Vernicia fordii leaf extract inhibited anthracnose growth by downregulating reactive oxygen species (ROS) levels in vitro and in vivo
- Published
- Accepted
- Received
- Academic Editor
- Marcello Iriti
- Subject Areas
- Biochemistry, Conservation Biology, Mycology, Toxicology
- Keywords
- Camellia oleifera, Leaf extracts, Vernicia fordii, Colletotrichum fructicola, Reactive oxygen species, Anthracnose, Pathogenic genes
- Copyright
- © 2024 Ge et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Vernicia fordii leaf extract inhibited anthracnose growth by downregulating reactive oxygen species (ROS) levels in vitro and in vivo. PeerJ 12:e17607 https://doi.org/10.7717/peerj.17607
Abstract
Background
Colletotrichum fructicola is a predominant anthracnose species in Camellia oleifera, causing various adverse effects. Traditional intercropping Vernicia fordii with C. oleifera may enhance anthracnose resistance, but the mechanism remains elusive.
Methods
We utilized UPLC-MS/MS and acid-base detection to identify the major antimicrobial alkaloid components in the V. fordii leaf extract. Subsequently, by adding different concentrations of V. fordii leaf extract for cultivating C. fructicola, with untreated C. fructicola as a control, we investigated the impact of the V. fordii leaf extract, cell wall integrity, cell membrane permeability, MDA, and ROS content changes. Additionally, analysis of key pathogenic genes of C. fructicola confirmed that the V. fordii leaf extract inhibits the growth of the fungus through gene regulation.
Results
This study discovered the alkaloid composition of V. fordii leaf extract by UPLC-MS/MS and acid-base detection, such as trigonelline, stachydrine, betaine, and O-Phosphocholine. V. fordii leaf extract successfully penetrated C. fructicola mycelia, damaged cellular integrity, and increased ROS and MDA levels by 1.75 and 2.05 times respectively, thereby inhibiting C. fructicola proliferation. By analyzing the key pathogenic genes of C. fructicola, it was demonstrated that the antifungal function of V. fordii leaf extract depends mainly on the regulation of RAB7 and HAC1 gene expression. Therefore, this study elucidates the mechanism of V. fordii -C. oleifera intercropping in strengthening anthracnose resistance in C. oleifera, contributing to efficient C. oleifera cultivation.
Introduction
Vernicia fordii, a biomass energy tree prevalent in China, has been distributed in 16 provinces and internationally in places such as America, Argentina, and Paraguay since the early 20th century (Zhang et al., 2019). V. fordii is a deciduous specious species that produces abundant fallen leaves in fall and winter (Zhang et al., 2014). Leaves of V. fordii possess bactericidal properties. Liu Liping’s research (Liu et al., 2009) indicates that V. fordii leaf extract (VFLE) exhibits potential antibacterial activity against Staphylococcus aureus, Escherichia coli, and Shigella dysenteroae. 80% ethanol extract from V. fordii leaf showed superior bacteriostasis. However, the components responsive to antimicrobial activity, mode of action, and inhibitory properties of fungi remain unknown.
Camellia oleifera, a valuable oil plant with a high monounsaturated fatty acid content, is of concern due to anthracnose causing failure rates of up to 50% and plant decline (Zeng et al., 2014; Li et al., 2016). In oil tea-producing regions, anthracnose reduces seed yield by 10–30% annually with extreme cases of 40–50% loss of yield, resulting in significant economic losses (Cao et al., 2023). The disease affects multiple plant organs and has prominent effects on various vegetables and fruits, including coffee, cocoa, avocado, mangos, mulberry, pistachio, and persimmon, so forth (Prihastuti et al., 2009; Rojas et al., 2010; Sanders & Korsten, 2003; Kwarteng et al., 2023; Xie et al., 2010; Yan et al., 2011; Yang et al., 2011). Mainly systemically applied fungicides such as carbendazim or metribuzin in China are inefficient due to a single mechanism of action causing fungicide resistance and environmental pollution. Moreover, these fungicides lack specific targeting of C. fructicola while showing broad-spectrum activity (He et al., 2022; Ma & Li, 2023).
In China, intercropping between V. fordii and C. oleifera trees dates back to Xu Guangqi’s “Agricultural Policy Book” in the Ming Dynasty. Intercropping of V. fordii with C. oleifera trees significantly enhances C. oleifera tree productivity compared to monoculture, and the anthracnose incidence rate of C. oleifera in the intercropped forests is lower than that of monoculture C. oleifera forests. The peak anthracnose occurrence time in C. oleifera coincides with the leaf-shedding phase of V. fordii. In mixed C. oleifera and V. fordii forests, the deciduous leaves of V. fordii serve as a natural canopy, containing bioactive constituents that impede the outbreak of C. oleifera anthracnose. Research indicates that intercropping of maize and Cynanchum bungei (He-Shou-Wu) effectively reduces the incidence rates of rust disease, leaf spot, downy mildew on Cynanchum bungei, and brown spot on Cynanchum bungei compared to monoculture. Furthermore, the flavonoid content increased by 8.42% and 6.58%, polyphenol content increased by 15.87% and 13.97%, and total steroidal glycosides content increased by 10.07% and 9.45% respectively in the intercropped treatments. Additionally, the yield of tuberous roots also increased by 14.5% and 13.8% respectively. These results indicate that the intercropping system of corn with Polygonum multiflorum is beneficial for reducing disease incidence (Shen et al., 2021). This may provide one explanation for the lower incidence rate of anthracnose in mixed C. oleifera and V. fordii forests compared to pure C. oleifera forests. Therefore, we speculate that certain V. fordii leaf components may inhibit C. fructicola and contribute to C. oleifera anthracnose relief. In our study, UPLC-MS/MS was used to identify the metabolites and major antifungal components of VFLE. Antifungal testing using C. oleifera anthracnose as the target sought to examine the effect of VFLE on C. oleifera anthracnose. The antifungal mechanism was investigated through mycelium growth observation, mycelium surface morphology analysis, mycelium cell wall integrity examination, mycelium cell membrane permeability assessment, mycelium pathogenicity assessment, and pathogenic gene expression analysis.
Materials and Methods
For this investigation, the certified local cultivar ‘Huatong No. 1’ of V. fordii (Hemsl.) Airy Shaw, derived from the germplasm conservation bank in Yongshun County, Hunan Province, was employed. Ten-year-old, uniform monocarpic plants were chosen and their leaves, flowers, and husks were preserved at −80 °C following collection. Analysis of leaf metabolite composition was initiated within one month of collection.
Test strain: Colletotrichum fructicola, the cause of anthracnose disease in C. oleifera, was provided by the Microbial Strain Conservation Experiment Center of Central South University of Forestry Science and Technology.
Leaves of C. oleifera: Immature leaves of C. oleifera were collected from the nursery on the roof of the Tree Building of Central South University of Forestry and Technology (Changsha, Hunan, China).
Preparation of extracts from V. fordii flowers, leaves, and husks
The samples of paulownia flowers, leaves, and fruit shells were ground with liquid nitrogen (30 Hz, 1.5 min) using an MM 400 grinder to powder form. After adding 200 mL of 80% acidic ethanol (pH adjusted to 3.0 with HCl) to each powder sample, further soaking was allowed for 24 h. After 1 h of sonication (SB-120DTN; 120W, Dongkang, Tianjin, China), the extract was filtered (DP-01; 20W, Scientz, Ningbo, Zhejiang, China). The extraction was then evaporated using a rotary evaporator, and the remaining paste was dissolved in three mL 0.1% DMSO before filtering through a 0.45 µm organic filter membrane (Liu et al., 2009).
Component identification (UPLC-MS/MS)
All chromatographic separations were carried out utilizing an ultra-performance liquid chromatography (UPLC) system coupled with an Agilent SB-C18 column (1.8 µm, 2.1 mm ×100 mm) (USA). The mobile phase consisted of solvent A (ultrapure water with 0.1% formic acid, v/v) and solvent B (acetonitrile), subjected to an elution gradient initiated at 5% solvent B and gradually increasing to 95% over 9 min, followed by a 1-min hold before decreasing to 5% and maintaining for 3 min. The flow rate was set at 0.35 mL/min, column temperature at 40 °C, and the injection volume was 4 µL.
The MS settings consisted of: an ESI source operating at a temperature of 550 °C, a mass spectrometer voltage of 5,500 V, and a gas curtain set at 206.8 kPa. CAD with high parameter settings was used in the triple quadrupole (QQQ) instrument, with each ion pair scanned and detected based on optimized declustering voltage and collision energy.
Quantification of major alkaloids in leaves of V. fordii
Concentrate a sufficient volume of VFLE into methanol to achieve a detection solution with a concentration of 10 µg/mL, utilizing an HPLC method concerninga standard curve consisting of trigonelline, stachydrine, O-phosphocholine, and betaine. The alkaloid content of V. fordii leaves was monitored using this procedure.
HPLC parameters included: Shimadzu Corporation’s Shim-pack XR-ODS III column (75 mm × 2.0 mm, 1.6 µm), a column temperature maintained at 35 °C, and a flow rate of 0.2 mL/min. The mobile phase consisted of solvent A (a 0.1% aqueous solution of formic acid) and solvent B (acetonitrile). An initial 5% B ratio was used for 6 min, rising linearly to 15%, followed by a 3-min equilibrium. Next, the B ratio dropped to 5% and remained steady for another 5 min.
Inhibition of extraction on pathogenicity of C. fructicola
The pathogenicity of the test strains was determined by conducting a detached leaf inoculation assay on C. oleifolia leaves, as described by reference Qian et al. (2009). Fresh and uniform-sized young leaves of C. oleifolia were collected and washed with distilled water. The leaves were then placed in Petri dishes lined with moist filter paper, with a piece of degreased cotton moistened with distilled water covering the leaf stalk. In the treatment group, a sterilized needle was heated using an alcohol lamp and cooled. Small holes were punctured on both sides of the C. oleifolia leaves, and in these holes, fungal mycelial plugs (6 mm in diameter) obtained from culture using 600 µg/mL extracts of V. fordii flowers, leaves, and fruit husks were inoculated. The control group consisted of six mm fungal mycelial plugs cultured on a standard PDA medium. The sealed Petri dishes were incubated at a temperature of 28 °C (GNP-9050; Sanfa, Shanghai, China). After 4 days of dark incubation at 28 °C, the leaves were removed, and the diameters of the fungal mycelial growth were measured using a vernier caliper with a cross measurement. Each treatment had three replicates. The average value was used to calculate the growth inhibition rate of the detached leaf lesions:
Inhibition rate of lesion growth %.
Study of the anti-potency of VFLE against C. fructicola
VFLE at various concentrations was treated in the culture medium where the C. fructicola fungus was then inoculated. The impact of VFLE on the parasitic mycelium of C. fructicola was evaluated by quantifying colony growth. The study used a PDB medium prepared using 3.5 g of potato dextrose agar + 100 mL of distilled water, sterilized at 121 °C for 20 min. Once cooled, VFLE at final concentrations of 0, 100, 200, 400, 600, 800, and 1000 µg/mL, respectively, was added. Fungal cubes (6 mm) of C. fructicola were placed and the medium was then incubated at 28 °C. Each test involved three repeats.
SEM observation
The mycelia of C. fructicola were cultivated in the presence of 600 µg/mL VFLE and subsequently subjected to sequential washing with phosphate buffer salt solution (PBS) solution on two occasions. Subsequently, the pellet was resuspended in PBS and subjected to centrifugation (5000 rpm, 4 °C, 5 min) to separate the supernatant. The recovered mycelial biomass was then fixed in a 3% glutaraldehyde solution at 4 °C for 24 h, after which it underwent thorough rinsing with sterile water for 20 min. Subsequently, the samples were subjected to graded ethanol dehydration, with sequential immersion in 30%, 50%, 70%, and 95% ethanol solutions for 20 min each, culminating in a final dehydration step in absolute ethanol for 45 min. The treated mycelia were subsequently freeze-dried, gold-coated, and examined under a scanning electron microscope (SEM-6380LV; Yishu, Shanghai, China) (Cai et al., 2014). All experiments had three replicates.
Evaluation of cell wall integrity and cell membrane permeability
One hundred milliliters of PDB were supplemented with 100, 200, 400, and 600 µg/mL of VFLE, respectively. Subsequently, a six mm mycelium mass of C. fructicola was added to the medium and cultivated for 3 d at 28 °C in an oscillating incubator with 160 rpm. Supernatant and mycelium were isolated to assess extracellular alkaline phosphatase (AKP) activity and cell wall integrity, respectively. Three replicates per treatment were performed.
To examine cell membrane permeability, 0.10 g of mycelium was weighed and steeped in one mL PBS (37 °C, 5 min) and then rinsed thrice. Electrical conductivity was determined using a conductivity meter (DDSJ-319L; INES, Shanghai, China). Three replicates were used for each treatment.
Assessment of oxidative stress
An amount of 0.10 g of mycelium was weighed and assayed for intracellular ROS levels using the non-specific ROS probe DCF-DA (Gemes et al., 2016). Absorbance was measured at 450 nm (ELISA reader, F-4500; Hitachi) to determine ROS concentration. MDA content was detected following the procedures outlined in the MDA assay kit purchased from Jiangsu Enzyme Free Company (China), with three replicates per treatment.
Cellophane penetration assay
Mycelia measuring 2 mm ×2 mm were excised from VFLE-cultured colonies of C. fructicola and transferred to cellophane-coated PDA plates. Plates were maintained for 48 h at 28 °C after which cellophane was removed. Three replicates per treatment were included.
Gene expression analysis
The PDB medium was supplemented with VFLE at final concentrations of 0, 100, 200, 400, and 600 µg/mL, followed by the addition of six mm mycelial discs. Cultures were incubated at 28 °C and 160 rpm for 48 h before samples were subjected to RNA extraction. RNA was designated as CK A, 0.5A, 1A, 2A, 3A, and preserved at −80 °C. Custom RT-qPCR primers (Table S1) were designed for the detection of HAC1, MKK1, RAB7, VAM7, and VPS39 gene expressions. qRT-PCR was conducted on the CFX96™ real-time PCR system (Bio-Rad, Hercules, CA, USA) with SYBR® Premix Ex Taq™ II (AG) using three replicates per treatment.
Statistical analysis
Each experiment was repeated thrice. Mass spectrometry data were processed utilizing the software SCIEX Analyst version 1.6.3. Experimental data, including AKP enzyme activity, extracellular conductivity, malondialdehyde content, reactive oxygen species (ROS) activity, anthracnose lesion size in detached leaves of C. oleifera, and the size of C. fructicola colonies on alkaloid standard culture plates, were processed and statistically analyzed via Excel 2016, IBM SPSS Statistics for one-way ANOVA, Image J, and Origin software. The expression levels of the selected genes were calculated using the 2−ΔΔCt method.
Results
Characterization of principal components in V. Fordii leaves
UPLC-MS/MS analysis identified 571 metabolites, with the percentage contribution of bioactive components such as phenolic acids, flavonoids, lignans, coumarins, tannins, alkaloids, and terpenoids exceeding 50%. Alkaloids and flavonoids have shown anti-bacterial and anti-inflammatory properties. The pH of the V. fordii leaves oil, tested in water and ethanol, was found to be above 8.0, indicating mainly alkaline properties (Table 1).
| Composition types | Composition | Characteristic component |
|---|---|---|
| Phenolic acid | Benzamide, Benzoic acid, 2-Phenylethanol, Salicylic acid*, Cinnamic acid, Protocatechuic acid*, Caffeic aldehyde, Terephthalic acid*, Coniferyl alcohol, Caffeic acid, Methyl 2,4-dihydroxyphenylacetate, Methyl ferulate, 2-Nitrophenol, 2,6-Dihydroxybenzoic acid, Anthranilic acid, Pyrogallol, Dibutyl phthalate*, Neochlorogenic acid*, Tetragallic acid, Ferulic acid, Methyl ferulate, Sinapinaldehyde, 2-Feruloyl-sn-glycerol, 1-Feruloyl-sn-glycero etc. 106 species in total. | Caffeoylnicotinoyl, Illoyltartaric acid |
| Flavone | Naringenin*, Phloretin, Phloretin-2′-O-(6′-O-xylosyl)glucoside Homoeriodictyol, Quercetin-3-O-robinobioside*, Quercetin*, Dihydroquercetin(Taxifolin), Catechin gallate*, 5,7,3′,4′,5′-Pentahydroxydihydroflavone, Gallocatechin-gallocatechin-gallocatechin, Isohyperoside*, Catechin gallate*, Quercetagetin-4′-Methyl Ether*, Robinetin*, 5,7,4′-Trihydroxyisoflavone-7-O-galactoside-rhamnose* Syringetin, Kaempferol-3-O-rhamnoside (Afzelin)(Kaempferin)*, Epicatechin gallate*, Naringenin-7-O-Rutinoside(Narirutin)*, Quercetin-3-O-glucoside (Isoquercitrin)*, Naringenin-4′-O-glucoside*, Dihydroquercetin(Taxifolin), Phloretin-2′-O-(6′-O-xylosyl)glucoside, Limocitrin (5,7,4′-trihydroxy-8,3′-dimethoxyflavone)* etc. 99 species in total. | — |
| Lignan | ClemaphenOl A, 4-Nitrocatechol, Pinoresinol-4-O-glucoside, Pinoresinol , Medioresinol-4′-O-(6′’-acetyl)glucoside, Pinoresinol-4-O-(6′-acetyl)glucoside etc. 9 species in total. | — |
| Coumarin | Methyl Brevifolincarboxylate, Scopoletin-7-O-glucuronide, Isofraxidin*, Scopoletin-7-O-glucoside (Scopolin), Isoscopoletin (6-Hydroxy-7-Methoxycoumarin), Fraxidin (8-Hydroxy-6,7-dimethoxycoumarin)* etc. 10 species in total. | — |
| Tannin | Ellagic acid, Geraniin, Gallic acid, Digallic Acid, Brevifolin, Gemin D, 1,3,6-Tri-O-galloyl-D-glucose*, 7-O-Galloyl-D-sedoheptulose, 3,3′,4-Trimethoxyellagic acid, 1,3,4,6-Tetra-O-Galloyl-D-Glucose, 2-O-Salicyl-6-O-Galloyl-D-Glucose, 1,2,3,4,6-Penta-O-Galloyl-D-Glucose etc. 31 species in total. | — |
| Alkaloid | Spermine, Trigonelline, Piperidine, N-Benzylmethylene isomethylamine, Tryptamine, Indole, Choline, Betaine*, Acetylcholine, Indole-3-carboxaldehyde, Histamine, Strictinin, Nicotianamine, O-Phosphocholine, trigonellin, Choline Alfoscerate, 6-Deoxyfagomine* etc. 39 species in total. | — |
| Terpenes | Ursonic acid*, Ketoursolic acid*, Camaldulenic acid, Pentadecanoic acid etc. 8 species in total. | 16 α-Hydroxytrametenolic acid |
Characteristics of key alkaloids in V. fordii leaves
Morphosate targeted metabolomics analysis established the substantial presence of several active alkaloid metabolites such as trigonelline, stachydrine, betaine, and O-phosphocholine (Table 2).
| Sequence | name of compound | Molecular weight/Da | Lon mode | The molecular weight of parent ion/Da | molecular weight of ion/Da |
|---|---|---|---|---|---|
| 1 | Trigonelline | 137.048 | + | 138.05 | 94.07 |
| 2 | Stachydrine | 143.095 | + | 144.1 | 84.1 |
| 3 | Betaine | 117.079 | + | 118.09 | 59 |
| 4 | O-phosphocholine | 184.073 | + | 184 | 125 |
Notes:
“+” Indicates positive ion mode.
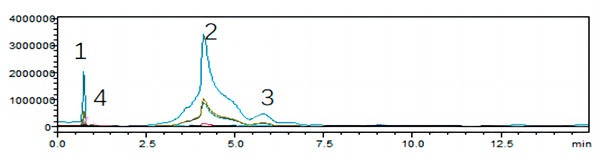
To unearth the predominant alkaloids in the antifungal efficacy of VFLE, a comprehensive HPLC analysis was performed. As shown in Fig. 1, the VFLE showed four peaks corresponding to standard compounds: trigonelline, stachydrine, betaine, and O-phosphocholine, respectively.
Peak area-based methods were used for quantitative analysis of trigonelline, stachydrine, betaine, and O-phosphocholine, revealing that VFLE exhibited the highest stachydrine content at 13.455 mg/L (Table 3).
V. fordii extracts inhibited mycelial growth
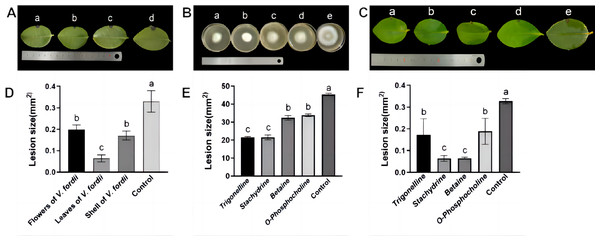
Observations in Figs. 2A and 2D illustrate that lesions on C. oleifera leaves treated with pathogenic fungi were reduced upon exposure to extracts of V. fordii flower, leaf, and husk, respectively, indicating a reduction in virulence of C. fructicola, thereby constraining lesion expansion. Importantly, VFLE decreased fungi infectivity to a significant extent, showing only 18.75% lesion area compared to the control group (Table 4). These findings suggest VFLE as an effective mitigant of C. oleifera anthracnose, laying the groundwork for future development.
The results of antifungal activity tests on C. fructicola by primary alkaloids from V. fordii are shown in Figs. 2B, 2E, and Table 5. Notably, all four alkaloid criteria significantly inhibited C. fructicola, with stachydrine and trigonelline showing superior suppression compared to betaine and O-phosphocholine, resulting in significantly reduced colony diameters compared to the control group. The efficacy experiment revealed reduced virulence of the fungus post-cultivation with alkaloid standards, yielding smaller lesions compared to untreated control (Fig. 2C). Specifically, stachydrine and betaine showed significantly higher inhibitory effects on lesion production than trigonelline and O-Phosphocholine, resulting in lesion areas only one-fourth the size of the control group (Fig. 2F). Thus, it appears that the primary bioactive alkaloids responsible for limiting C. fructicola growth in VFLE are trigonelline and betaine.
Figure 1: HPLC chromatograms of VFLE extract.
Peaks 1: Trigonelline; Peak 2: Stachydrine; Peak 3: Betaine; Peak 4: O-phosphocholine.VFLE inhibition concentration
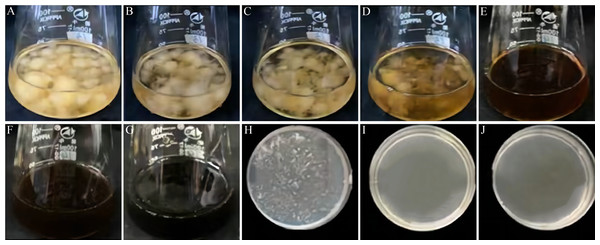
VFLE’s effect on C. fructicola growth, at various concentrations, is demonstrated in Fig. 3 and Table 6. C. fructicola mycelia ceased growth after 2 d steeped in a PDB liquid medium with VFLE concentration reaching 600 µg/mL (Fig. 3E). On further incubation for two more days on PDA plates with VFLE above 600 µg/mL, C. fructicola growth completely ceased at a concentration of 800 µg/mL (Fig. 3I). This illustrates VFLE’s potent inhibition of C. fructicola growth and its positive antifungal effects.
| Sequence | name of compound | Retention time | Qualitative ion pair | Regression equation | Compound content (mg/L) |
|---|---|---|---|---|---|
| 1 | Trigonelline | 0.92 | 118.1>42.1 | Y = 170332X+2.26225e+006 | 3.874 |
| 2 | Stachydrine | 3.56 | 137.8>92.0 | Y=386728X+1.06064e+006 | 13.455 |
| 3 | Betaine | 5.79 | 145.1>58.0 | Y = 26589.0X+310872 | 4.698 |
| 4 | O-Phosphocholine | 0.94 | 83.8>124.9 | Y = 123731X+1.16866e+006 | 2.588 |
Figure 2: Inhibition of growth of C. fructicola mycelium by V. fordii extracts.
(A) Map of C. oleifera lesions post-V. fordii treatment, treatments a-d: V. fordii application on flowers, leaves, and husks, and a control; (B) colony growth pattern with treatments a-e: Trigonelline, Stachydrine, Betaine, O-Phosphocholine, and a control; (C) in vitro leaf lesion growth; (D) spot sizing analysis for defining C. oleifera lesion pathogenicity; (E) colony diameter alteration; (F) lesion size modifications, treatments a-e: trigonelline, stachydrine, betaine, O-phosphocholine, and a control. The values represent the means ± standard deviations. Statistical analysis was performed using t-test. The lowercase letters indicate a significant difference at the 0.05 level, and the same letters indicate insignificant differences.| Extract | Diameter of colony /mm | Inhibition rate of colony growth /% | The disease of spot area /mm2 | Inhibition rate of disease spot growth/% |
|---|---|---|---|---|
| Flower of V. fordii | 34.88 ± 0.26 b | −8.89 | 0.21 ± 0.19 b | 34.37 |
| Leaf of V. fordii | 23.73 ± 0.26 b | 25.91 | 0.06 ± 0.13 c | 81.25 |
| Husk of V. fordii | 22.61 ± 1.91 b | 29.40 | 0.18 ± 0.16 b | 43.75 |
| CK | 32.03 ± 0.36 ab | 1.32 0.45 a |
| Alkaloid | Diameter of colony /mm | Inhibition rate of colony growth /% | The disease of spot area /mm2 | Inhibition rate of disease spot growth /% |
|---|---|---|---|---|
| Trigonelline | 23.76 ± 0.48 c | 46.00 | 0.17 ± 0.25 b | 45.16 |
| Stachydrine | 23.92 ± 1.32 c | 46.17 | 0.06 ± 0.05 c | 80.64 |
| Betaine | 34.50 ± 1.36 b | 22.07 | 0.07 ± 0.02 c | 80.64 |
| O-Phosphocholine | 34.88 ± 0.78 b | 21.22 | 0.18 ± 0.21 b | 42.93 |
| Control | 46.35 ± 0.29 a | 0 | 1.32 ± 0.15 a | 0 |
Notes:
The values represent the means ± standard deviations. Statistical analysis was performed using t-test. The lowercase letters indicate a significant difference at the 0.05 level, and the same letters indicate insignificant differences.
Figure 3: Effect of VFLE on mycelial growth of C. fructicola.
(A–G) 0, 100, 200, 400, 600, 800, 1000 µg/mL VFLE on liquid culture of C. fructicola, respectively; (H–J) 600, 800, 1000 µg/mL VFLE on solid culture of C. fructicola, respectively.| Concentration (µg/mL) | 0 | 100 | 200 | 400 | 600 | 800 | 1000 |
|---|---|---|---|---|---|---|---|
| PDB Culture 2d | ++ | ++ | ++ | ++ | – | – | – |
| PDA Culture 2d | ++ | ++ | ++ | ++ | + | – | – |
Notes:
“++” indicates vigorous colony growth in the medium; “+” indicates small colony growth; “-” indicates no colony growth. Three biological replicates for each treatment.
VFLE compromised the cell wall and membrane of C. fructicola
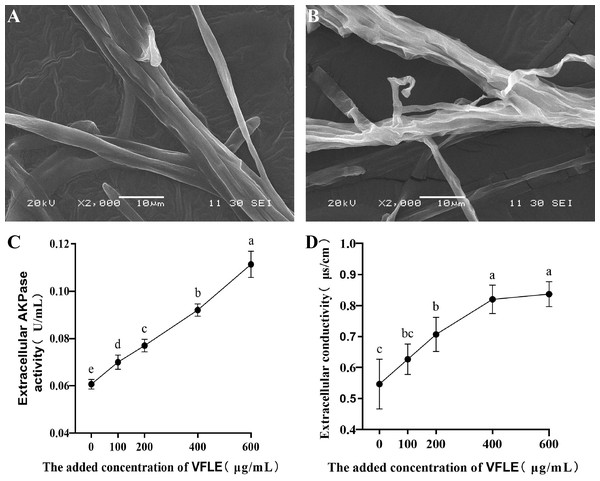
To examine the anti-fungal effects of VFLE on C. fructicola, cell membrane integrity and wall integrity were examined. Initially, C. fructicola mycelium subjected to various treatments was visualized by scanning electron microscopy. The surface morphologic alterations induced by VFLE are depicted in Fig. 4. The untreated control group presented a smooth, complete, and intact structure, showing even thickness and robust growth (Fig. 4A). Conversely, with 600 µg/mL VFLE, there were rough, irregular, and prominent wrinkles (Fig. 4B). Next, wall damage was estimated through AKP activity. The experiment showed dramatic augmentation in the VFLE-treated group, reaching 0.111 ± 0.005 U/mL, significantly higher than the untreated control group (0.07 ± 0.09 U/mL, p < 0.05) (Fig. 4C). This significant increase signifies compromised cell wall integrity, causing AKP release from the periplasmic space into the extracellular space. In addition, the cell membrane’s response was evaluated by investigating relative electrical conductivity shifts. As shown in Fig. 4D, the treated group showed an appreciable increase in relative electrical conductivity compared to the untreated control group, and the conductivity escalated progressively with VFLE addition. Noteworthy, a sharp increase within the 200–400 µg/mL VFLE dose range was seen, subsequently reaching a plateau with escalating concentration, indicating saturated electrolyte leakage and slow conductivity increment.
Figure 4: Impact of VFLE on cell wall integrity and membrane permeability of C. fructicola.
(A) C. fructicola mycelium in control group; (B) C. fructicola mycelium treated with 600 µg/mL; (C) AKP change under VFLE treatment; (D) alteration in electrolyte leakage content under VFLE treatment. The values represent the means ± standard deviations. Statistical analysis was performed using t-test. The lowercase letters indicate a significant difference at the 0.05 level, and the same letters indicate insignificant differences.VFLE induced an increase in oxidative stress in fungal mycelia
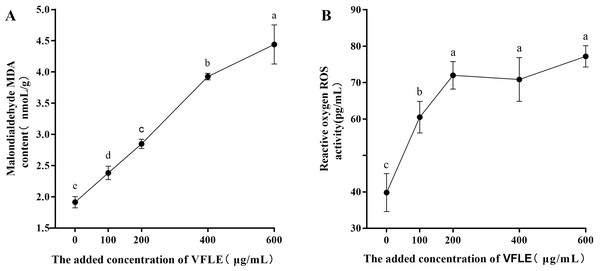
Considerable increases in malondialdehyde (MDA) were observed in the VFLE-treated mycelium, as depicted in Fig. 5. Notably, the MDA levels escalated with elevated VFLE concentration, particularly at 200–400 µg/mL inducing substantial apoptosis of C. fructicola mycelium. Furthermore, the introduction of 100 µg/mL VFLE to the medium resulted in a marked rise in the mycelium’s reactive oxygen species (ROS) content, suggesting the accumulation of ROS post-VFLE treatment in C. fructicola mycelium.
VFLE influenced the colonization capacity of C. fructicola
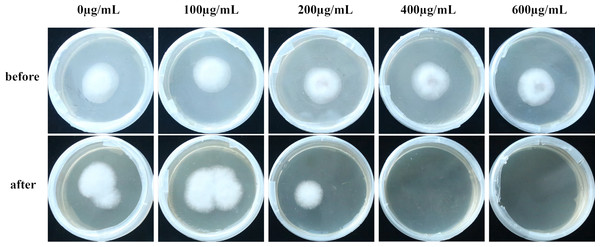
Cellophane mimicry of C. oleifera served as a substrate on which cultures with varying levels of VFLE were inoculated. Observations demonstrated that post-cellophane removal, non-VFLE, and 100 and 200 µg/mL VFLE-treated fungi consistently formed colonies. Conversely, 400 and 600 µg/mL VFLE-treated strains failed to proliferate (Fig. 6), suggesting VFLE-restricted C. fructicola penetration.
Figure 5: Effect of VFLE on ROS and MDA content of C. fructicola.
(A) Propylene glycol MDA content; (B) ROS activity. The values represent the means ± standard deviations. Statistical analysis was performed using t-test. The lowercase letters indicate a significant difference at the 0.05 level, and the same letters indicate insignificant differences.Figure 6: Effect of VFLE on penetration of C. fructicola cellophane.
Before: Cellophane membrane colonies; After: Cellophane membrane colonies removal.VFLE affected the expression of key pathogenic genes
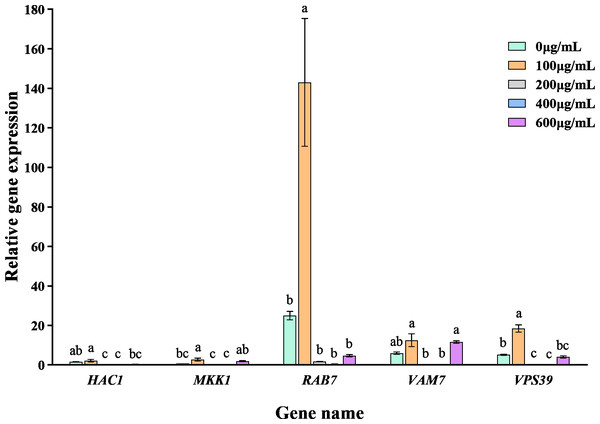
The addition of varying concentrations of VFLE significantly altered the expression levels of five major pathogenic genes in C. fructicola (Fig. 7). These genes showed their peak expression at an extract concentration of 100 µg/mL, then gradually declined with increased extract concentration. This observation indicates that VFLE, at a concentration of 100 µg/mL, amplifies the pathogenicity of C. fructicola in C. oleifera. However, as VFLE concentration increases, C. fructicola growth in C. oleifera is inhibited, resulting in reduced pathogenicity. The most profound effect was observed for RAB7, followed by HAC1 and VPS39.
Figure 7: Transcriptional changes of five pathogenic genes following VFLE addition.
The values represent the means ± standard deviations. Statistical analysis was performed using t-test. The lowercase letters indicate a significant difference at the 0.05 level, and the same letters indicate insignificant differences.Discussion
Rotation and intercropping strategies increase plant resistance to pathogens, enhance antifungal activity, and thus reduce disease incidence (Han et al., 2016; Yu et al., 2019). Intensive tea plantations benefited from the inclusion of climate-resilient crops (sorghum, cowpea) and aromatic volatile blends of semen cassiae, marigold, and flemingia as companion crops, mitigating disease and pest risks (Pokharel et al., 2023). Intercropping pest and disease suppression can be attributed to leaf and root extract components and volatiles such as DPT, DMT, and MP that proved vital in suppressing Panama banana disease in breeder practice (Li et al., 2020). Studies suggest that extracts from V. fordii leaf, flower, and husk effectively suppress anthracnose from C. oleifera, with leaf extracts showing enhanced efficacy (Liu et al., 2021). The V. fordii leaf-shedding phase was consistent with anthracnose infection peaks in C. oleifera, with interplantation significantly improving C. oleifera productivity compared to monoculture. In addition, intercropped forests were less prone to anthracnose infection than monocultural forests (Zhang et al., 2019). The abundant alkaloids in V. fordii leaves inhibited anthracnose, with stachydrine and betaine generally more potent against C. fructicola. Although the precise role of alkaloid volatiles has yet to be fully elucidated, their potential significance merits further comprehensive investigation.
Alkaloids are a diverse class, essential for biological activities, especially those related to antifungal activity. Luo et al.’s (2020) meticulous studies have definitively established the remarkable antifungal properties of Coptis alkaloids, particularly their inhibitory effects on Trichophyton rubrum and Trichophyton mentagrophyte. This antifungal activity is specifically mediated by magnoflorin, which underscores the significant potential of these alkaloids in the treatment and prevention of fungal infections (Luo et al., 2020). Magnoflorine served as a potent suppressor of T. rubrum hyphal membrane integrity, neurotransmission, and cell proliferation. In contrast, isoquinoline alkaloids showed optimal inhibitory action against Magnaporthe oryzae. After treatment with sanguinarine, M. oryzae hyphal cell turgor was depleted, leading to bending, collapse of mycelia, and eventually damage to the cell membrane. In addition, reactive oxygen species production, mitochondrial membrane potential, and nuclear morphometry declined, ultimately impairing membrane function and hyphal proliferation (Zhao et al., 2019). The results of this study show that increased extracellular alkaline protease activity, malonaldehyde content, ROS content, and extracellular relative electrical conductivity of C. fructicola were reported at different VFLE concentrations, suggesting detrimental effects on cell wall integrity and irreversible DNA, protein, and cell membrane damage leading to pathogen cell death. Studies have highlighted the significance of CfRAB7 proteins, CfHAC1, CfVAM7, CfMKK1, and CfVPS39 in C. fructicola pathogenesis (Wu, Li & Li, 2022; Yao et al., 2019). Furthermore, ΔCfrab7 exerted enhanced sensitivity to H2O2 stress, demonstrating CfRAB7 involvement in the ROS response of fruit anthrax (Wu, Li & Li, 2022). Increased ROS indices lead to increased levels of expression of these pathogenicity genes, particularly CfRAB7, in the early stages. A modest dose increased CfRAB7 protein activity while consistently high doses resulted in a significant reduction in the CfRAB7 gene and reduced pathogenicity. The inability of cells expressing high concentrations of VFLE to penetrate cellophane supports impairment in VFLE-induced C. fructicola pathogen production.
Additionally, H2O2 regulates gene expression via redox-based epigenetic modification (Bosch-Presegue et al., 2011; Cyr & Domann, 2011; Kreuz & Fischle, 2016), causing transcriptional activation of redox-sensitive transcription factors like AP-1, and NRF2, CREB, HSF1, HIF-1, TP53, NF- κB, NOTCH, SP1, SCREB-1 and FOXO family (Schreck, Rieber & Baeuerle, 1991; Allen & Tresini, 2000; Finkel & Holbrook, 2000; Bonello et al., 2007; Matsuzawa & Ichijo, 2008; Akasaki et al., 2014; Marinho et al., 2014; Espinosa-Diez et al., 2015; Weidinger & Kozlov, 2015). Furthermore, H2O2 affects posttranscriptional processing by regulating both cap-dependent and cap-independent translation (Stoneley & Willis, 2004; Li et al., 2010; Zhang et al., 2012). Our research suggests that with an elevated concentration of more than 100 µg /mL, the expression of HACl in C. fructicola of C. oleifera declined, implicating that VFLE may impair CfHAC1 transcription factor activity by boosting ROS levels. Li, Zhang & Li (2021) identified a t-SNARE protein CfVAM7 regulated by CfHAC1, affecting C. fructicola growth, stress response, autophagy, and pathogenicity by controlling the hyphal vacuole fusion process. Studies further demonstrated that CfVPS39, a CfVAM7-interacting protein, was also implicated in C. fructicola growth, autophagy, and pathogenicity regulation (Li, Zhang & Li, 2021). Our data revealed that while changes in the expression of CfVAM7 and CfVPS39 genes lacked discernable patterns, their trend remained consistent, potentially related to the interaction between CfVAM7 and CfVPS39. Although CfMKK1 expression often fluctuates within treatment groups, it suggests that CfMKK1 is not the primary pathogenic gene repressed by VFLE.
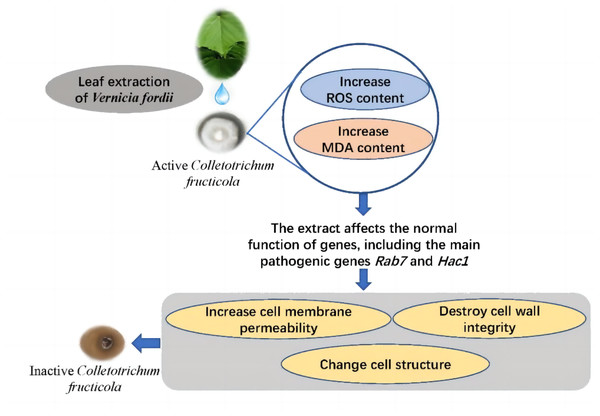
Conclusion
Ethanol extracts from V. fordii flowers, leaves, and husks potently inhibit C. fructicola’s activity, specifically VFLE. Notably, VFLE displays the most potent antifungal effect, surpassing an 80% inhibition rate at a concentration of 600 µg/mL. The primary antifungal alkaloids of VFLE are hydrothylline and betaine. Experiments reveal VFLE’s ability to permeate mycelium cells, elevating ROS levels and malondialdehyde content. Thus, pronounced downregulation of key pathogenic genes such as RAB7 and HAC1 occurs, representing the primary targets of VFLE in inhibiting anthracnose in C. oleifera. Further changes include increased cell membrane permeability, compromised cell wall integrity, and altered cell structure culminating in cell death (Fig. 8). These findings provide a solid scientific basis for the development of novel plant anthracnose inhibitors using V. fordii extracts.