Green nanobiocatalysts: enhancing enzyme immobilization for industrial and biomedical applications
- Published
- Accepted
- Received
- Academic Editor
- Abdelwaheb Chatti
- Subject Areas
- Biochemistry, Bioengineering, Biotechnology, Molecular Biology
- Keywords
- Nanomaterials, Food industry, Biofuel, Green synthesis, Nanobiocatalyst, Immobilization
- Copyright
- © 2024 Khafaga et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Green nanobiocatalysts: enhancing enzyme immobilization for industrial and biomedical applications. PeerJ 12:e17589 https://doi.org/10.7717/peerj.17589
Abstract
Nanobiocatalysts (NBCs), which merge enzymes with nanomaterials, provide a potent method for improving enzyme durability, efficiency, and recyclability. This review highlights the use of eco-friendly synthesis methods to create sustainable nanomaterials for enzyme transport. We investigate different methods of immobilization, such as adsorption, ionic and covalent bonding, entrapment, and cross-linking, examining their pros and cons. The decreased environmental impact of green-synthesized nanomaterials from plants, bacteria, and fungi is emphasized. The review exhibits the various uses of NBCs in food industry, biofuel production, and bioremediation, showing how they can enhance effectiveness and eco-friendliness. Furthermore, we explore the potential impact of NBCs in biomedicine. In general, green nanobiocatalysts are a notable progression in enzyme technology, leading to environmentally-friendly and effective biocatalytic methods that have important impacts on industrial and biomedical fields.
Introduction
Nanobiocatalyst represent a novel technological advancement that combines advanced nanotechnology with biotechnology, resulting in synergistic integration (Budhiraja et al., 2022; Villalba-Rodríguez et al., 2023; Ayub et al., 2023; Samaddar & Banerjee, 2023; Thirumavalavan, Settu & Lee, 2017). Enzymes are adaptable biocatalysts that practically all organisms use to control their metabolism and ensure their survival (Dev, Srivastava & Karmakar, 2018). Compared with chemical processes, which are carried out under extremely harsh conditions and result in unwanted side by-products, biocatalysts demonstrate remarkable selectivity and specificity for their substrates under moderate conditions (Singh et al., 2023). The use of soluble enzymes as environmentally friendly catalysts may be impeded by their limitations, including inability to be reused, increased susceptibility to various denaturing agents, elevated cost, instability in large-scale processing, conformational changes, lack of reusability, and inapplicability in fixed-bed reactors (Dadi et al., 2023b; Mohidem et al., 2023; Kalayci et al., 2024). The production of NBCs involves the integration of enzyme molecules onto carriers composed of nanomaterials to enhance the specific chemical kinetics and substrate selectivity (Oke et al., 2023). This integration has the potential to significantly boost the engineering capabilities, stability, and activity of enzymes in many bioprocessing applications. Previous studies have demonstrated the existence of nanostructured materials that conform to the NBCs regulations. Nanostructured materials with large surface areas have been developed for NBCs, providing enhanced enzyme loading and reduced mass transfer resistance (Dadi, Celik & Ocsoy, 2020). Examples include nanoporous materials, electrospun nanofibers, and magnetic nanoparticles (Kim, Grate & Wang, 2008; Najeeb et al., 2021; Hammed et al., 2022). Nanostructured materials possess various advantageous characteristics, including the ability to change their dimensions on the nanoscale. The ability to change many attributes, such as the size of nanoparticles, the thickness of nanofibers or nanotubes, and the diameter of nanopores, can be achieved by implementing this control mechanism. The uniform size distribution of nanomaterials and their resemblance in size to enzyme molecules, coupled with their beneficial properties such as magnetism and conductivity, have brought about significant advancements in nanobiocatalytic methodologies across different domains of enzyme technology. These developments have resulted in enhanced enzyme characteristics in nanobiocatalytic systems, particularly stability and activity (Ge, Lei & Zare, 2012). The green synthesis method for creating nanomaterials is efficient and requires less time. Green synthesis of nanomaterials can be accomplished by employing a wide variety of resources, such as bacteria, viruses, algae, fungi, yeast, plants, and plant-derived compounds. It is expected that creating nanoparticles using biological principles will be simple, cost-effective, safe, and environmentally friendly (Gautam et al., 2023). The production of nanoparticles by using plants has drawn a lot of attention, mostly because it is an easy process that does not require complex steps, such as maintaining a microbial culture or numerous purification stages (Khafaga et al., 2023a, 2023b). Green chemistry is a novel approach to the subject of nanosynthesized materials. It facilitates the synthesis and application of safe substitutes for nanomaterials, including organic-inorganic nanoflowers, biogenic nanoparticles, and biopolymers. These nanosupports, which are also known as biomaterials, have drawn a lot of interest because of their unique characteristics. Biomaterials are distinguished by their distinct physicochemical features and enriched surface with functional groups, in addition to their economical, environmentally friendly, and rapidly scaled-up synthesis. By taking advantage of these unique qualities, enzyme immobilization is made significantly easier, proving that naturally-derived materials are superior host-platforms than standard nanomaterials. Green nanotechnology is introduced by the creation of completely sustainable nanobiocatalytic systems, which bridge the gap between bioprocesses and industrial applications (Mohtar et al., 2019; Gkantzou et al., 2021). The purpose of this review is to highlight the use of nanobiocatalysts in industrial and biomedical applications, whose synthesis involves integrating nanomaterials produced through green synthesis to avoid the toxicity of nanomaterials produced through other methods. This enhances the activity of the free enzyme through immobilization.
Techniques of enzyme immobilization
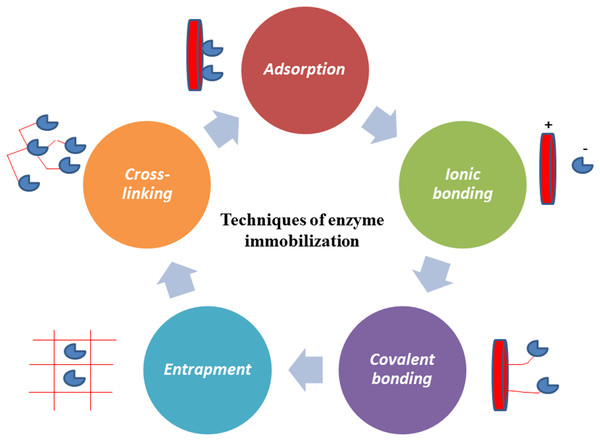
By immobilizing enzymes, their mobility is restricted, leading to several benefits including increased catalytic activity, improved stability, and the ability to be reused multiple times (Cui et al., 2023; Mohidem et al., 2023). It can be concluded that enzyme immobilization improves biocatalyst characteristics and productivity, making them attractive for a variety of applications (Mohamad et al., 2015). Therefore, immobilization technology is commonly used to improve the overall effectiveness of enzyme catalysis (Kashefi, Borghei & Mahmoodi, 2019; Batool et al., 2024). This versatile technique has applications across a spectrum of industrial sectors, including the medical, detergent, food, textile, pharmaceutical (Rossino et al., 2022), and water treatment industries (Mohammadi et al., 2023; Maghraby et al., 2023). Various immobilization methods, including crosslinking, covalent binding, adsorption, ionic bonding, and entrapment, have been employed in this context (Mohamad et al., 2015; An et al., 2020; Maghraby et al., 2023; Dadi & Ocsoy, 2024), as illustrated in Fig. 1.
Figure 1: Enzyme immobilization using different techniques cross-linking, covalent binding, adsorption, ionic bonding and entrapment.
Adsorption
Enzyme adsorption onto solid supports relies on weak interactions, including Van der Waals forces, electrostatic attractions, and hydrophobic interactions. The process involves immersing the support material in an enzyme solution for a specific duration, under conditions that preserve enzyme activity. Subsequently, unbound enzyme molecules are washed away using a buffer solution. A significant disadvantage of the adsorption method is the weak physical attachment of enzymes to the support material. This can lead to enzyme desorption or leaching under fluctuating conditions such as temperature, pH, or ionic strength changes. Additionally, biosensors employing adsorbed enzymes often exhibit poor operational and storage stability due to both enzyme leaching and the nonspecific adsorption of other molecules onto the transducer surface, causing signal contamination and interference (Nguyen & Kim, 2017). Effective enzyme adsorption hinges on the presence of specific active groups on the carrier material, facilitating interactions between the enzyme and the support. However, in the absence of such groups, carrier modification can be employed to introduce functionalities that promote enzyme attachment. By tailoring these interactions based on protein structure and matrix charge, it is possible to achieve strong adsorption while minimizing enzyme distortion. Although this strategy allows for the utilization of various carrier materials, compatibility between the enzyme and the support is crucial. Optimal adsorption necessitates specific conditions, with enzyme carrier affinity playing a pivotal role (Maghraby et al., 2023).
Ionic bonding
Utilizing ionic interactions between enzymes and charged support materials, ionic bonding offers a simple, cost effective, and reversible immobilization approach. The success of this technique relies on the principle of electrostatic attraction, where enzymes with an opposite charge to the support material bind effectively (Maghraby et al., 2023). The reversibility of ionic bonding is a notable advantage, as the enzyme can be readily desorbed by altering the pH or employing salting out methods. Maintaining an optimal pH throughout the reaction is crucial, and the stable charge of the immobilization matrix facilitates easy control of the solution’s acidity or alkalinity (Maghraby et al., 2023). Successful application of ionic forces for enzyme immobilization requires careful consideration of two key parameters: the pH of the reaction solution and the isoelectric point (pI) of the enzyme. The enzyme’s surface charge, whether positive or negative, is determined by the difference between its pI and the solution’s pH. This charge difference enables immobilization through ionic and strong polar interactions with an oppositely charged support surface (Nguyen & Kim, 2017).
Covalent bonding
A widely employed and robust method for enzyme immobilization, covalent binding involves the formation of strong, stable linkages between enzymes and support materials such as porous silica, polyacrylamide, agarose, or porous glass. This technique offers numerous benefits, including enhanced enzyme durability, efficient recovery for reuse, and increased stereospecificity, leading to improved stability (Maghraby et al., 2023). For effective covalent immobilization, it is crucial to select a functional group on the enzyme that is not directly involved in its catalytic activity. Common binding sites include the side chains of amino acids such as lysine (with its ε-amino group), cysteine (possessing a thiol group), and aspartic or glutamic acids (containing carboxylic acid groups). A variety of enzyme functional groups can participate in covalent coupling, encompassing amino, carboxyl, phenolic, sulfhydryl, thiol, imidazole, indole, and hydroxyl groups (Nguyen & Kim, 2017). The process of covalently attaching an enzyme to a solid support typically involves a two-step approach. Firstly, the support surface is activated using linker molecules like glutaraldehyde or carbodiimide. These multifunctional reagents act as bridges, forming covalent bonds between the support and the enzyme. The initial step involves the formation of a self-assembled monolayer (SAM) on the support surface, while the subsequent step establishes a covalent linkage between the pre-activated support and the enzyme. The choice of linker molecule depends on the nature of the support surface, whether it is an inorganic material, a natural or synthetic polymer, or a membrane, as well as the specific immobilization protocol, such as direct attachment to the transducer surface or binding to a thin membrane affixed to the transducer (Nguyen & Kim, 2017). Despite its advantages, covalent attachment carries the risk of enzyme denaturation due to the chemical modifications often required to introduce suitable functional groups. Additionally, this method typically necessitates a high volume of bioreagents while achieving relatively low enzyme loading capacities (approximately 0.02 g of enzyme per gram of support matrix). Although covalent immobilization significantly enhances enzyme stability, it can lead to reduced activity in affinity-based reactions, often resulting in poor reproducibility. Compared to adsorption, the process is more time-consuming, requiring longer incubation periods for SAM formation and subsequent enzyme linkage, which can span several hours. The complexity of the procedure also necessitates careful control of chemical purity to ensure the homogeneity of the SAM layer. The following sections will briefly outline the most common methods for covalent enzyme immobilization on functionalized surfaces, focusing on the activation of carboxyl and amino groups.
Activation of carboxylic groups
Carbodiimides, characterized by the functional group RN=C=NR, facilitate the formation of covalent bonds between carboxyl groups (−COOH) on the support material and amino groups (−NH2) present on the enzyme. To enhance immobilization efficiency, N-hydroxysuccinimide (NHS) can be introduced alongside the carbodiimide prior to the enzyme coupling step, acting as a stabilizing intermediate.
Activation of amino groups
Glutaraldehyde serves as another effective activating agent for enzyme immobilization. This process involves an initial Schiff base reaction between the amine-functionalized support and one of the aldehyde groups of glutaraldehyde. Subsequently, the second aldehyde group of glutaraldehyde forms a covalent bond with an amine group on the enzyme, creating a stable linkage (Nguyen & Kim, 2017).
Entrapment
Entrapment, an irreversible immobilization technique, involves confining enzymes within a network of fibers through either covalent or non-covalent interactions, effectively creating a cage-like structure around the enzyme molecules (Maghraby et al., 2023). Unlike methods that directly attach enzymes to a support surface, entrapment immobilization involves embedding enzymes within a polymeric network. This network allows for the diffusion of substrates and products while restricting the movement of the enzyme molecules, thus creating a confined environment for catalysis. The entrapment process typically consists of two steps: first, the enzyme is mixed with a monomer solution, and then the monomer solution undergoes polymerization, either through a chemical reaction or by modifying experimental conditions, to form the entrapping network (Nguyen & Kim, 2017). As enzymes do not directly interact with the polymers in the entrapment strategy, the risk of denaturation is minimized. This technique boasts several advantages, including high enzyme loading capacity, cost-effective fabrication, enhanced mechanical stability of the entrapped enzymes, and reduced mass transfer limitations. Moreover, the encapsulation material can be modified to create an optimal microenvironment with a suitable pH, polarity, and amphiphilicity for enzyme activity. The most commonly employed method for enzyme entrapment involves the gelation of polycationic or polyanionic polymers through the addition of multivalent counterions. However, there are also drawbacks associated with this technique. For example, increased matrix thickness due to extensive polymerization can hinder mass transfer, limiting substrate diffusion to the enzyme’s active sites. Additionally, large pore sizes in the support material can result in enzyme leakage. Other limitations include potential damage to the support during polymerization and, in some cases, a lower enzyme loading capacity. Several entrapment procedures, such as photopolymerization, the sol-gel method, and electropolymerization, offer alternative approaches with varying advantages and limitations (Maghraby et al., 2023).
Cross-linking
Cross-linking presents an irreversible enzyme immobilization method that relies on the formation of intermolecular covalent bonds between enzyme molecules. This process, facilitated by multifunctional reagents acting as linkers, results in the creation of three-dimensional cross-linked enzyme aggregates. Notably, the immobilized enzymes exist freely within the reaction mixture, without being bound to a solid support (Nguyen & Kim, 2017). Various cross-linking strategies have been developed, including cross-linked spray-dried enzymes, cross-linked aggregates, and cross-linked dissolved enzymes. The latter method involves the intermolecular cross-linking of enzymes in their crystalline form using glutaraldehyde. This technique offers several advantages for industrial biotransformations, including control over particle size (ranging from 1 to 100 μm), resistance to organic solvents and high temperatures, high stability, efficient recycling with optimal catalytic activity, and high volumetric efficiency. However, its requirement for highly purified enzymes in crystalline form makes it a costly approach. In contrast, cross-linked enzyme aggregates are formed through simple precipitation in an aqueous solution, resulting in physical aggregates of protein particles. This method is versatile, allowing for the simultaneous co-immobilization of multiple enzymes and offering cost-efficiency. However, the aggregates exhibit poor mechanical stability. Lastly, the cross-linked spray-dried enzyme method finds application in certain industrial settings but faces limitations due to the potential for reversible enzyme deactivation during the spray-drying process (Maghraby et al., 2023).
Effect of immobilization techniques and experimental conditions on enzyme activity
Despite its benefits, enzyme immobilization presents certain challenges. These include the economic burden associated with carrier materials, the cost and complexity of immobilization techniques, and the expenses and logistical difficulties involved in disposing of spent biocatalysts. Additionally, immobilized enzymes often exhibit reduced activity compared with their free counterparts owing to mass transfer limitations, potential inactivation during immobilization procedures, and fouling issues. Furthermore, some degree of empiricism remains in immobilization methodologies, requiring optimization for specific applications (Bié et al., 2022). Recent advancements have addressed some of the limitations associated with enzyme immobilization. The use of nanostructured materials, with dimensions similar to large biomolecules, as enzyme carriers has proven effective in mitigating mass transfer limitations. Additionally, the small particle size of these materials provides a larger surface area, leading to increased enzyme loading and, consequently, enhanced activity. Furthermore, improved enzyme stability was observed upon immobilization onto the nanostructured supports. Moreover, strategies for achieving controlled reversibility of the immobilization process have been developed, offering greater flexibility and control over biocatalyst usage (Bié et al., 2022). Furthermore, it is essential to consider several factors when designing an immobilization protocol. These include the inherent properties of the support material, the specific functional groups on both the support and the enzyme that will participate in the immobilization process, and the choice of immobilization technique itself, as each method presents unique advantages and drawbacks (Boudrant, Woodley & Fernandez-Lafuente, 2020). To comprehensively evaluate the impact of immobilization on enzyme activity, it is crucial to gather and analyze relevant experimental data. While the presentation of such data may vary depending on the specific immobilization technique employed, it should consistently encompass the progression of enzyme activity throughout the entire immobilization period. Initially, it is essential to establish a baseline by monitoring the activity of the free enzyme under conditions identical to those used for immobilization, preferably in the absence of any support material or using an inert support as a control. Subsequently, tracking the activity within the supernatant allows for the determination of the percentage of enzyme successfully immobilized, assuming the enzyme remains active and stable under the immobilization conditions. Finally, monitoring the activity within the immobilization suspension provides insights into the direct effects of immobilization on enzyme function. By analyzing these three activity profiles, a comprehensive understanding of the enzyme immobilization process can be achieved, elucidating the dynamic interactions between the enzyme and the support material within the immobilization environment (Boudrant, Woodley & Fernandez-Lafuente, 2020).
Advantages of enzyme immobilization
First, it is important to recall why immobilized enzymes are generally the optimal forms for the use of these biocatalysts.
Stabilization
The stability of appropriately folded enzymes ranged from 5 to 20 kcal/mol. This is the rationale behind the widely held belief that enzymes are more fragile than chemical catalysts are. Prior to the advent of recombinant DNA, the addition of stabilizers, chemical modification, and immobilization on solid supports have been the main approaches available for additional stabilization. Immobilization, contrary to common beliefs, does not usually result in considerable stability. It should be noted that immobilization can provide both storage and operating stability (Gupta et al., 2011). To maximize enzyme stability and reactivity under immobilization conditions, the catalytically active tertiary structure of the enzyme must be preserved (Mohamad et al., 2015).
Recovery and reusability
Recovery after use differs from reusability in that it relates to how easily the immobilized enzymes may be removed/separated from the reaction components for reuse. To ensure maximum reusability, an appropriate procedure must be carefully defined before reusing or cleaning in-place activities. Centrifugation or use of membranes is an obvious option when using solid supports. The use of smart carriers enables the development of stimuli-sensitive immobilized enzymes that can operate as homogeneous catalysts yet easily dissociate from the reaction mixture, which is a heterogeneous catalyst. The use of magnetic carriers facilitates the separation of enzymes mounted on solid substrates by using magnetic fields. Magnetic nanocarriers are exciting and advantageous in this context (Gupta et al., 2011). Magnetic carriers have various attractive features that render them suitable for a wide range of biomedical applications. These include high surface area, large surface-to-volume ratio, easy separation using external magnetic fields, and efficient mass transfer (Bilal et al., 2019, 2022).
Flexibility of bioreactor design
The most frequently employed carriers for immobilization are porous micron-sized particles. It was quickly observed that the inside surface of the beads had a significantly greater surface area than the outside surface in such instances. Because the enzyme dimensions were nanoscale and considerably smaller than the pores of such beads, a greater amount of enzyme was consistently immobilized inside the beads. Enzyme molecules are entrapped or encapsulated inside beads during entrapment/encapsulation. This issue may be less severe when low molecular weight substrates are used. However, macromolecular substrates do not operate effectively because these large molecules cannot readily reach the enzyme molecules in the bead core (Gupta et al., 2011).
Disadvantages of enzyme immobilization
One of the advantages of enzyme immobilization is that it allows the enzymes to attach to larger molecules. However, a key drawback of using immobilized enzymes is that their activity tends to diminish during immobilization, particularly when they are linked to macromolecular substrates. Diffusion restrictions, enzyme leaching, high cost, and scalability are the significant disadvantages associated with this method. Hence, it is essential to consistently try to improve the catalytic efficiency, durability, recyclability, and ease of recovery of immobilized enzymes, enabling their wider use in many fields, such as catalysis, adsorption, medicine, food processing, and biofuel generation (Mohidem et al., 2023). The research conducted over the past decade has focused on overcoming these limitations. It has been found that immobilizing enzymes on nanomaterials can create a suitable matrix with improved characteristics, including increased thermal and pH stability, enhanced storage stability, reusability, and higher enzymatic activity (Villalba-Rodríguez et al., 2023). After immobilization via entrapment, covalent immobilization, and physical adsorption, multimeric enzymes were stabilized. The relationship between the support and enzyme is related to this impact. The maximum number of enzyme subunits with the maximum support area is bound by covalent immobilization and physical adsorption. Hydrophobic or ionic interactions after entrapment of the enzyme reduce the formation of inactive intermolecular aggregation as well as the dissociation of the protein into its constituent subunits. During immobilization, enzymes may denature and cease to function. Distortions are the cause of this, particularly if there are several interactions between the matrix and enzyme. It has been suggested that secondary structural alterations may result from protein immobilization on solid substrates. Proteins that undergo these modifications acquire a β-sheet rather than an α-helical structure. Conversely, distortions can cause changes in the characteristics of biocatalysts (Guzik, Hupert-Kocurek & Wojcieszyńska, 2014). Encapsulation and entrapment techniques are frequently employed; however, a major drawback of these approaches is the leakage of enzymes and the slow diffusion of substrates and products within the supporting material. Physical adsorption and electrostatic interactions can be easily employed for immobilization. However, there are concerns related to nonspecific protein binding and enzyme loss throughout the process. Chemical coupling approaches are superior to physical adsorption and entrapment methods in terms of protecting the enzyme activity; however, they are more complex. Furthermore, these approaches are limited by the disadvantage of possible denaturation and deactivation due to alteration of the three-dimensional structure of the protein induced by several binding points. In summary, the practical use of traditional immobilization procedures is now restricted, mostly because of a decrease in enzyme activity following immobilization. This is caused by alterations in the conformational integrity of the enzyme, resulting in the depletion of its dynamic characteristics (Fathi et al., 2019).
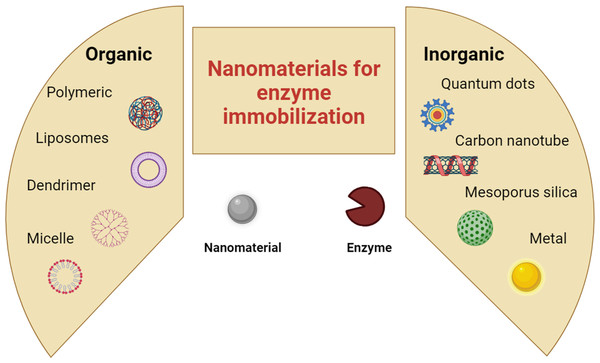
Nanomaterials for enzyme immobilization
Enzymes are ubiquitous catalysts that enable changes in chemical species in biological systems (Singh et al., 2020). The exceptional activity, specificity, and selectivity of enzymes make them potential biocatalysts for a wide range of applications including biocatalysis, biosensors, and biomedicine (Sillu, Kaushik & Agnihotri, 2021). Biocatalysts encourage green processes owing to their low chemical consumption and lack of harmful byproducts (Husain et al., 2011). Several inorganic and organic compounds, including silica (Correro et al., 2017), carbon, gold, and other metals, have been utilized (Shrestha et al., 2016; Ulu, Koytepe & Ates, 2016; Liu et al., 2016; Hajar & Vahabzadeh, 2016; Corell Escuin et al., 2017; Shafi, Ahmed & Husain, 2021), as illustrated in Fig. 2. Recent advances in nanotechnology have yielded numerous nanoscale carriers suitable for enzyme immobilization. The process of assembling enzyme molecules onto nanomaterial carriers to enhance the chemical kinetics and substrate selectivity is termed nanobiocatalyst formation. Functionalized nanocarriers allow the creation of organized enzyme structures, transforming them into nanoscale systems for information storage and processing. Recent research has indicated that nanostructured materials align well with the requirements of nanobiocatalysts, offering a multitude of advantages. These include high reusability, facile modification, swift separation facilitated by magnetic properties, and a substantial surface area conducive to high enzyme immobilization (Husain et al., 2011; Ding et al., 2015). This approach enables the creation of a microenvironment around the enzyme catalysts, thereby fostering optimal reaction efficiency. Immobilizing enzymes with nanostructured carriers offers a substantial extension of the biocatalyst life cycle, consequently reducing the overall cost of the biocatalytic process (Betancor & Luckarift, 2008). NBCs differ greatly from ordinary immobilized enzymes in terms of preparation, catalytic performance, and application potential. To date, functional nanomaterials, such as nanofiber (NF) scaffolds (du Plessis et al., 2013), nanotubes (NTs) (Wang & Jiang, 2011; Yadav et al., 2019), nanoparticles (NPs) (Klibanov, 1979), nanocomposites (NCs) (Tran, Chen & Chang, 2012), and nanosheets (NSs) (Ma et al., 2012) have been employed to create NBCs (Sigurdardóttir et al., 2018). Particle size can significantly influence enzyme immobilization. Smaller particles have a larger surface area, which means that there is more potential space for enzyme attachment (Sigurdardóttir et al., 2018). Materials with nonporous nanoscale structures provide attractive enzyme-loading capacity. The degree to which a surface is “crowded” once enzymes are immobilized determines the protein’s ability to continue functioning biologically. When more enzyme molecules are added to a given surface, their activity, also known as immobilization efficiency, first increases. This is because thermodynamic factors cause enzyme molecules to try to maximize contact when there are few molecules on a large surface, which causes them to undergo conformational distortion. Once an optimal value is reached, subsequent increases in enzyme density cause the activity to decrease because conformational deactivation occurs again when the surface becomes overly packed with protein molecules. Therefore, to optimize enzyme loading, a large surface area is beneficial (Gupta et al., 2011). Nonporous nanosized materials provide a high loading capacity for enzymes with very small mass-transfer limitations (Gupta et al., 2011). Porous materials are commonly used as enzyme immobilization supports. The ideal pore size is 3–5 times that of the protein (Gao et al., 2010). Traditionally, macroporous materials with pore sizes greater than 50 nm have been considered the best support materials because they provide no spatial limitations for enzyme molecules entering such large pores (Bayne, Ulijn & Halling, 2013). Because highly porous nanoparticles with ultrahigh surface areas are of great interest, silica is still commonly used (Sigurdardóttir et al., 2018). Recently, there has been a notable focus on mesoporous silica materials in both academic and industrial sectors because of their highly organized and customizable pore structures, as well as their abundant surface silanol groups. Their extensive surface areas and large pore volumes may permit the entry of enzyme molecules, allowing enzymes to be reused and enhancing their activity, selectivity, and operational stability. Therefore, they have been used in the field of enzyme immobilization (Gao et al., 2010). Ultrafine metal nanoparticles (UMNPs) with a narrow size distribution are thought to provide a higher density of active sites available for catalysis and make the surface atoms more reactive, thereby greatly increasing atom efficiency and lowering the cost of precious-metal catalysts. One of the primary factors in catalysis is the high surface-to-volume ratio of the nanocatalysts. “ultrafine MNPs” refers to particles less than 3 nm in size. Unfortunately, because of their high surface energy, UMNPs are prone to aggregation during catalytic processes, resulting in the loss of catalytic activity and recyclability. This instability also makes UMNPs thermodynamically unstable. Consequently, the key to selective and increased activity lies in controlling the size, shape, and dispersion of UMNPs. Although many studies have attempted to develop simple and effective methods for producing a variety of MNPs, there are currently relatively few techniques that can be used to produce uniformly small MNPs. Furthermore, the smooth surface of the UMNPs contributed significantly to the enhanced catalytic activity. Owing to its simplicity and reproducibility in preparing UMNPs, the colloidal method, which involves reducing metal precursors in solution in the presence of various organic capping agents such as dendrimers, oleylamines, and polyvinyl pyrrolidone, has been extensively studied to produce well-dispersed UMNPs and prevent their agglomeration (Zhu & Xu, 2016). A higher enzyme density and higher localized avidity, improved mass transport, increased surface curvature, favorable surface functional group interactions, and favorable enzyme orientation for enzyme–substrate interactions, or an optimized enzyme–substrate trajectory, are the five mechanisms that are essential to enhanced enzymatic activity with enzymes immobilized onto nanomaterials (Ding et al., 2015). For a number of reasons, immobilizing enzymes on nanomaterials may improve their efficacy. Enzyme density, orientation, and configuration can all be altered by modifying the surface chemistry of nanomaterials (Ardao et al., 2012). Target-specific avidity has been demonstrated when it is possible to regulate the mobility of the enzyme-nanomaterial system as well as the configuration and density of the enzyme on the nanoparticle (Tassa et al., 2010). When several enzymes are linked to a single nanomaterial as opposed to free enzyme, the localized density of enzymes in a given volume can increase considerably (Zhang et al., 2011). Additionally, nanomaterial shape can have a significant effect on the enzymatic enhancing process. These materials enable for larger center-to-center distances between neighboring attached enzymes while minimizing negative protein-to-protein interactions since nanomaterials/nanotubes maintain higher radii of curvature due to their smaller dimensions. Additionally, nanomaerial shape can have a significant effect on the enzymatic enhancing process. Because of their smaller size, nanotubes and nanomaterials have greater radii of curvature. Thus, these materials minimize undesirable protein-to-protein interactions while permitting larger center-to-center separations between neighboring attached enzymes. Additionally, by carefully adjusting the chemistry of the enzyme attachment, enzyme orientation can be regulated, enabling the immobilized enzyme’s substrate-binding pocket to be strategically oriented away from the toward the affected substrate and the nanomaterial surface. Additionally, through Brownian motion, the nanomaterial inherent mobility improves the interactions between the substrate and the enzyme. Nanomaterial immobilized enzyme activity can also be enhanced by secondary interactions at the nanomaterial–enzyme interface, which are partly caused by substrate–nanomaterial attraction through mechanisms including electrostatic attraction (Algar et al., 2012).
Figure 2: Nanomaterials for enzyme immobilization.
Nanomaterials for nanobiocatalysis include graphene quantum dots as a new substrate for immobilization of glucose oxidase (GOx), carbon nanotubes and micelle were used for lipase immobilization, trypsin was immobilized by mesoporus silica or gold, poly(methyl-methacrylate) was reported as the support for the immobilized urease, liposomes for CRISPR/Cas9 enzyme immobilization, and new dendrimers with a ferrocene core were produced by means of a divergent method for the immobilization of the glucose oxidase enzyme. (Own creation, made with the help of https://www.biorender.com/).Toxicity of nanomaterials during enzyme immobilization
The direct handling of nanomaterials with significantly harmful effects can lead to environmental and health issues. For example, pure powdered carbon nanotubes (CNTs) can be dangerous. Analyzing the toxicity of certain nanomaterials is crucial before using nano-supports as precautions. Transition metal-based catalysts are more expensive owing to their limited applications, and often require hazardous solvents as reaction media, which can induce secondary toxicity. Therefore, monitoring the effects of nanomaterials on the environment and determining their exposure levels during production are crucial. Artemia salina was used as the test organism to evaluate the toxic effects of untreated diclofenac and naproxen solutions, as well as solutions modified with encapsulated or adsorbed laccase, at room temperature (Zdarta et al., 2019). Untreated naproxen and diclofenac solutions had effective concentrations (EC30) of approximately 20% and 25%, respectively, indicating the substantial toxicity of these medicinal formulations. Following immobilization with NBCs, toxicity levels were considerably reduced, with the EC30 ranging from 60% to 85%. When encapsulated laccase was used instead of the adsorbed enzyme, the EC30 values were greater. This suggests that the effluent posed less risk, making encapsulation a more suitable immobilization technique for reducing the rate of toxicity. The industrial and academic sectors, along with regulatory bodies and legal authorities, should identify the benefits and hazards of nanomaterials to maximize their large-scale use, while maintaining the integrity of the environment and human health (Reshmy et al., 2021).
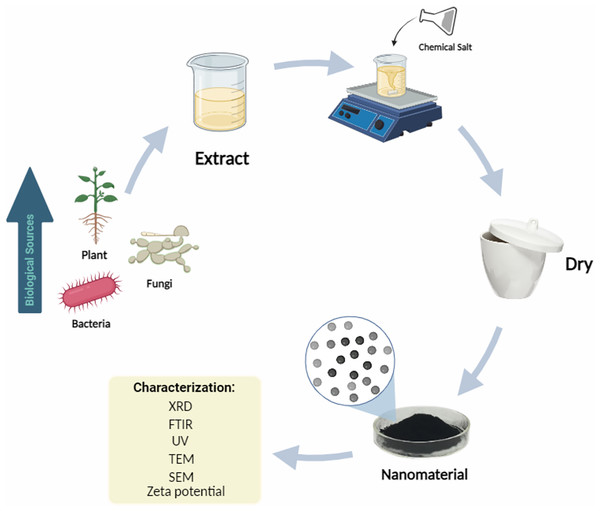
Green synthesis of nanomaterials
Recently, an increasing number of studies have focused on the synthesis of nanoscale metals using chemical, physical, and green approaches (Wang et al., 2007; Horwat et al., 2011). Green synthesis technologies are progressively replacing physical and chemical approaches (Tippayawat et al., 2016; Alsammarraie et al., 2018; Rashwan et al., 2022; Nagy et al., 2022; Jahan et al., 2023; Khafaga et al., 2023a, 2023b) owing to difficulties with significant energy usage (Horwat et al., 2011), toxic and hazardous chemical emissions (Hoag et al., 2009), and the use of complex equipment and synthetic environments (Baruwati, Polshettiwar & Varma, 2009; Ahmed & Ikram, 2015). Physical methods such as UV radiation (Wojnarowicz et al., 2018), aerosols (Smirniotis, Boningari & Inturi, 2018), and thermal decomposition (Ahmed et al., 2016a) require high temperatures and pressures (Ahmed et al., 2016b). On the other hand, green synthesis uses natural and ecologically favorable components. Some green materials can be utilized as both end-capping agents and dispersants (Devi et al., 2019), thereby saving energy and preventing the use of poisonous and dangerous chemicals. Green synthesis is primarily based on microorganisms (Subramaniyam et al., 2015; Arsiya, Sayadi & Sobhani, 2017), leaf extracts derived from leaves (Chahardoli, Karimi & Fattahi, 2018; Leili, Fazlzadeh & Bhatnagar, 2018; Devi et al., 2019; Koca et al., 2020), flowering plants (Thovhogi et al., 2015, 2016; Sone et al., 2020), and seeds from diverse plants (Dhand et al., 2016; Gao et al., 2016) as illustrated in Fig. 3. Green materials contain polyphenols and proteins, which can function as reducing agents instead of chemical reagents, to reduce metal ions to a lower valence state (Can, 2020). Green synthesis offers several benefits over chemical and physical methods, as it is non-toxic (Devi et al., 2019; Srivastava, Ildusovich Kharisov & Kharissova, 2021), pollution-free (Alsammarraie et al., 2018), and economically and environmentally friendly (Kataria & Garg, 2018). Bacterial cells are well suited for nanoparticle synthesis because of their capacity to survive and multiply under stressful conditions, including high metal concentrations, which may be linked to their unique resistance mechanisms. Bacterial strains that are not naturally resistant to high metal concentrations can be used to produce nanoparticles. Microbe-produced nanoparticles have a wide range of applications in bioremediation, bioleaching, biocorrosion, and biomineralization. In addition to bacteria, fungi and algae are two other sources that may produce nanoparticles in an environmentally beneficial manner. Because they can produce many bioactive chemicals, fungi are often used to reduce and stabilize the production of nanoparticles on a large scale while maintaining their shapes and sizes under control. However, algae may produce a variety of bioactive chemicals, pigments, and proteins that function as capping agents in the synthesis process, assisting in salt reduction (Khafaga et al., 2023b). The creation of environmentally friendly nano-supports for enzyme immobilization is crucial from the perspective of sustainability. Environmentally friendly nanoparticle-immobilized enzymes exhibit increased stability, reusability, and retention of catalytic activity (Ariaeenejad et al., 2023), as shown in Table 1.
Figure 3: Green synthesis of nanomaterials.
Own creation, made with the help of https://www.biorender.com/.| Nanomaterial | Biological source | Enzyme | Ref. |
|---|---|---|---|
| Hybrid zinc oxide-iron oxide | Olea europaea leaf aqueous extract | Lipase | Fotiadou et al. (2021) |
| ZnF-CA | Psidium guava leaves | Lipase | Fahim et al. (2024) |
| Polyaniline coated silver | Ziziphus mauritiana fruit extract | Yeast alcohol dehydrogenase | Alam et al. (2015) |
| Iron oxide | Bauhinia tomentosa leaf extract | Pancreatic lipase | Lakshminarayanan et al. (2021) |
| Silver | Fagonia indica aqueous extract | Glucoamylase | Syed et al. (2016) |
| Co-doped NiO | S. persica leaf extract | Metagenome-derived laccase | Ariaeenejad et al. (2023) |
| Zinc oxide | Cayratia pedata leaf extract | Glucose oxidase | Jayachandran, T.R. & Nair (2021) |
| ZnO nanoparticles decorated on the surface of polyindole (PIn)-multi-walled carbon nanotube | Neem leaves extract | Glucose oxidase | Inamuddin et al. (2020) |
| ZnO-modified carbon paste electrode | Zingiber officinale root | Glucose oxidase | Dönmez (2020) |
| Cysteine-Functionalized Silver | Green tea extract | Lipase | Dutt & Upadhyay (2018) |
| Poly-L-lysine modified silver | Syzigium aromaticum extract | Laccases | Fauzia Farheen Zofair et al. (2023) |
| NiO | Salvadora persica leaf extract | Laccase | Ariaeenejad et al. (2023) |
Organic–inorganic hybrid nanoflower as nanobiocatalyst
The process of creating functional organic–inorganic hybrid nanoflowers (FNFs), which are organic–inorganic nanostructures in the form of flowers made of proteins or enzymes and Cu2+ ions in a phosphate-buffered saline (PBS) solution. Nanoflower development involves key steps. In the nucleation step, Cu2+ ions combine with phosphate to form the primary copper phosphate nanocomplex (Cu3(PO4)2). During the growth step, the amide groups in the protein backbone preferentially bind to the Cu2+ ions in the Cu3(PO4)2 nanocomplexes to form flower petals. In the final step, the petals stick together to form a nanoflower (Celik et al., 2018). Organic–inorganic hybrid nanoflowers in the form of flowers have the potential to be employed in a variety of applications such as industrial biocatalysts, biosensors, bioanalytical instruments, biomedicine, and biofuel cells (Yilmaz et al., 2022; Kalayci et al., 2024; Aslan et al., 2024).
Multi-enzymatic nanobiocatalyst
The creation of multi-enzymatic NBCs is a rapidly developing and valuable technology for the synthesis of fine chemicals and other goods with added value. There are a number of strategies and techniques for creating multi-enzyme NBCs, but the most effective is the immobilization of these enzymes on different types of support materials. When building multi-enzyme NBCs, there are a variety of factors to consider, including the stability and reusability of enzymes, as well as their activity. It is commonly recognized that several enzymes acting together in a cascade cause natural reactions within cells (Sheldon & Woodley, 2018). To mimic this, many enzymes are immobilized on an appropriate carrier or support in a process known as a multi-enzyme cascade. However, enzymes can also be linked by a linker, in which case a carrier or support would not be necessary (Ren et al., 2019). To create effective biocatalytic processes, Xu et al. (2020) recently studied the application of several support materials for the immobilization of multiple enzymes. These include inert inorganic materials, such as silica and TiO2, graphene, carbon nanotubes, metals and organic ligand complexes, DNA nanostructures, and other types of polymers (Xu et al., 2020). Zhang et al. (2021a) presented the classification, synthesis conditions, functional characteristics, and industrial uses of enzyme-inorganic hybrid nanoflowers (HNF), a new material for immobilizing multiple enzymes. They suggested that this is a highly significant new field for nanoimmobilization of several enzymes (Zhang et al., 2021a).
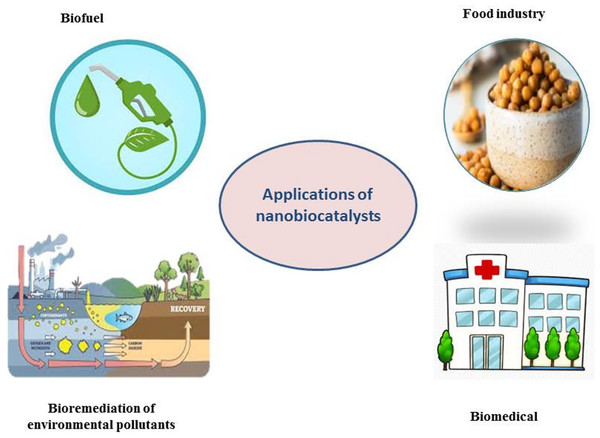
Applications of nanobiocatalysts
Nanobiocatalysts have a variety of uses in the industry and biomedicine owing to their capacity to be engineered, stability, effectiveness in bioprocessing, and ease of recovery after use (Najeeb et al., 2021; Reshmy et al., 2021), as shown in Table 2. NBCs represent an individual category of nanomaterials that provide significant promise and assurance across various domains, including the food industry (Safdar et al., 2023), biofuel manufacturing (Kumar, Morya & Datta, 2019), medicine, bioremediation, and other related fields, as illustrated in Fig. 4. These attributes are generally linked to intrinsic stability, bioprocessing efficiency, engineering adaptability, and ease of downstream recovery (Reshmy et al., 2021). Because they avoid the usage and production of hazardous materials and utilize fewer chemicals, green bioprocessing techniques supported by NBC have great promise. Enzyme immobilization is becoming increasingly popular in this field as a means of maintaining biocatalysts in chemical and environmental settings (Reshmy et al., 2021).
| Nanomaterial | Enzyme | Advantage | Application | Ref. |
|---|---|---|---|---|
| Chitosan microparticles | Penicillium decumbens naringinase | Enhanced operational stability | Food industry | Bodakowska-Boczniewicz & Garncarek (2019) |
| Poly (2-hydroxyethyl methacrylate)-micro particles | α-amylase: Aspergillus oryzae | Increased pH and thermal stability | Food industry | Del Arco et al. (2021) |
| Silica coated Fe3O4 particles | Pseudomonas fluorescens lipase | Comparable performance to commercial lipase | Food industry | Del Arco et al. (2021) |
| Carboxyl-functioned magnetic | Glucoamylase | Enhanced hydrolysis and stability | Food industry | Misson, Zhang & Jin (2015) |
| Magnetic chitosan | Pectinase | Enhanced thermo stability and 74% turbidity reduction | Food industry | Bilal & Iqbal (2019) |
| Silica-coated Fe3O4 | Lipase | Excellent reusability | Biodiesel production | Amini et al. (2017) |
| Polyacrylonitrile-coated Fe3O4 | Aspergillus oryza ST11 lipase | 95% yield with enhanced operational stability | Biodiesel production | Paitaid & H-Kittikun (2020) |
| Polyporous amines grafted magnetic cellulose beads | Candida antarctica lipase B | 92.3% yield, Biodegradable/biocompatible support | Biodiesel production | Zhang et al. (2020) |
| Chitosan Fe3O4 macroparticles | Lactobacillus reuteri nucleoside 2′-deoxyribosyltransferase | High conversion rate, Higher thermostability | Deoxynucleosides and Ara nucleosides synthesis | Fernández-Lucas et al. (2013) |
| Dendrimer-grafted flower like Fe3O4 microcarriers | Penicillin G acylase | Better thermostability and reusability | Penicillin G potassium salt hydrolysis | Li et al. (2018) |
| Commercial MagReSyn® NTA microparticles | T. brucei purine deoxyribosyltransferase | High atom economy and efficiency, excellent recyclability | Synthesis of various therapeutic nucleoside | Arco et al. (2019) |
| Silica | Horseradish peroxidase | Improved biosensor/biodetection | Biosensor | Yang et al. (2010) |
| Concanavalin A–agnetic nanoparticles | β-galactosidase | Improved biodetection | Biosensor | du Plessis et al. (2013) |
Figure 4: Different applications of nanobiocatalysts (lipase immobilized for biodiesel production, oxidoreductases immobilized for removal of water pollutants, pectinase immobilized for juice processing and liposome as a carrier for enzymes).
Own creation, made with the help of https://www.biorender.com/.Food industry
The food industry places high priority on food safety. Therefore, only food-grade enzymes, including those derived from microorganisms and their products that are generally recognized as safe (GRAS), are used as immobilizers. Magnetic nanoparticles have recently been investigated in the food sector because of their potential to increase the stability and activity of certain enzymes. For diverse food applications, enzymes such as papain, naringinase, cellulase, lipase (Asmat, Anwer & Husain, 2019), amylase, trypsin, and isomerase have been immobilized on magnetic nanoparticles. For example, magnetic nanoparticles have been employed as stabilizers for pectinase and cellulase to aid in the extraction of color from orange peel. Magnetic nanoparticles were surface-functionalized with (3-aminopropyl) triethoxysilane and crosslinked with glutaraldehyde (Verma, Abraham & Puri, 2020). Compared to free enzymes, nanoimmobilized enzymes significantly increased carotenoid pigment extraction, with a nine-fold increase at ideal reaction conditions of pH 5.0 and 50 °C. Moreover, the immobilized enzymes retained approximately 85% of their catalytic activity after three cycles, indicating their potential for multiple applications. Graphene nanosheets have been used as nanocarriers for β-galactosidase, originating from chickpeas. This study showed improved affinity compared to that of the native enzyme. Response surface methodology has been used to optimize immobilization (Singh et al., 2014). NBCs have been used to degrade the raffinose family of oligosaccharides, which can lead to flatulence of soybean-based foods. The NBCs remained stable after six hydrolysis cycles using genuine substrates. To detect lactose levels in meals, β-galactosidase produced from pea plants was covalently bound to the surface of gold nanoparticles (AuNPs). A cysteamine-glutaraldehyde spacer arm was inserted on the nanoparticle surface, which enhanced the enzyme stability and catalytic activity for up to 6 months. Magnetic enzymes produced by pectinases, cellulases, and hemicellulases are commonly used to clear fruit juices. For example, Penicillium oxalicum (PoPase) was adsorbed onto magnetic corn-starch microspheres and used to minimize pectin-induced cloudiness in apple purees (Jiménez-Sánchez et al., 2017). Dal Magro et al. (2018) achieved grape juice clarity using immobilized enzyme systems such as pectinase-cellulase (Rohapect 10 L) or mCLEAs (CLEA-MP*). Rhamnose and naringinase are used in the production of fruit juices to decrease the bitter taste caused by flavonoids in different types of citrus fruits. To develop an enhanced pathway, RhmnasemCLEAs were created using polyethyleneimine (PEI), which functions as an enzyme linking agent. Recently, two separate magnetic NBCs using Aspergillus Niger-derived naringinase have been formulated for grapefruit juice dehydration (Bodakowska-Boczniewicz & Garncarek, 2019). The magnetic NBCs exhibited exceptional endurance in highly acidic and elevated-temperature environments and maintained their efficacy after ten repeated cycles with no more than a 20% decrease. Acosta et al. (2020) found that the use of hypoxanthine-guanine-xanthine phosphoribosyltransferase from Thermus thermophilus for enzyme immobilization resulted in the production of inosinic and guanosine acids, which enhanced the flavor of food products. These results suggest that NBCs could be a valuable tool for improving the quality of nutritious food products through enzyme immobilization.
Biofuel
The current ecological problems and reduction in non-renewable energy sources have led to the development of a more environmentally friendly method for producing biofuels (Pandit et al., 2023; Khan et al., 2023), which entails the use of enzymatic technologies for bioprocessing (Das et al., 2018; Rawat, Choubey & Bajpai, 2023). In contrast to traditional alkaline-catalyzed methods, biodiesel production processes that use lipases are characterized by decreased energy consumption and increased eco-friendliness (Singh, Paritosh & Vivekanand, 2021). Furthermore, nanobiocatalysts can be used for cellulose hydrolysis to yield fermentable sugars suitable for bioethanol production (Sillu & Agnihotri, 2020). Magnetic nanostructures (Parandi et al., 2023), graphene oxide, nano-silica, and metal-organic frameworks have been developed as nanocarrier hosts to enhance enzyme activity and loading during lipase immobilization (Madamwar et al., 2019; Asmat et al., 2020). This was revealed in a recent study by Bilal et al. (2021). The physical adsorption of polyacrylonitrile biohybrid nanofibers resulted in a 23-fold increase in lipase activity of Pseudomonas cepacia. Bolina et al. (2018) developed ion-exchange supports to improve biofuel production efficiency. Silica-based nanoparticles were functionalized with (3-glycidyloxypropyl) trimethoxysilane and activated with glycine. This allowed for the immobilization of T. lanuginosus lipase through adsorption. These promising research findings pave the way for the commercialization of the NBC technology for biofuel production. Immobilization of the β-glucosidase enzyme, in particular, has been found to reduce cellobiose inhibition in cellulosic bioethanol synthesis, resulting in up to four-fold higher bioethanol production via enzyme aggregation compared to covalent binding, as demonstrated by Deng et al. (2020). Although NBCs in biofuel manufacturing are still in their early stages, their involvement has great potential to stimulate future research and innovation in bioprocessing engineering.
Bioremediation of environmental pollutants
Enzyme-based bioremediation is a more efficient and rapid means of removing toxins from the environment than the other processes. Aqueous pollutant degradation can be effectively achieved using oxidoreductases such as laccases, which are both effective and cost-effective (Mahmoodi & Abdi, 2019). Recent research has shown that NBCs or laccase-mCLEAs can be used successfully to remove non-phenolic and phenolic chemicals from aqueous media, with increased substrate specificity afforded by mediators (Sadeghzadeh et al., 2020). Using silica-coated magnetic microbeads and bovine serum albumin as a protein co-feeder, laccase-mCLEAs were prepared, resulting in efficient elimination of non-phenolic and phenolic bioactive components (Del Arco et al., 2021). Primožič et al. (2020) reported that Chitosan/1- ethyl-3-[3-(dimethylamino) propyl carbodiimide as a crosslinker to generate laccase-mCLEAs with improved activity recovery. The use of mesoporous supports in monolithic microreactors enables direct interaction between the reaction medium and pollutant adsorption, thereby facilitating biocatalytic degradation for effective bioremediation, as suggested by Zdarta et al. (2019). The use of NBCs in wastewater treatment can serve as a standalone system because of the significant role of enzymes such as laccases, tyrosinases, lignin, phenol peroxidases, and horseradish peroxidase (HRP) in bioremediation processes, particularly in the elimination of pollutants, such as dyes and phenolic compounds. The use of these methods presents several advantages, including low energy consumption, high stability, and moderate-temperature functionality while limiting sludge production. Employing NBCs is considered an efficient approach to decolorizing dye-contaminated water sources (Dadi et al., 2023a). One study tested the efficacy of covalently immobilized laccase in reducing the color of textile effluents with diverse organic chemicals and salts utilized during dyeing (Yavaşer & Karagözler, 2021). Bello-Gil et al. (2018) showed the successful decolorization of Direct Yellow 106, Direct Red 105, and Direct Black 112 when inhibitors are present, such as salts, dispersants, and tensoactives, using immobilized bacterial laccase from Escherichia coli on polyhydroxy butyrate beads. In recent years, the use of biocatalysts in solid waste remediation has gained popularity, such as immobilized cutinase, PETase, and lipase to degrade plastic wastes, as well as proteases and related hydrolases to treat municipal, food, and organic wastes. Schwaminger et al. (2021) presented a novel NBC utilizing iron oxide nanoparticles supported by the PETase enzyme that exhibits high enzymatic loads and effective decomposition of PET substrates that can be magnetically recycled after up to ten cycles while retaining approximately half of their original activity level. Girelli & Scuto (2021) recently reported that magnetic porous carbon NF are highly suitable for enzyme immobilization and subsequent bioremediation. They suggested that carbon-derived mesoporous magnetic biocomposites could be produced from low-cost renewable sources and could serve as a feedstock for this purpose. Zhang et al. (2020) employed magnetic carbon nanocarriers derived from Luffa sponges to accomplish the adsorption of laccase, which was subsequently used to remove bisphenols through enzymatic action. Samak et al. (2018) successfully eliminated Congo red, an active textile dye, using a biocatalyst consisting of laccase and a magnetic graphene oxide nanosheet functionalized with amino groups. In this process, CotA laccase acted as an adsorbent. Lin et al. (2015) used chitosan/CeO2 microspheres to covalently immobilize laccase and employed CeO2 as a redox catalyst for the decolorization of orange II and methyl red dyes. These studies highlight the potential of NBCs in diverse bioremediation procedures, and indicate that enzyme technology could advance this area of research. From a global perspective, the global community faces extremely difficult barriers to water supply (Chandrasekara & Pashley, 2017; Dolan et al., 2021). Currently, aquatic environments are contaminated worldwide due to a wide variety of industrial and human activities. Therefore, it is necessary to effectively use technologies that are already available for wastewater treatment in addition to developing new procedures and recommendations for the reuse of wastewater streams (Oprčkal et al., 2017; Prasad Dey, Mishra & Sen, 2017). Recently, enzyme-catalyzed polymerization and precipitation have been considered for the treatment of aqueous phenols (al-Kassim et al., 1994), mostly peroxidases (Tsutsumi, Haneda & Nishida, 2001). Several studies have suggested that laccases may be more effective than peroxidases at eliminating phenolic pollutants from water and wastewater. Laccases oxidize phenolic moieties in wastewater pollutants using molecular oxygen as a substrate (Kudanga et al., 2011). The treatment of wastewater appears to be successful, according to the findings of one study that focused on laccase biocatalysts. According to the findings of this study, laccase biocatalysts can convert bisphenol A (BPA) even in environmentally relevant quantities in biologically treated wastewater. Furthermore, they have significantly higher stability in wastewater than free enzymes. In addition, laccase-nanoparticle conjugates have the potential to be effective biological wastewater treatment agents and are suitable for large-scale manufacture of biocatalysts (Hommes et al., 2012). However, several investigations have focused on immobilized oxidoreductases for the biodegradation of dangerous organic contaminants. The immobilization of oxidoreductases for wastewater treatment has shown promising results in at least one of these studies. According to the findings of this study, oxidoreductases are capable of biodegrading hazardous organic pollutants, the majority of which consists of dyes, pharmaceuticals, phenols, and bisphenols, and the clearance rate can reach up to 90 percent in some cases. Therefore, immobilized oxidoreductases show promise as a potential strategy for water purification in the near and distant future (Zdarta et al., 2021).
Biomedical
Advanced nanosystems, particularly nanobiocatalysts, have shown outstanding potential for biomedical research. Notable uses include effective therapeutic effects on inflammatory diseases and cancer, development of drug delivery systems, and design of non-invasive clinical diagnostics (Villalba-Rodríguez et al., 2023). The identification of biomarkers is essential for monitoring human health and for making a variety of diagnoses, including cancer. Numerous detection methods are currently available, but many require significant setup time or are vulnerable to interference from molecules diluted in the samples (Fadhel et al., 2017). Several nanotechnology-based techniques have been developed to improve detection resolution without sacrificing specificity and to reduce expenses and processing time. For instance, a limit of detection (LOD) of 3.8 mol/L for procedures involving 10 samples was established for a FRET-based sensor for inorganic phosphate in urine samples. The phosphate in the sample interacts with molecules of cetyltrimethylammonium bromide bound to gold nanoparticles and displaces the luminous probe, the terbium-ethylenediaminetetraacetic acid complex ([Tb-EDTA]-1). Depending on the phosphate concentration, this competition causes a change in the fluorescence intensity. Similarly, gold nanostructures combined with bovine serum albumin (BSA) and coated with graphene oxide and folic acid have been employed for glutathione sensing. Competition between the antioxidant molecules glutathione and BSA causes photobleaching of nanostructures. Glutathione is closely associated with numerous types of cancer (Wong et al., 2021). Similarly, functionalized nanostructures have been combined with enzymes, such as horseradish peroxidase (Xianyu et al., 2021). Human immunodeficiency virus (HIV) DNA biomarkers can be detected using glucose oxidase (Long et al., 2016). Notably, there is a glucose sensor that uses biofuel made from a mixture of HRP and GOx as catalytic energy (Chansaenpak et al., 2021), although its application is determined by enzymatic activity and the stability of polystyrene nanostructures (Long et al., 2016), gold (Phiri, Mulder & Vorster, 2019; Xianyu et al., 2021), and manganese oxide (Xu et al., 2021), which reduce sample manipulation, increase reaction velocity, and boost repeatability and reusability (Ripoll et al., 2020). Hu et al. (2019) developed a collaborative system consisting of liquid metal nanoparticles and glucose oxidase, referred to as “LM@GOX”. The treatment uses a combination of two therapeutic methods, namely starvation and photothermal therapy, to treat cancer (Hu et al., 2019). Chronic inflammation has received significant attention in the field of immunology owing to its association with several diseases. Currently, several drugs reduce and assist in reducing inflammation; however, their efficacy is frequently limited by the absorption systems inside the body (Fleit, 2014). Polymeric nanoparticles carrying drugs have several advantageous biological characteristics, including biocompatibility and mucoadhesiveness (Romero et al., 2013; Kost et al., 2015). Zhang et al. (2021b) developed a new nanomaterial by incorporating uricase and catalase into zeolitic imidazolate framework-8 (ZIF-8), which was then coated with a neutrophil membrane. This nanomaterial has been used to treat specific types of inflammatory arthritis. The enzymatic system exhibited exceptional specificity and demonstrated the ability to digest up to 90% uric acid, as shown by both in vitro and in vivo experiments. Furthermore, this system effectively decreases inflammation and achieves a high level of therapeutic efficacy (Zhang et al., 2021b).
Conclusions
Nanobiocatalysts represent a highly promising technological advancement with the opportunity to bring about transformative changes across numerous industries. These catalysts are flexible, making them applicable in diverse industrial sectors. NBCs offer numerous advantages over conventional biocatalysts. Immobilizing enzymes into nanomaterials improves their stability, activity, and selectivity. NBCs are currently at a developmental stage, although they have the capacity to exert a substantial influence on the global landscape. As further investigation in this domain persists, it is expected that NBC will play a progressively significant role across various sectors. NBCs have considerable potential for novel technological advancement.