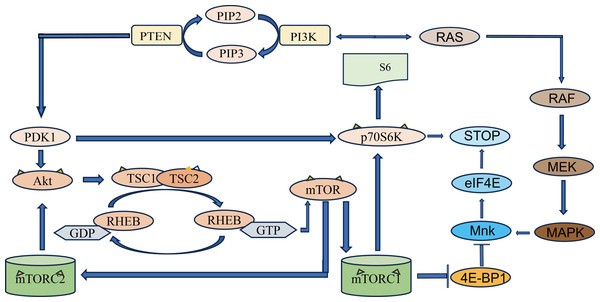
Phosphorylation is one of the most common PTMs (Singh et al., 2017). Tyrosine kinases such as focal adhesion kinase (FAK) (Liu et al., 2023), fibroblast growth factor receptor 1 (FGFR1) (Wang et al., 2018) and cyclin-dependent kinases are involved in protein phosphorylation or dephosphorylation pathways that promote the occurrence and development of cancer by inducing cancer cell proliferation, inhibiting cancer cell apoptosis, inducing cancer cell invasion and metastasis, inducing cancer cell angiogenesis, and inducing cancer stem cell proliferation, and so on (Liu et al., 2021a). Therefore, targeting phosphorylation pathway is one of the potential pathways for anticancer drug development. PI3K/Akt/mTOR and Ras/MAPK are two important signaling pathways implicated in PCa development (Fig. 1) (D’Abronzo & Ghosh, 2018). Eukaryotic translation initiation factor 4E (eIF4E) is phosphorylated by mitogen-activated protein kinase-interacting kinases 1 and 2 (Mnk1/2) in response to rapamycin (mTOR), and phosphorylation of eIF4E increases the rate of oncogene translation (such as c-Myc and Akt) and promotes drug resistance (such as everolimus and gemcitabine) in prostate cancer (D’Abronzo & Ghosh, 2018). Extracellular signal-regulated kinase 2 (ERK2) phosphorylates the leukemia suppressor receptor (LIFR) S1044 and subsequently activates the protein kinase B (AKT) signaling pathway to phosphorylate AKT S473, which induces the expression of a number of proliferation and transformation genes (Shao et al., 2019). COPS3, the third subunit of the COP9-CSN complex, is highly expressed in PCa tissues and promotes the epithelial-mesenchymal transformation (EMT) of PCa by increasing the phosphorylation level of P38 MAPK, thereby promoting the proliferation, migration and invasion of prostate cancer cells (Zhu et al., 2019). Tousled-like kinase mediates the phosphorylation of NEK1, an amitotic gene A-associated kinase 1, leading to DNA damage and promoting the development of CRPC (Khalil et al., 2022). ErbB-2 is phosphorylated by Src kinase and phosphorylated by AKT, which promotes PCa cell proliferation and migration through PI3K/AKT (Gao et al., 2016). In PCa, loss of nuclear FOXP3 is usually accompanied by low expression of TSC1, which induces c-Myc transcription and protein phosphorylation to synergistically increase c-MYC expression and activate mTOR signaling (Wu et al., 2019). Overexpression of wild-type (WT) EphA7 in PCa cells resulted in reduced tumor volume and increased tumor apoptosis in primary tumors compared to site-mutated phosphorylated EphA7 (Li et al., 2017).
Figure 1: Schematic diagram of PI3K/Akt/mTOR signaling pathway.
PTEN, phosphatidylinositol 3-kinase (PI3K) mediates the production of phosphatidylinositol 4, 5-diphosphate (PIP2) from phosphatidylinositol triphosphate (PIP3). PIP3 recruits PDK1, a protein with PH domain, phosphorylates and disrupts tuberculosis complex 1/2 (TSC1-TSC2) through Akt, and phosphorylates mTOR through RHEB. The two complexes that mTOR proteins are involved in are mTOR protein complex 1 (mTORC1) and mTOR protein complex 2 (mTORC2). mTORC1 increases protein translation by phosphorylation of its two direct targets and P70S6K. The arrows represent promotion, and the others represent inhibition.Targeting phosphorylation sites for diagnosis and treatment of prostate cancer
Based on the above molecular mechanisms by which phosphorylation affects prostate cancer, targeting these targets and pathways can be developed for clinical applications in prostate cancer. Phosphorylation controls the androgen receptor (AR) and the PTEN/PI3K/AKT/mTOR axis (McAllister et al., 2020). Cyclin-dependent kinase (CDK) is a serine/threonine kinase that plays a key role in cell cycle progression and has been proposed as a promising target for cancer therapy (Wang, Bode & Zhang, 2023). CDK1 and AKT phosphorylate Ser81 and Ser213 of AR, respectively, while phenethyl caffeic acid (CAPE) reduces the protein levels and activity of CDK1 and AKT and inhibits the phosphorylation of Ser81 and Ser213 on AR, thereby regulating the stability of AR, implying the possibility of using CAPE as a treatment for advanced PCa (Kuo et al., 2019). The Proviral Integration site for Moloney murine leukemia virus (PIM) kinases are a family of oncogenic Ser/Thr kinases (Casillas et al., 2018). An unbiased proteomic screen identified Abl-interactor 2 (ABI2), an integral member of the wave regulatory complex (WRC), as a PIM1 substrate, Ser183 is phosphorylated by PIM1 to promote prostate cancer invasion, while PIM inhibitors can reduce prostate cancer metastasis (Jensen et al., 2023). Androgen deprivation therapy (ADT) is commonly used to treat advanced prostate cancer. Nonetheless, most patients progress to castration-resistant prostate cancer (CRPC) after being treated by ADT. PCa develops a key restrictive androgen biosynthetase of CRPC, 3β-hydroxysteroid dehydrogenase type 1 (3βHSD1) (Hettel & Sharifi, 2018). BMX is a member of TEC family nonreceptor tyrosine kinase. In the presence of dehydroepiandrosterone (DHEA), bone marrow kinase on the X chromosome (BMX) is activated and phosphorylates 3βHSD1 to form an active dimer that promotes the conversion of DHEA to dihydrotestosterone (DHT) (Hettel & Sharifi, 2018). DHT interacts with AR to promote the progression of PCa to CRPC. The BMX inhibitor zanubrutinib can effectively block this process, thereby inhibiting (Qiu, 2023). ErbB-2 can be used as a biomarker for invasive PCa when hyperphosphorylated (Chuang et al., 2010). Androgens induce an increase in p66Shc protein, a 66 kDa proto-oncogene of Src and collagen homolog (Shc), resulting in increased cell ROS levels, ROS oxidation and inactivation of cellular prostatic acid phosphatase (cPAcP), preventing cPAcP from dephosphorylating ErbB-2. This results in ErbB-2 activation and the CRPCa phenotype of PCa cells. Therefore, inhibition of ErbB-2 with antiandrogens significantly reduces the risk of tumor recurrence in PCa xenotransplantation in mouse (Miller, Ingersoll & Lin, 2019). Therefore, using ErbB-2 as the target and combining various methods to treat PCa is worthy of further study. LQB-118 is a pterocarpanquinone with proven antineoplastic activity, which regulates the proliferation, death and migration/invasion of PCa cells via a negative regulator of the Akt/GSK3 signaling pathway. As a result, LQB-118 may be used in the treatment of metastatic prostate cancer, either alone or in combination with another chemotherapeutic agent (Martino et al., 2023). Serine/threonine protein phosphatase 5 (PPP5C) is a member of the protein serine/threonine phosphatase family that regulates the phosphorylation of protein serine/threonine residues and activates or inactivates the corresponding substrates. PPP5C is highly expressed in PCa tissues. After the downregulation of PP5C expression, the phosphorylation of ERK1/2 and JNK is significantly increased, which leads to the arrest and apoptosis of tumor cells in G0/G1 phase, thus inhibiting cell proliferation in PCa cells. Therefore, PPP5C may become a new diagnostic biomarker and therapeutic target for PCa (Lv et al., 2018). For a summary of studies on the impact of protein phosphorylation on the diagnosis, treatment, and prognosis of PCa, please refer to the Table S2.
Glycosylation
Glycosylation influences the molecular mechanism of prostate cancer
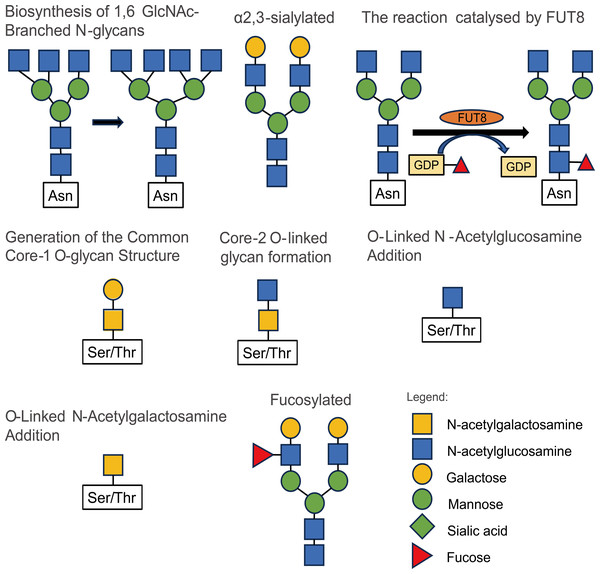
The modification of glycosylation is mainly catalyzed and regulated by various glycosyltransferases and glycosidases (Samaržija, 2021), which play an important role in the origin and development of malignant tumors. Most tumor markers used in clinical applications are glycoproteins (Butler & Huang, 2021). Studies on the influence of glycosylation modification on the occurrence and development of prostate cancer mainly include 2, 6-sialylation, core fucosylation, branched N-glycans, and LacdiNA (GalNAcβ1–4GlcNAc) glycosylation (Kałuza, Szczykutowicz & Ferens-Sieczkowska, 2021). The structures and types of these glycosylation modifications are illustrated in Fig. 2.
Figure 2: Some types and structures of glycosylation.
ST6 β-galactoside α-2,6-sialtransferase 1 (ST6GAL1) is an enzyme that catalyzes the addition of 2,6-linked sialic acids to terminal N-linked glycans. Its upregulation in prostate cancer has been found to promote tumor growth and metastasis (Garnham et al., 2019; Gratacós-Mulleras et al., 2020). Growth differentiation factor 15 (GDF15) is a member of the TGF-β superfamily with immunomodulatory functions, and its high expression is often associated with cancer progression. High expression of GDF15 is associated with poor survival in prostate cancer patients. GDF15 N70 is modified by N3H4F3 and N6H3F2, and the glycosylation of GDF15 is completely inhibited when cells are treated with the N-linked glycosylation inhibitor Tunicamycin (TM). GDF15 inhibits EGFR activity, and GDF15 N70 glycosylation can reduce the inhibition of EGFR, thereby leading to the development of castration resistance, which provides a good basis for the future development of selective inhibitors based on GDF15 glycosylation for the treatment of CRPC (Wang et al., 2022). Neural cell adhesion molecule L1 (L1CAM) was found to be a mediator of the pro-invasive effects of FUT8. L1CAM is a highly glycosylated protein known to regulate cell attachment, invasion, and migration in several cancers. α (1,6) Fucosylaminotransferase (FUT8) is an enzyme involved in N-glucosylfucosylation. Higher levels of L1CAM cleavage were observed in FUT8-silenced cells, whereas FUT8 overexpression reduced L1CAM cleavage. Thus, FUT8 drives cancer cell invasion and tumor metastasis, in part due to reduced cleavage of core-fucosylated L1CAM (Mao et al., 2023). Meanwhile, FUT8 mediates glycosylation of several proteins including EGFR, TGF-beta receptor (TGFBR), E-cadherin, PD1/PD-L1, and β1-integrin, and plays an important role in promoting the malignant phenotype of tumor cells (Mao et al., 2023).
Targeting glycosylation sites for diagnosis and treatment of prostate cancer
These studies suggest that further research into protein glycosylation may lead to the development of new biomarkers or drugs for the diagnosis and treatment of prostate cancer. The level of core fucosylated glycoproteins in the serum of prostate cancer patients is significantly increased, and core fucosylated prostate-specific antigen (PSA) shows potential as a diagnostic biomarker for distinguishing prostate cancer from other prostate diseases, such as benign prostatic hyperplasia (BPH) (Liao et al., 2021; Höti et al., 2020). Serum prostate-specific antigen (sPSA) could not distinguish between poorly differentiated, moderately differentiated, and highly differentiated PCa. The integration of N-glycosylation profiles and prostate volume changes into a single urinary glycosylation profile marker (UGM) capable of distinguishing between BPH, PCa and prostatitis has high potential as a PCa biomarker (Vermassen et al., 2015a; Vermassen et al., 2015b). Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) has been employed to detect changes in O-glycans in prostate cancer cells, including increased O-glycan levels, decreased complex O-glycans, and increased sialylation of O-glycans (Bai et al., 2020). The N-acetylgalactosaminyltransferase 7 (GALNT7) belongs to a large family of 20 GALNT isoenzymes and is characterized for exclusively glycosylating proteins/peptides that already carry GalNAc moieties added by other GALNT-isoenzymes. GALNT7 can alter the O-glycosylation of membrane and secreted proteins. It has been found that the levels of GALNT7 in urine and blood of patients with CRPC are higher than normal, and its diagnostic value exceeds that of PSA alone (Scott et al., 2023). Treatment with Myc inhibitors (10074-G5 or 10058-F4) induces the IRE- α-XBP1s pathway to trigger fructose-6-phosphoamidotransferase-1 (GFAT1) and increased protein glycosylation. When Myc inhibitors are used in combination with GFAT-1 inhibitors (DON), there is a synergistic effect in inhibiting prostate cancer cell proliferation and migration, suggesting that targeting Myc and GFAT-1 is a novel approach that may represent a strategy for the treatment of prostate cancer (Zhang et al., 2023).
Ubiquitination and deubiquitination
Ubiquitination is the process of attaching ubiquitin to a target protein, involving three enzymes: ubiquitin-activating enzyme E1, ubiquitin-conjugating enzyme E2, and ubiquitin ligase E3. Proteins can undergo either monoubiquitination or multiubiquitination. Multiubiquitination occurs when one of the seven lysine residues of ubiquitin is linked to another ubiquitin (Cockram et al., 2021). The process of ubiquitination is a reversible ATP-dependent reaction, with the removal of ubiquitin from the target protein referred to as deubiquitination (Samaržija, 2021).
Ubiquitination influences the molecular mechanism of prostate cancer
Speckle-type POZ protein (SPOP), the most commonly mutated tumor suppressor gene in human primary prostate cancer (Gang et al., 2019), is an E3 ubiquitin ligase that inhibits tumor growth by breaking down cancer-promoting substrates. Wild-type (WT) SPOP induces the expression of caprin1,3-phosphoinositol-dependent protein kinase-1 (PDK1) (Shi et al., 2019). SPOP can hinder tumor growth by inhibiting the activity of AKT kinase (Jiang et al., 2021). Additionally, it can facilitate the degradation of heterogeneous nuclear ribonucleoprotein K (HnRNPK) by promoting its ubiquitination, thereby inhibiting the proliferation of prostate cancer cells (Wu et al., 2021). The SPOP/CUL3/RBX1 complex inhibits PCa progression through ubiquitination of cyclin E1 (Ju et al., 2019). However, mutant SPOP has different effects. It promotes the degradation of transcription factor 2 (ATF2), leading to the proliferation and migration of prostate cancer cells (Ma et al., 2018). Additionally, mutant SPOP can increase androgen production, androgen receptor (AR) activation, and the growth of PCa cells (Shi et al., 2021). The ubiquitin-conjugating enzyme E2S (UBE2S) regulates the stability of p16 and β-catenin through K11-linked ubiquitination, thereby promoting the migration and invasion of PCa cells (Peng et al., 2022). The ubiquitination process requires ATP and hexokinase (HK) is the first rate-limiting enzyme in glycolysis (Nogueira, Patra & Hay, 2018). Docetaxel can activate the expression of hypoxia-inducing factor 1 (HIF-1) and thereby increase the expression of SUMO-specific protease 1 (SENP1), which mediates HK2 desumoylation and promotes HK2 binding to mitochondria (Shangguan et al., 2021). Melatonin reduces the expression of SENP1, which mediates the desumoylation of HDAC1, a key factor in AR transcription activity (Hao et al., 2022). Furthermore, F-box and WD repeat domain containing 2 (FBXW2) can target EGFR for ubiquitination and degradation, thereby inhibiting the proliferation and metastasis of PCa cells (Zhou et al., 2022). USP16 and USP33, as the deubiquitinating enzymes of c-Myc, regulate the proliferation of PCa cells by deubiquitinating and stabilizing the expression of c-Myc. By down-regulating the expression of USP16 and USP33, the growth of PCa cells in vitro was significantly inhibited in vivo (Ge et al., 2021; Ding et al., 2021). Ovarian tumor deubiquitinase 6A (OTUD6A) is highly expressed in prostate cancer tissues, and OTUD6A stabilizes Brg1 and AR expression by removing FBXW7-mediated polyubiquitination of the K27 junction of Brg1 and the SPOP-mediated K11 junction of AR (Fu et al., 2022). BRCA1-associated protein 1 (BAP1) is a deubiquitinating enzyme that can inhibit prostate cancer progression by stabilizing the expression of PTEN and downregulating the PI3K-Akt pathway (Deng et al., 2021).
Targeting ubiquitination sites for diagnosis and treatment of prostate cancer
The use of protein ubiquitination and deubiquitination to affect protein stability can also achieve the goal of disease treatment. Nobiletin, a compound, has the ability to specifically promote the degradation of AR-V7 through the K48-linked ubiquitination form of the AR splice variant 7. It achieves this by preventing the interaction of the deubiquitinating enzymes USP14 and USP22 with AR-V7. In addition, Nobiletin also enhances the sensitivity of CRPC to enzalutamide, effectively inhibiting the growth of CRPC (Liu et al., 2021b). UBC9 mediates the SUMOylation of signal transducer and activator of transcription 4 (STAT4). Inhibiting UBC9 with 2-D08 can promote the activation of tumor-associated macrophage (TAM) and CD8 T cells, preventing the progression of PCa (Xiao et al., 2023). USP14 is one of the related proteins of kinesin family member 15 (KIF15) and acts as a deubiquitinating enzyme, preventing AR and AR-V7 degradation, thereby increasing prostate cancer resistance to enzalutamide (Gao et al., 2021). At the same time, lncRNA PCBP1 antisense RNA 1 (PCBP1-AS1) can also stabilize USP22-AR/AR-V7 complex formation, enhance AR and AR-V7 deubiquitination, and promote CRPC progression and resistance to enzalutamide (Zhang et al., 2021b). The ubiquitin-specific peptidase 1 (USP1) functions as a deubiquitinating enzyme, while SNS-032 as a kinase inhibitor, inducing apoptosis and downregulating USP1 expression, thereby inhibiting PCa proliferation (Liao et al., 2022). Moreover, overexpression of USP7, USP10 and USP12 in PCa cells is associated with poor prognosis in PCa patients and may be used as prognostic markers (Guo et al., 2023). Furthermore, one of the induced degradation techniques developed for target proteins is PROTAC (Proteolytic Targeting Chimera) technology by forming ternary complexes that link the target proteins with E3 ligases (Qi et al., 2021). AR degraders developed using PROTAC technology, such as ARD-61, ARV-110, ARD-2128, and ARD-266, have shown significant inhibitory effects on cancer cell proliferation and have the potential to overcome drug resistance (Yedla et al., 2023; Kargbo, 2022). Most prostate cancer patients treated with docetaxel develop resistance to docetaxel, and nuclear protein 1 (NUPR1), a stress-inducible transcription factor, confers docetaxel resistance to prostate cancer cells, suggesting that NUPR1 plays a role in docetaxel resistance (Schnepp et al., 2020). Additionally, some RNAs are involved in the ubiquitination process. For instance, circ_0006156 regulates S100A9 protein expression via the ubiquitination process, thereby inhibiting R transfer in PCa cells (Zhang et al., 2022a). Detailed studies on the impact of protein ubiquitination on prostate cancer diagnosis, treatment, and prognosis can be found in the Table S3.
Acetylation and deacetylation
The acetylation of proteins is the transfer of the acetyl group to the protein by the acetyl donor (e.g., acetyl-coA) under the catalysis of acetyltransferase. The reverse process is called deacetylation (Samaržija, 2021).
Acetylation modification affects prostate cancer
Tumor protein D52 (TPD52) can be acetylated by lysine acetyltransferase 2B (KAT2B), creating antagonism with histone deacetylase 2 (HDAC2) and preventing the interaction between TPD52 and the HSPA8 member, resulting in tumor growth impairment. This represents a target for PCa treatment (Fan et al., 2021). Lysine acetyltransferase 2A (KAT2A) acetylates AR and induces its translocation from the cytoplasm to the nucleus, resulting in increased transcription activity of the AR target gene PSA, thereby increasing resistance to abiraterone (Lu et al., 2021). CBP/P300 mediates the acetylation of HOXB13, AR, JMJD1A, SKP2 and other proteins and promotes the emergence and development of PCa, making it the key factor of antiandrogen resistance of CRPC(Waddell, Huang & Liao, 2021; Nguyen et al., 2022; Xu et al., 2020; Rezaeian et al., 2023). CBP/P300-related factors can promote the degradation of β-catenin through acetylation and thus inhibit prostate cancer progression (Zhou et al., 2019). Furthermore, in CRPC, carnitine palmitoyl transferase 1 A (CPTIA), a rate-limiting step that catalyzes the conversion of acyl coenzyme A to acyl carnitine, provides acetyl groups to histones to promote tumor growth and anti-androgen resistance (e.g., enzalutamide) (Joshi et al., 2019). In patients with advanced PCa, the degree of acetylation of the H3 in their tissues is significantly increased (Puzyrenko et al. , 2022). Furthermore, N-α-acetyltransferase 10 (NAA10), as an acetyltransferase that acetylates both N-terminal amino acid and internal lysine residues of proteins, which has been found to promote the proliferation and migration of prostate cancer cells, as well as induce autophagy (Kim et al., 2020). On the other hand, acetyl-CoA acetyltransferase 1 (ACAT1), known as a protumor factor in prostate cancer, has been shown to promote the occurrence and development of the disease by inhibiting autophagy and eliminating reactive oxygen species (Guan et al., 2022). SIRT5 inhibits PI3K and mediates PI3K/AKT/NF-B signaling to suppress prostate cancer metastasis (Choi et al., 2022). Moreover, SIRT5 promotes the activity of the MAPK signaling pathway through ACAT1, enhancing the proliferative, migratory, and invasive abilities of prostate cancer cells (Guan et al., 2020).
Acetylation modification may be used as a therapeutic target in prostate cancer
Since acetylation modification affects the occurrence and development of prostate cancer, acetylation modification can also achieve the purpose of treating prostate cancer. Docetaxel, a semisynthetic taxane, has exhibited significant single-agent activity against prostatic tumors (Pienta, 2001). However, drug resistance and toxicity often occur during treatment (Aragon-Ching & Madan, 2018). Therefore, it is clear that acetylation and deacetylation of proteins affect the sensitivity of prostate cancer to drugs. Kruppel-like factor 5 (KLF5), a member of the KLF family, regulates the expression of a large number of novel target genes and is involved in a variety of cellular functions including desiccation, proliferation, apoptosis, autophagy and migration. Transforming growth factor-β (TGF-β) can induce a process known as acetylation of Kruppel-like factor 5 (KLF5) (Ac-KLF5), which promotes bone metastasis in PCa by activating the C-X-C chemokine receptor type 4 (CXCR4). The use of the CXCR4 inhibitor AMD3100 has been shown to increase tumor sensitivity to docetaxel and inhibit bone metastasis in PCa (Zhang et al., 2021a). Ac-KLF5 also plays a role in regulating prostate development (Zhang et al., 2020). Nitazoxanide which is an anti-parasitic drug with potent antiviral activity as an inhibitor can suppress Ac-KLF5-induced bone metastasis in PCa by regulating KLF5 function (Huang et al., 2023). SIRT3 is involved in regulating the acetylation level of various proteins (such as HSD17B4, ACO2, etc.) to affect protein stability, and it can serve as a diagnostic marker for predicting PCa progression (Huang et al., 2020). Notably, mitochondrial aconitase (ACO2), a nuclear encoded tricarboxylate cycling enzyme, has significantly increased activity. AR can regulate the expression of SIRT3 by binding to steroid receptor coactivator 2 (SRC-2). In the absence of SRC-2, the expression of SIRT3 is enhanced, and the acetylation of ACO2 is reduced. Increased expression of SRC-2 and decreased expression of SIRT3 serve as genetic markers for the accumulation of prostate cancer metastases (Sawant Dessai et al., 2021). Phosphoenolpyruvate carboxykinase subtype 2 (PCK2), which has complex functions in addition to regulating glucose metabolism, may promote tumorigenesis by reducing acetyl-CoA levels through a reduction in the TCA cycle. PCK2 is therefore a potential therapeutic target for aggressive prostate tumours (Zhao et al., 2017).
Methylation
Protein methylation is an important epigenetic modification (Li et al., 2022). Studies have shown that some key genes in prostate cancer cells are altered by methylation modification, such that a change in the activity of the genes leads to the development of prostate cancer (Zhu et al., 2021).
SET domain protein 2 (SETD2) mediates the expression of Zeste homolog 2 (EZH2) and promotes EZH2 degradation, which prevents PCa metastasis. However, metastasis is promoted when SETD2 is absent (Yuan et al., 2020). As a methyltransferase, EZH2 mediates the methylation of ERG and enhances its transcriptional and carcinogenic activity (Sugiura et al., 2021). The administration of the EZH2 inhibitor GSK343 inhibits ERG methylation and tumor growth in PCa mouse models (Zoma et al., 2021). DNA methyltransferase 1 (DNMT1) promotes the emergence and metastasis of PCa by inhibiting the transcription of tumor necrosis factor receptor-associated factor 6 (TRAF6), which mediates EZH2 ubiquitination (Li et al., 2022).
Histone methyltransferase has become an important therapeutic target in oncology. Telomere silencing 1-like disruptor (DOT1L) is overexpressed as a histone methyltransferase in PCa tissues and is associated with a poor prognosis (Baratchian et al., 2022). It impairs the mobility of PCa cells and organoids. When DOT1L is knocked out or the inhibitors EPZ004777 or EPZ5676 are used, the expression of MYC decreases and the expression of HECTECT domain E3 ubiquitin protein ligase 4 (HECTD4) and MYCBP2 is regulated, ultimately promoting the degradation of AR and MYC (Baratchian et al., 2022). Methylated H3 blocks antiandrogen resistance (Baratchian et al., 2022).
Succinylation
Succinylation by transferring a succinyl group to a residue of the target protein in an enzymic or non-enzymic manner (Dai et al., 2022). Therefore, the level of succinylation is mainly regulated by succinyl donor, succinyltransferase, and desuccinylase (Lu & Han, 2022). Succinylation alters rates of enzymes and pathways, especially mitochondrial metabolic pathways (Yang & Gibson, 2019), thus linking metabolic reprogramming with various pathological disorders including cancers (Dai et al., 2022). However, little has been reported on the role and value of succinylation modification of the lysine site in prostate cancer.
The level of succinylation in PCa tissues was significantly increased and the level of succinylation correlated with the Gleason score and PDL1 expression level (Zhang et al., 2022b). C-terminal binding protein 1 (CTBP1) is a corepressor in gene transcription regulation and is highly expressed in prostate cancer tissues. CTBP1 promotes migration of prostate cancer cells. E-cadherin (CDH1), a transmembrane glycoprotein that connects epithelial cells at adherent junctions, exerts its tumour suppressing role mainly by sequestering β-catenin from its binding to LEF (Lymphoid enhancer factor)/TCF (T cell factor) (Wong et al., 2018). CDH1 functions as a substrate of CTBP1. KAT2A mediates succinylation of CTBP1 and inhibits the transcription activity of CTBP1 on CDH1 and thus play a role in cancer promotion (Zhou et al., 2023). In addition, desuccinylation also plays an important role in prostate cancer. For example, SIRT5, a nicotinamide adenine dinucleotide (NAD)-dependent desuccinylase, significantly reduced expression levels of SIRT5 and significantly increased succinylation at lactate dehydrogenase A (LDHA) lysine 118 (K118su) in aggressive PCa cells. As a substrate of SIRT5, LDHA-K118su significantly increased migration and invasion of PCa cells (Kwon et al., 2023).
Fish oil (FO) composed of omega-3 polyunsaturated fatty acids (omega-3 PUFA) affects the succinylation of glutamate-oxaloacetic aminotransferase 2 (GOT2), which may inhibit PCa progression by interfering with aspartate synthesis and nucleotide production. This provides the basis for further investigation of succinylation and GOT2 as potential drug targets for future PCa treatment (Jiang et al., 2023).
Lipidation
Protein lipid modification mainly includes cysteine palmitoylation, N-terminal glycine myristoylation, and cysteine isoprenylation (Samaržija, 2021).
Of the enzymes involved in lipidation, FASN, a key enzyme responsible for de novo lipid synthesis required for cancer cell development, catalyzes the synthesis of malonyl coenzyme A (MCoA) and acetyl coenzyme A (ACoA) from procondensation and stores the palmitate by converting excess carbon uptake into fatty acids. It is responsible for the acylation of key regulatory switches in most signal transduction energy pathways and plays a central role in energy homeostasis (Schmidt, Ballman & Tindall, 2007). FASN increases prostate cancer cell adhesiveness, impairs HGF-mediated cell migration and reduces three-dimensional (3D) invasion by mediating actin cytoskeletal remodeling downstream of palmitoylated atypical GTPase RHOU (De Piano et al., 2021).
Increased expression of FASN is associated with poor prognosis in PCa, and a 5 α-reductase inhibitor (dutasteride) inhibits FASN expression in prostate cancer cells (Schmidt, Ballman & Tindall, 2007). Caveolin-1 promotes androgen resistance by upregulating acetyl-CoA carboxylase-1 (ACC1) and FASN expression and lipid synthesis and promotes the proliferation and metastasis of PCa cells (Karantanos et al., 2016). FASN inhibitor (IPI-9119) and demonstrated that selective FASN inhibition antagonises CRPC growth through metabolic reprogramming and leads to decreased protein expression and transcriptional activity of full-length AR (AR-FL) and AR-V7 (Zadra et al., 2019).
Lysophosphatidylcholine Acyltransferase1 (LPCAT1) can also mediate lipid modification of proteins. LPCAT1 is overexpressed in CRPC relative to primary prostate cancer. LPCAT1 was found to mediate CRPC growth via nuclear re-localization and Histone H4 palmitoylation in an androgen-dependent fashion, increasing mRNA synthesis rates. And LPCAT1 overexpression led to CRPC cell resistance to treatment with paclitaxel. Therefore, LPCAT1 as a viable therapeutic target of CRPC (Han et al., 2020).
Lactylation
Lactic acid is an abundant metabolite in the tumor microenvironment, is secreted by cancer-associated fibroblasts, and can be absorbed by cancer cells to maintain mitochondrial metabolism (Ippolito et al., 2022). However, lactate modification is a novel protein modification that was only reported in 2019 (Zhang et al., 2019), so there are relatively few studies. HIF1 introduces lactic acid into PCa cells via monocarboxylate transporter 1 (MCT1), and HIF1 lactylation enhances transcription of KIAA1199 and promotes prostate cancer angiogenesis (Luo et al., 2022). In addition, regulation of histone lactylation is also a potential PCa therapy target (He et al., 2023). For example, tumor cells treated with PI3K inhibitors or anti-PD-1 antibodies (aPD-1) reduce lactate production and inhibit lactylation of histone proteins within tumor-associated macrophages (TAMs), resulting in phagocytic activation (Chaudagar et al., 2023). Absence of the Numb/Parkin pathway in prostate cancer leads to metabolic reprogramming, a significant increase in lactic acid production and subsequent upregulation of histone lactylation and neuroendocrine-associated gene transcription, a promising therapeutic target for cancer cell plasticity modulation of histone lactylation (He et al., 2023).
Interaction of various PTMs
There are many forms of mutual regulation of PTMs between proteins, such as the interaction between ubiquitination and phosphorylation, the interaction between acetylation and ubiquitination, and the interaction between phosphorylation and lipidation.
In PCa tissues, overexpression of prostatic leucine zipper (PrLZ) can promote cell growth and migration (Zeng et al., 2018), and Cullin 3/SPOP can mediate ubiquitination and degradation of PrLZ, thereby regulating prostate cancer progression (il - 6th). Activation of ERK1/2 expression prevents SPOP-mediated degradation of PrLZ phosphorylation at Ser40 (Fan et al., 2022). Protein acetylation is also associated with protein degradation. Early studies demonstrated that proteins with free α-amino groups can be degraded by ATP-dependent ubiquitin degradation, and that ubiquitin-mediated protein degradation can be prevented when the N-terminal α-amino group is acetylated (Xia et al., 2020; Hwang, Shemorry & Varshavsky, 2010). P21-activated kinase 6 (PAK6) in the inner mitochondrial membrane promotes sirtuin protein 4 (SIRT4) ubiquitination degradation, while SIRT4 abolishes acetylation of adenine nucleotide translocase 2 (ANT2) to promote ANT2 ubiquitination degradation. PAK6 directly phosphorylates ANT2 to inhibit prostate cancer cell apoptosis, and the phosphorylation and deacetylation modification of ANT2 are mutually regulated, thereby promoting PCa progression (Ding et al., 2022). Both CDK4/6 and CDK2 can phosphorylate RB, and RB phosphorylation decreases the interaction between HDAC5 and RB (Zhou et al., 2021). CBP/p300 interacts with the Glu/ASP-rich C-terminal domain transactivator 2 (CITED2) and binds to polymer complexes (NCL, p300, PRMT5) to drive nucleolar protein (NCL) methylation and acetylation, thus inducing NCL regulate translocation. AKT is activated to drive the EMT and cell migration (Shin et al., 2018). Phenethyl isolipoate (PEITC) is a natural compound derived from horseradish that regulates histone acetylation, activates the PI3K/AKT pathway, and phosphorylates PI3K to regulate prostate cancer cell development (Wu et al., 2020). SPOP regulates lipid metabolism by reducing FASN expression and fatty acid synthesis, thereby inhibiting tumour progression (Gang et al., 2019).
The forkhead box protein A1 (FOXA1) is an invasion suppressor in prostate cancer. EZH2 prevents FOXA1 ubiquitination by enhancing FOXA1 methylation and increasing FOXA1 stability (Park et al., 2021). Simultaneously, the deubiquitinating enzyme USP7 also interacts with FOXA1 to reduce the ubiquitination of FOXA1, and the use of EZH2 and USP7 inhibitors (GSK-126 and EPZ-6438) inhibits the growth of PCa (Li et al., 2020). Acetylation of LIFR K620 is dependent on AKT production and promotes PCa progression through phosphorylation of LIFR S1044, which activates the AKT pathway and recruitment of 3-phosphoinositol-dependent protein kinase 1 (PDPK1) and PTEN loss connected is. This represents a biomarker to monitor the progression of PCa (Ding et al., 2022). The retinoblastoma protein (RB) binds to HDAC, and when HDAC5 is absent, it increases prostate cancer cell resistance to the CDK4/6 inhibitor palbocinb. Baicalein can regulate fatty acid metabolism and induce cell apoptosis by activating the AKT-SREBP1-FASN signalling network in human PCa cells, showing potent anti-tumour effects. Therefore, it may be a promising candidate for anticancer drug development (Sun et al., 2022). Eriobotrya japonica (EJCE) blocks SREBP-1/FASN-driven metabolism (Hsieh et al., 2021). Targeting FASN or in combination with AR pathway inhibitors (SCD1 and AR) is likely to be a combined drug strategy (Galbraith, Leung & Ahmad, 2018). The combination of orlistat (a FASN inhibitor) and radiotherapy significantly reduced NF- κB activity and associated downstream proteins in both prostate cancer cells, and the combination therapy showed the best tumour suppression (Chuang et al., 2019). By restricting histone lactylation and HIF1A expression in PCa cells, Evodiine blocks lactate-induced angiogenesis and further enhances Sema3A transcription while inhibiting PD-L1 transcription. Evodiine is a promising agent for anti-angiogenesis therapy or immunotherapy of PCa (Yu et al., 2023).
Conclusion and Perspective
In summary, post-translational modification of proteins plays an important role in cellular processes by regulating cell signalling, protein localisation and maintaining cellular function by altering protein structure and function. However, protein modifications such as protein methylation, succinylation and lactylation have been less studied in prostate cancer. At present, these studies only show that protein succinylation, lactylation and methylation modifications can be used as promoting factors in prostate cancer, and do not conduct in-depth studies on the role these modifications play in prostate cancer and its treatment. Therefore, we can reasonably assume that the specific regulatory role of these modifications in prostate cancer may become a new direction of prostate cancer targeted therapy. In the future, it may be possible to use PROTAC technology to directly mediate protease degradation of target proteins for succinylation, lactylation and methylation modification, or to mediate other types of modified enzymes to competitively target the sites of succinylation, lactylation and methylation modification. and provide a new vision for gene therapy. The PROTAC technology can be used to design proteins that are difficult to target with traditional small molecule drugs and therefore has great potential in drug development. With this technology, some protein targets that have traditionally been considered “undruggable” can be targeted, providing an entirely new therapeutic approach.
Now, with advances in biotechnology such as high-throughput sequencing, proteomics and metabolomics, these technologies can be used to screen for new biomarkers to reduce prostate cancer mortality (Khoo et al., 2021). High-throughput sequencing technologies have identified millions of genetic mutations in a wide range of human diseases. The combination of functional features such as PTMs with genetic mutations can distinguish disease-associated mutations and provide potential molecular targets for new therapeutic strategies (Peng et al., 2020). Mass spectrometry can be used to detect and quantify proteins in prostate secretions, urine and blood to assess disease status (Khoo et al., 2021). For example, fucosylated (Takahashi et al., 2016) and n-glycosylated (Yang et al., 2015) N-glycans of the protein haptoglobin can be used as biomarkers for prostate cancer. Biomarkers for succinylation, lactylation and methylation modifications can therefore be developed using these techniques. Kinase inhibitors, methyltransferase inhibitors, deacetyltransferase inhibitors and ubiquitin ligase inhibitors have achieved remarkable success in clinical use (Zhai et al., 2022). Mass spectrometry-based proteomics is a powerful approach for system-wide characterisation of PTMs, helping to identify drug targets, elucidate drug mechanisms of action and personalise treatment (Khoo et al., 2021).
Irreversible post-translational modification of proteins that promote the migration and proliferation of tumour cells, by comprehensively analysing different post-translational modification patterns, a new PTMI model was established that can accurately predict the clinical prognosis and treatment response of colorectal cancer (CRC) patients (Liu & Zhu, 2023). New models can also be developed for prostate cancer, such as GlycoPAT, but only for glycosylation changes in prostate cancer (Liu et al., 2017). Therefore, it may be possible in the future to develop a simulation platform for the computational assessment of methylation, succinylation and lactylation in prostate cancer. A detailed study of post-translational protein modifications not only helps us to understand the mechanisms of prostate carcinogenesis, but also opens up new opportunities in the biopharmaceutical field. Therefore, it is hoped that by studying the aberrant changes in post-translational modifications of proteins, new markers associated with prostate cancer can be discovered and new diagnostic methods and treatment strategies can be developed.