Optimization of polysaccharide conditions and analysis of antioxidant capacity in the co-culture of Sanghuangporus vaninii and Pleurotus sapidus
- Published
- Accepted
- Received
- Academic Editor
- Paripok Phitsuwan
- Subject Areas
- Biotechnology, Food Science and Technology, Mycology
- Keywords
- Sanghuangporus vaninii, Pleurotus sapidus, Co-culture, Fungal polysaccharide, Antioxidant capacity
- Copyright
- © 2024 Lu and Liu
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Optimization of polysaccharide conditions and analysis of antioxidant capacity in the co-culture of Sanghuangporus vaninii and Pleurotus sapidus. PeerJ 12:e17571 https://doi.org/10.7717/peerj.17571
Abstract
Fungal polysaccharides are commonly utilized in the food industry and biomedical fields as a natural and safe immune modulator. Co-culturing is a valuable method for enhancing the production of secondary metabolites. This study used intracellular polysaccharide (IPS) content as a screening index, co-culturing seven different fungi with Sanghuangporus vaninii. The seed pre-culture liquid culture time was selected through screening, and conditions were assessed using single factor experimentation, a Plackett-Burman (PB) design, and response surface methodology (RSM) optimization. RSM optimization was conducted, leading to the measurement of antioxidant capacity. Results indicated that the co-culture of S. vaninii and Pleurotus sapidus exhibited the most effective outcome. Specifically, pre-culturing S. vaninii and P. sapidus seed cultures for 2 days and 0 days, respectively, followed by co-culturing, significantly increased IPS content compared to single-strain culturing. Further optimization of co-culture conditions revealed that yeast extract concentration, liquid volume, and S. vaninii inoculum ratio notably influenced IPS content in the order of yeast extract concentration > liquid volume > S. vaninii inoculum ratio. Under the optimal conditions, IPS content reached 69.9626 mg/g, a 17.04% increase from pre-optimization co-culture conditions. Antioxidant capacity testing demonstrated that co-cultured IPS exhibited greater scavenging abilities for DPPH and ABTS free radicals compared to single strain cultures. These findings highlight the potential of co-culturing S. vaninii and P. sapidus to enhance IPS content and improve antioxidant capacity, presenting an effective strategy for increasing fungal polysaccharide production.
Introduction
Sanghuangporus spp. are a type of large edible and medicinal fungi that belong to the family Hymenochaetaceae and the genus Sanghuangporus (Zhou et al., 2016). The medicinal properties of this fungus were first documented in traditional Chinese medicine texts, such as ‘Shen Nong’s Materia Medica,’ and are internationally recognized for their potent anti-cancer effects (Wu et al., 2012, 2022). Sanghuang contains a range of bioactive compounds, including polysaccharides (Yang et al., 2020), flavonoids (Wu et al., 2019), polyphenols (Liu et al., 2017), terpenoids (Rajachan et al., 2020), steroidal compounds (Thanh et al., 2018), and nitrogen-containing compounds (Li et al., 2015). Among these, polysaccharides are one of the key bioactive components responsible for the pharmacological effects of Sanghuang (Wang et al., 2022). This fungus exhibits various pharmacological properties, such as anti-tumor effects (Chakraborty et al., 2019), anti-inflammatory properties (Hou et al., 2020), hepatoprotective effects (Yuan et al., 2019), immune-modulating properties (Meng, Liang & Luo, 2016), and antioxidant activity (Cai et al., 2019). The efficacy of this fungus has garnered significant interest in both pharmaceutical research and the health product industry, both domestically and internationally. Sanghuangporus vaninii (S. vaninii) has become one of the most sought-after varieties of this fungus in the market due to its ease of cultivation and strong biological activity.
Pleurotus sapidus (P. sapidus) is an edible species that is classified under the genus Pleurotus and the family Pleurotaceae (Song et al., 2017). The Pleurotus genus demonstrates a wide range of species diversity, with approximately 773 recorded entries as of December 17, 2023 (source: http://www.indexfungorum.org/Names/Names.asp). However, only about 50 of these species are widely accepted (Guzmán, 2000). Pleurotus species are rich in nutrients such as polysaccharides, proteins, amino acids, and fatty acids. These components play a crucial role in enhancing immune function, delaying aging, combating fatigue, exhibiting anti-tumor activity, regulating blood sugar and lipid levels, and contributing to chemoprevention (Xu et al., 2014; Im et al., 2014; Lin et al., 2014).
Microbial co-culturing involves culturing two or more strains (such as fungus-fungus, fungus-bacteria, and bacteria-bacteria) in the same vessel (Mantravadi et al., 2019), which is known to enhance the production of secondary metabolites (Bertrand et al., 2014; Caudal, Tapissier-Bontemps & Edrada-Ebel, 2022). Guo et al. (2021) demonstrated that co-culturing Sanghuangporus lonicericola and Cordyceps militaris resulted in significantly higher mycelial polysaccharide yields compared to monocultures, with increases of 69.95% and 59.63%, respectively. Wu et al. (2021) observed that co-culturing Ganoderma lucidum and Flammulina velutipes led to reduced biomass production but increased extracellular polysaccharide (EPS) production compared to monocultures. Therefore, microbial co-culturing is an effective strategy for enhancing polysaccharide production in fungi.
Response surface methodology (RSM) is a valuable tool for exploring the relationship between different variables and for optimizing culture medium components and co-culture conditions. Successful cases have demonstrated increased production of secondary metabolites in microbial co-cultures using RSM strategies. For instance, Pan et al. (2023) used RSM to develop a statistical model for the co-culture conditions of Streptomyces albulus IFO 14147 and Corynebacterium glutamicum CICC 10064, resulting in a 31.47% higher yield of ε-poly-L-lysine at 27.07 ± 0.47 g/L compared to the single-strain yield. Similarly, Li et al. (2020) optimized the co-culture conditions of Trichoderma atroviride SG3403 and Bacillus subtilis 22 using RSM, achieving a 54.22% inhibition rate against Fusarium graminearum in the optimized co-culture fermentation broth, a 9% improvement over the original conditions. These findings highlight the ability of RSM to induce and enhance the production of active secondary metabolites in microbial co-cultures.
In this study, a strain of P. sapidus was screened and obtained for co-cultivation with S. vaninii to enhance intracellular polysaccharide (IPS) content. A Seed pre-culture liquid culture time of S. vaninii and P. sapidus was also screened. Single-factor experiments were conducted to optimize concentrations of glucose, yeast extract powder, MgSO4·7H2O, KH2PO4, as well as initial pH, inoculum volume, proportion of S. vaninii inoculum volume, and liquid volume. Key factors were identified through a Plackett-Burman (PB) design, followed by RSM to determine the antioxidant capacity of IPS in the co-culture and monoculture under optimal conditions. This research presents a novel culture strategy for enhancing fungal polysaccharide production and paves the way for the advancement of technologies related to fungal co-culturing and polysaccharide production.
Materials and Methods
Test strains
Sanghuangporus vaninii (ST), Pleurotus sapidus (BLZ), Pleurotus ostreatus (P8), Flammulina filiformis (BJZ), Irpex lacteus (BPCJ), Hericium erinaceus (HT3), Lentinus edodes (XG), and Panellus edulis (DM) were obtained from the Institute of Edible and Medicinal Fungi, Agricultural college, Yanbian University, Yanji, Jilin, China. Each strain was identified using a specific code.
Culture media
The agar plate culture medium used in this study consisted of 200 g/L potato, 20 g/L glucose, and 20 g/L agar.
The liquid culture medium contained 5 g/L yeast extract, 20 g/L glucose, 1.5 g/L KH2PO4, and 0.5 g/L MgSO4·7H2O.
Activation of strains
Following removal from the 4 °C refrigerator, the test strains were allowed to equilibrate at room temperature for 2 h to restore their vitality. Subsequently, clumps measuring 0.5 cm × 0.5 cm were aseptically taken using an inoculation spatula and transferred to a fresh plate medium. The petri dishes were then placed in a smart artificial incubator set at 28 °C for incubation.
Seed culture fluid
Under aseptic conditions, a 9 mm hole was punched to extract one activated fungal mycelial block of I. lacteus, P. sapidus, and P. ostreatus, as well as five fungal mycelial blocks of S. vaninii, H. erinaceus, F. filiformis, L. edodes, and P. edulis, which were then transferred into the liquid filling medium. The liquid culture medium, with a volume of 100 mL, was placed in a constant temperature shaking incubator set at 28 °C and 150 rpm for cultivation. Irpex lacteus was cultured for 4 days, while P. sapidus and P. ostreatus were cultured for 5 days, and S. vaninii, H. erinaceus, F. filiformis, L. edodes, and P. edulis were cultured for 7 days. Once the culture was complete, it was homogenized into a suspension using an adjustable high-speed homogenizer to create a seed liquid.
Liquid co-culture
The seed liquid was transferred to a liquid medium with a volume of 80 mL, based on an inoculum volume of 20% (v/v), to create the seed pre-culture liquid. Each strain was then individually cultured in a liquid medium with a filling volume of 80 mL using an inoculum volume of 20% (v/v). Sanghuangporus vaninii was used as a consistent strain. Subsequently, S. vaninii and other strains were each inoculated, with an inoculation volume of 10% (v/v), from the seed pre-culture liquid and transferred to a liquid medium with a volume of 80 mL for co-culturing. The shake flasks were then placed in a constant temperature shaking incubator set at 28 °C and 150 rpm for a duration of 7 days.
Seed pre-culture liquid culture times screening
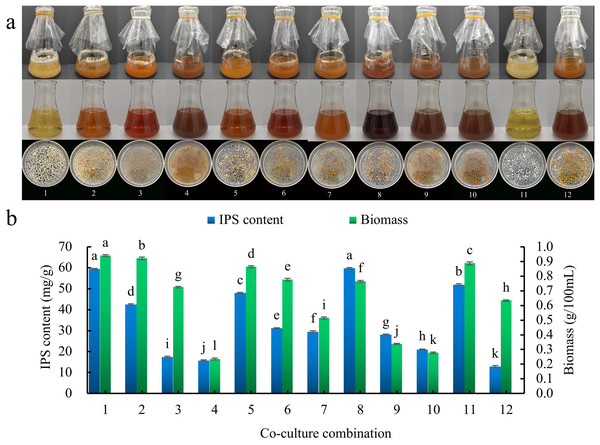
As shown in Table 1, liquid co-culture experiments were conducted with S. vaninii and P. sapidus. Combinations 1–10 involved a S. vaninii and P. sapidus co-culture, combination 11 was a P. sapidus monoculture, and combination 12 was a S. vaninii monoculture. The subsequent operational procedures remained consistent. All shake flasks were incubated in a constant temperature shaking incubator at 28 °C and 150 rpm for a duration of 7 days.
| Combination | ST seed pre-culture liquid culture time (d) | BLZ seed pre-culture liquid culture time (d) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 1 | 1 |
| 3 | 2 | 2 |
| 4 | 3 | 3 |
| 5 | 1 | 0 |
| 6 | 2 | 1 |
| 7 | 3 | 2 |
| 8 | 2 | 0 |
| 9 | 3 | 1 |
| 10 | 3 | 0 |
| 11 | – | 7 |
| 12 | 7 | – |
Note:
Combinations 1–10 are ST and BLZ co-culture, combination 11 is BLZ single culture, and combination 12 is ST single culture.
Single-factor experiment
This experiment investigated the effect of eight factors on IPS content in the co-culture. The fundamental culture conditions included glucose concentration (20 g/L), yeast extract concentration (5 g/L), MgSO4·7H2O concentration (0.5 g/L), KH2PO4 concentration (1.5 g/L), initial pH (natural), inoculation volume (v/v, 20%), S. vaninii inoculum ratio (50%), and liquid loading volume (100 mL).
The levels of each factor in the single-factor experiment were as follows: glucose concentration (10, 20, 30, 40, 50, 60, 70 g/L); yeast extract concentration (2, 5, 8, 11, 14, 17, 20 g/L); MgSO4·7H2O concentration (0.2, 0.5, 1, 1.5, 2, 2.5, 3 g/L); KH2PO4 concentration (0.5, 1, 1.5, 2, 2.5, 3, 3.5 g/L); initial pH (3, 4, 5, 6, 7, 8, 9, 10, 11); inoculation volume (v/v, 5, 10, 15, 20, 25, 30, 35 mL); S. vaninii inoculum ratio (20%, 30%, 40%, 50%, 60%, 70%, 80%); liquid loading volume (50, 75, 100, 125, 150, 175, 200 mL).
PB design
The PB design identified the essential factors that impacted the IPS content. These factors are represented as X1−X8 in Table 2. Each independent variable was assigned two levels, high (+) and low (−), while the IPS content was the responsive variable.
| Variables | Levels | |||
|---|---|---|---|---|
| Factor | Name | Unit | −1 | +1 |
| X1 | Glucose concentration | g/L | 24 | 30 |
| X2 | Yeast extract concentration | g/L | 6.4 | 8 |
| X3 | KH2PO4 concentration | g/L | 0.8 | 1 |
| X4 | MgSO4·7H2O concentration | g/L | 1.6 | 2 |
| X5 | Initial pH | – | 7.2 | 9 |
| X6 | Liquid loading volume | mL | 80 | 100 |
| X7 | Inoculation volume | % | 8 | 10 |
| X8 | ST inoculum ratio | % | 56 | 70 |
RSM optimization design
Based on the results of the single factor experiment and PB design, an RSM experiment was conducted. The independent variables in this experiment were yeast extract concentration, liquid loading volume, and S. vaninii inoculum ratio, while the responsive variable was IPS content. The levels of each factor are shown in Table 3.
| Variables | Levels | ||||
|---|---|---|---|---|---|
| Factor | Name | Unit | −1 | 0 | +1 |
| A | Yeast extract concentration | g/L | 5 | 8 | 11 |
| B | ST inoculum ratio | % | 60 | 70 | 80 |
| C | Liquid loading volume | mL | 75 | 100 | 125 |
IPS content determination
IPS content was determined using the methods outlined by Wang (2021), with slight modifications. The cultured fermentation broth was filtered using four layers of gauze. The mycelial balls were then rinsed with distilled water and dried in a drying oven at 60 °C until a constant weight was achieved. After cooling, the mycelium balls were weighed to obtain their biomass. Subsequently, the dried mycelium balls were crushed and ground through a 60-mesh standard sieve, and samples were collected for the determination of IPS content.
The hot water extraction method was used in this experiment for IPS extraction. A sample weighing 0.3 g was accurately measured and placed in a centrifuge tube. Distilled water was added to achieve a material-to-liquid ratio of 1:30 (g/mL). The centrifuge tube was then placed in a 90 °C water bath for a 2-h extraction period. Afterward, the mixture was centrifuged at 8,000 r/min for 30 min to collect the supernatant. This extraction process was repeated twice, and the supernatants were combined and concentrated in an electrothermal constant temperature drying oven to one-fifth of the original volume. Next, four times the volume of absolute ethanol was added, and the mixture was allowed to stand at 4 °C for 12 h. The mixture was then centrifuged at 8,000 r/min for 30 min to collect the precipitate, which was then placed in a drying box to completely evaporate the ethanol. Distilled water was added to redissolve the precipitate, resulting in the IPS test solution.
For the glucose standard solution (0.1 mg/mL), 0.1 g of glucose standard was weighed and then placed into a 100 mL beaker. Distilled water was then added to dissolve and dilute the solution to 1,000 mL and then stored at 4 °C. Next, 0, 0.2, 0.4, 0.6, 0.8, and 1 mL of the glucose standard solution were pipetted into separate test tubes. Distilled water was added to each test tube to reach a total volume of 1 mL and then each test tube was shaken well. After that, 1 mL of 5% phenol and 5 mL of concentrated sulfuric acid were added to each test tube in sequence. After being mixed well, the test tubes were bathed in boiling water for 15 min. Finally, the test tubes were cooled quickly under running water and then sat at room temperature for 10 min. The absorbance value was measured at a wavelength of 490 nm using distilled water as a blank. The glucose standard curve was plotted using the glucose content (mg) as the abscissa and the absorbance value as the ordinate. The linear regression equation of the glucose standard was Y = 10.185X − 0.0453 (R2 = 0.9927). The IPS content was calculated according to the following formula:
In the formula: m1—glucose content calculated from the standard curve, mg; V1—constant volume, mL; N—dilution factor; m—sample mass, g; V—volume of sample solution taken during measurement, mL.
Measurement of antioxidant capacity
Measurement of DPPH free radical scavenging capacity
For measuring DPPH free radical scavenging capacity, the methods described by Zeng et al. (2012) were followed, with slight adjustments. Samples were accurately weighed and diluted with distilled water to create solutions of varying concentrations. DPPH was weighed precisely and mixed with absolute ethanol to create a 0.1 mmol/L DPPH solution. Subsequently, 2 mL of a sample solution at a specific concentration was mixed with 2 mL of the DPPH solution, thoroughly mixed, and incubated in the dark at 30 °C for 30 min. The absorbance at 517 nm, denoted as A1, was then measured. The absorbance when distilled water was used instead of the DPPH solution was recorded as A2, and the absorbance when distilled water was used in place of the sample solution was recorded as A0. The DPPH free radical scavenging rate was calculated using the following formula:
Measurement of ABTS free radical scavenging capacity
For measuring ABTS free radical scavenging capacity, the methods of Jo, Cho & Chun (2021) were followed, with slight modifications. Equal amounts of 7 mmol/L ABTS solution and 2.45 mmol/L K2O8S2 solution were mixed evenly and left to stand in the dark at room temperature for 12 h to obtain the ABTS mother solution. The ABTS mother solution was then diluted with distilled water until its absorbance at 734 nm was 0.7 ± 0.02, recorded as A0, to obtain the ABTS working solution. Sample solutions of different concentrations (1 mL each) were mixed evenly with 4 mL of ABTS working solution, placed in the dark at 30 °C for 6 min, and the absorbance at 734 nm was measured and recorded as A. The ABTS free radical scavenging rate was calculated using the following formula:
Statistical analysis
Data enumeration was performed using Excel, and single factor variance analysis (ANOVA) was conducted using SPSS 26.0 software. PB and RSM experiments were conducted using Expert 13 software. *p < 0.05 and **p < 0.01 indicated significant and highly significant differences, respectively. Each experiment was replicated three times.
Results
Enhancement of IPS content by co-cultivation of S. vaninii and P. sapidus
In this study, seven different strains were chosen to be co-cultured with S. vaninii. Strain S. vaninii was co-cultured with the seven candidate strains, resulting in the inhibition of S. vaninii growth, while all candidate strains exhibited normal growth (Fig. 1). As shown in Table 4, when S. vaninii was co-cultured with the candidate strains, only the IPS content of I. lacteu, P. sapidus, and H. erinaceus co-cultures was higher than the IPS content of the individual strains. The IPS content of the S. vaninii and P. sapidus co-culture was 370.05% higher than that of S. vaninii alone and 11.4% higher than P. sapidus alone. Additionally, the biomass of the S. vaninii and P. sapidus co-culture was 59.57% higher than that of S. vaninii alone and 5.67% higher than P. sapidus alone. Based on these results, S. vaninii and P. sapidus were chosen to enhance the IPS content of further co-culture experiments.
Figure 1: ST was purely cultured and co-cultured with each of the strains to be screened in liquid.
| No. | Test strains | Biomass (g/100 mL) | IPS content (mg/g) |
|---|---|---|---|
| 1 | ST | 0.5775 ± 0.0150ef | 12.7286 ± 0.3774k |
| 2 | BPCJ+ST | 0.5377 ± 0.0165g | 21.3047 ± 0.6351h |
| 3 | BPCJ | 0.5640 ± 0.0112fg | 20.1241 ± 0.6093i |
| 4 | P8+ST | 0.8174 ± 0.0251c | 23.1432 ± 0.4273fg |
| 5 | P8 | 0.8174 ± 0.0176c | 25.1078 ± 0.6944d |
| 6 | HT3+ST | 0.4223 ± 0.0093h | 24.5098 ± 0.1535de |
| 7 | HT3 | 0.3406 ± 0.0102i | 23.7068 ± 0.5603ef |
| 8 | DM+ST | 0.6848 ± 0.0171d | 13.3412 ± 0.3346k |
| 9 | DM | 0.5987 ± 0.0148e | 15.7817 ± 0.2976j |
| 10 | BJZ+ST | 0.4367 ± 0.0083h | 16.3433 ± 0.4364j |
| 11 | BJZ | 0.7005 ± 0.0238d | 22.7538 ± 0.5149g |
| 12 | XG+ST | 0.0420 ± 0.0051j | 20.1931 ± 0.3185i |
| 13 | XG | 0.0503 ± 0.0063j | 27.1388 ± 0.6252c |
| 14 | BLZ+ST | 0.9215 ± 0.0322a | 59.8302 ± 0.8376a |
| 15 | BLZ | 0.8729 ± 0.0181b | 53.7090 ± 0.8054b |
Note:
Different letters indicate significant differences in means (p < 0.05).
Screening of S. vaninii and P. sapidus seed pre-cultures for culture time
Different fungi exhibit varying growth rates, and the efficacy of fungal co-cultures are greatly influenced by the timing of inoculation. To ensure a thorough co-culturing of fungi with different growth rates, the inoculation epochs of S. vaninii and P. sapidus were screened.
There were 10 co-culture combinations of S. vaninii and P. sapidus (combinations 1–10). When the seed pre-culture solution intervals of S. vaninii and P. sapidus were the same, the color of the fermentation filtrate gradually darkened and the size of the bacterial balls decreased as culture time increased, showing a more pronounced inhibitory effect. The color of S. vaninii bacterial balls shifted from yellow to orange-red, while the color of P. sapidus bacterial balls changed from white to yellow or yellow brown (Fig. 2A). Additionally, with longer seed pre-culture medium culture times, both the biomass and IPS content exhibited a decreasing trend (Fig. 2B). Combination 1 showed significantly higher biomass compared to combination 8, although there was no significant difference in the IPS content between the two combinations. The co-culture effect was more prominent in combination 8 than in combination 1. Consequently, combination 8 was chosen as the test combination, where the seed pre-culture fluids of S. vaninii and P. sapidus were cultured for 2 days and 0 days, respectively, before co-culturing. In combination 8, the IPS content was 4.68 times higher than that of the S. vaninii culture alone (combination 12) and 1.15 times higher than that of the P. sapidus culture alone (combination 11), while the biomass was 1.21 times higher than that of the S. vaninii culture alone and 0.86 times higher than that of the P. sapidus culture alone.
Figure 2: Seed pre-culture liquid culture times screening.
(A) Liquid culture of different co-culture combinations; (B) effect of different co-culture combinations on IPS content and biomass. Different lowercase letters indicate significant differences in means (p < 0.05). Combinations 1–10 are S. vaninii and P. sapidus co-culture, combination 11 is P. sapidus pure culture, and combination 12 is S. vaninii pure culture.Single-factor experiments
Glucose concentration screening
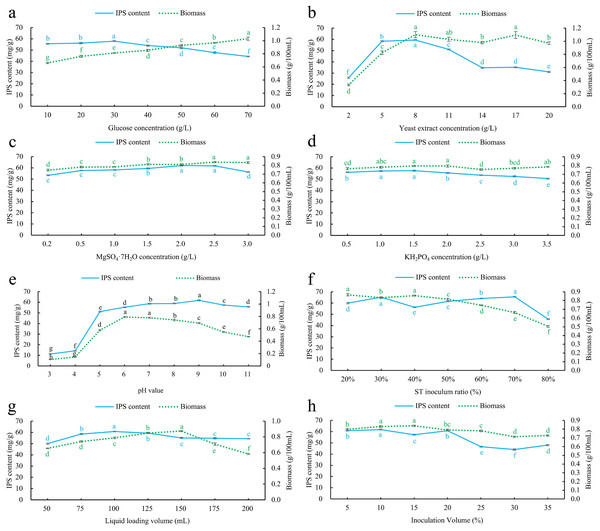
According to the results (Fig. 3A), the IPS content showed a gradual increase within the glucose concentration range of 10–30 g/L. At a glucose concentration of 30 g/L, the IPS content reached its highest value of 57.9784 ± 0.4536 mg/g. However, within the 30–70 g/L glucose concentration range, the IPS content decreased. Conversely, the biomass consistently increased from 0.6604 ± 0.0100 g/100 mL to 1.0310 ± 0.0455 g/100 mL within the 10–70 g/L glucose concentration range. Based on the selection criterion of IPS content, the optimal glucose concentration was determined to be 30 g/L.
Figure 3: Effect of single factor index screening on co-culture IPS content and biomass.
(A) Glucose concentration; (B) yeast extract concentration; (C) MgSO4·7H2O concentration; (D) KH2PO4 concentration; (E) pH; (F) S. vaninii inoculum ratio; (G) Liquid loading volume; (H) inoculum volume. Different lowercase letters indicate significant differences in means (p < 0.05).Yeast extract concentration screening
The results indicate (Fig. 3B) that both the IPS content and biomass increased as the yeast extract concentration increased in the 2–8 g/L range. At a yeast extract concentration of 8 g/L, both the IPS content and biomass reached their maximum values, measuring 59.4230 ± 0.4821 mg/g and 1.1008 ± 0.0902 g/100 mL, respectively. However, when the yeast extract concentration exceeded 8 g/L and ranged from 8–20 g/L, the IPS content showed a decreasing trend, while the biomass remained relatively stable. Therefore, the optimal yeast extract concentration was determined to be 8 g/L.
MgSO4·7H2O concentration screening
The results indicate (Fig. 3C) that there was an increasing trend in IPS content as MgSO4·7H2O concentration increased in the 0.2–2 g/L range. The IPS content reached its highest value of 62.0891 ± 0.4404 mg/g when the concentration of MgSO4·7H2O was 2 g/L, after which it decreased. Similarly, the biomass showed an increasing trend as MgSO4·7H2O concentration increased in the 0.2–2.5 g/L range. The biomass reached its peak value of 0.8379 ± 0.0079 g/100 mL when the concentration of MgSO4·7H2O was 2.5 g/L and then remained stable. Based on the selection criterion of IPS content, the optimal concentration of MgSO4·7H2O was determined to be 2 g/L.
KH2PO4 concentration screening
As KH2PO4 concentration increased from 0.5 to 1.5 g/L of KH2PO4, both IPS content and biomass showed an increasing trend. However, at a KH2PO4 concentration of 1.5 g/L, both the IPS content and biomass reached their maximum values, measuring 57.6404 ± 0.5523 mg/g and 0.7956 ± 0.0053 g/100 mL, respectively. As KH2PO4 concentration increased from 1.5 to 3.5 g/L, IPS content decreased, while biomass initially decreased and then increased (Fig. 3D). Therefore, the optimal concentration of KH2PO4 was determined to be 1.5 g/L.
Initial pH screening
The concentration of IPS showed an increasing trend across the pH range of 3–9. Initially, the IPS concentration was relatively low within the pH range of 3–4. However, it rapidly increased as the pH increased from 4 to 5, followed by a gradual increase within the pH range of 5–9. The highest IPS content of 61.7806 ± 0.5062 mg/g was observed at pH 9, after which it started to decrease. Conversely, biomass showed an upward trajectory within the pH range of 3–6, reaching its highest value of 0.7904 ± 0.0104 g/100 mL at pH 6 before starting to decline (Fig. 3E). Based on the evaluation of IPS content, the optimal pH was determined to be nine.
Sanghuangporus vaninii inoculum ratio screening
Within the 20–80% inoculation ratio range of S. vaninii, the IPS content showed an overall ascending-descending-ascending-descending trend. At a 30% and 70% inoculation ratio, the IPS content reached peak values of 65.2716 ± 0.3909 mg/g and 65.5855 ± 0.4270 mg/g, respectively, with no significant difference between these two peaks (Fig. 3F). Conversely, biomass consistently decreased. Due to the faster mycelial growth rate of P. sapidus compared to S. vaninii, the biomass increased as the proportion of P. sapidus inoculation increased. Therefore, to establish a more comprehensive co-culture state of the two strains and maximize their interaction, the optimal inoculation ratio for S. vaninii was determined to be 70%.
Liquid loading volume screening
The IPS content showed an increasing trend as the liquid volume increased in the 50–100 mL range. At a liquid volume of 100 mL, the IPS content reached its peak at 60.7687 ± 0.3657 mg/g, and then decreased as liquid volume increased. Similarly, the biomass increased as the liquid volume increased from 50 to 150 mL, reaching its highest value of 0.8736 ± 0.0064 g/100 mL before declining (Fig. 3G). Based on the evaluation criterion of IPS content, the optimal liquid volume was determined to be 100 mL.
Inoculum volume screening
IPS content showed an ascending-descending-ascending-descending trend as inoculum volume increased from 5% to 35%. At inoculum volumes of 10% and 20%, the IPS content reached peak values of 61.8494 ± 0.4158 mg/g and 60.8487 ± 0.4427 mg/g, respectively, with a significant difference between these two peaks. Conversely, biomass increased as inoculum volume increased from 5% to 15%, reaching its highest point at 15% with a value of 0.8378 ± 0.008 g/100 mL, after which it started to decline (Fig. 3H). Based on the evaluation criterion of IPS content, the optimal inoculum volume was determined to be 10%.
The optimal culture conditions were determined through Single-factor experiments, with the following parameters: glucose concentration of 30 g/L, yeast extract concentration of 8 g/L, MgSO4·7H2O concentration of 2 g/L, KH2PO4 concentration of 1.5 g/L, pH of 9, S. vaninii inoculum ratio of 70%, Liquid loading volume 100 mL, and inoculum volume of 10%.
PB design
In this study, a PB design strategy was used to systematically investigate the effects of different elements on IPS content in the co-culture and identify which factors significantly affected IPS content. The experimental design and interpretation of results were conducted using Design Expert 13 software. The specific methodologies and outcomes of the experiments are presented in Table 5.
| Run. order | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | Response value |
|---|---|---|---|---|---|---|---|---|---|
| Glucose concentration (g/L) | Yeast extract concentration (g/L) | KH2PO4 concentration (g/L) | MgSO4·7H2O concentration (g/L) | Initial pH | Liquid loading volume (mL) | Inoculation volume (%) | ST inoculum ratio (%) | IPS content (mg/g) | |
| 1 | 30 | 8 | 0.8 | 1.6 | 9 | 100 | 8 | 56 | 52.9959 |
| 2 | 24 | 6.4 | 1 | 1.6 | 9 | 100 | 8 | 70 | 44.9510 |
| 3 | 24 | 8 | 0.8 | 2 | 7.2 | 100 | 10 | 70 | 58.0327 |
| 4 | 30 | 6.4 | 0.8 | 1.6 | 7.2 | 100 | 10 | 70 | 64.0629 |
| 5 | 30 | 6.4 | 1 | 2 | 7.2 | 80 | 8 | 70 | 52.0004 |
| 6 | 24 | 6.4 | 0.8 | 1.6 | 7.2 | 80 | 8 | 56 | 58.4561 |
| 7 | 24 | 8 | 1 | 1.6 | 9 | 80 | 10 | 70 | 29.6220 |
| 8 | 30 | 8 | 0.8 | 2 | 9 | 80 | 8 | 70 | 33.7195 |
| 9 | 30 | 6.4 | 1 | 2 | 9 | 100 | 10 | 56 | 64.1685 |
| 10 | 24 | 6.4 | 0.8 | 2 | 9 | 80 | 10 | 56 | 59.6148 |
| 11 | 30 | 8 | 1 | 1.6 | 7.2 | 80 | 10 | 56 | 43.6575 |
| 12 | 24 | 8 | 1 | 2 | 7.2 | 100 | 8 | 56 | 48.8338 |
A variance analysis was performed on the experimental data, and the results are shown in Table 6. The high values of the model confirmation coefficient R2 and the adjusted coefficient Adj R2 indicate that the model fits well and the results are highly reliable. Among the elements studied, X2, X6, and X8 had significant influences on the response variable. The extent of their influence was ranked as X2 > X6 > X8. Therefore, future experiments will focus on examining the effects of these three elements on the response variable to determine the optimal culture conditions.
| Source | Sum of squares | F-value | p-value | |
|---|---|---|---|---|
| Model | 1,329.81 | 10.17 | 0.0413* | |
| X1 | Glucose concentration | 10.26 | 0.63 | 0.4861 |
| X2 | Yeast extract concentration | 486.32 | 29.76 | 0.0121* |
| X3 | KH2PO4 concentration | 158.77 | 9.72 | 0.0526 |
| X4 | MgSO4·7H2O concentration | 42.65 | 2.61 | 0.2046 |
| X5 | Initial pH | 133.14 | 8.15 | 0.0649 |
| X6 | Liquid loading volume | 261.1 | 15.98 | 0.0281* |
| X7 | Inoculation volume | 66.28 | 4.06 | 0.1375 |
| X8 | ST inoculum ratio | 171.3 | 10.48 | 0.0479* |
| R2 | 0.9644 | |||
| Adj R2 | 0.8696 | |||
Note:
RSM optimization experiment
RSM experimental design and results
This investigation used the Box-Behnken central composite design strategy with adjustable variables including yeast extract concentration, S. vaninii inoculum ratio, and liquid loading volume. A three-factor, three-level experimental design was implemented to refine the culture conditions, with IPS content as the response parameter. The results are presented in Table 7. The generated IPS content was evaluated and fitted using Design Expert 13 software.
| Run. order | Variables | Response value | ||
|---|---|---|---|---|
| A: Yeast extract concentration (g/L) | B: ST inoculum ratio (%) | C: Liquid loading volume (mL) | IPS content (mg/g) | |
| 1 | 5 | 70 | 125 | 61.6408 |
| 2 | 5 | 80 | 100 | 60.8699 |
| 3 | 11 | 70 | 125 | 34.7527 |
| 4 | 8 | 70 | 100 | 66.7635 |
| 5 | 8 | 60 | 125 | 47.3028 |
| 6 | 11 | 60 | 100 | 50.6033 |
| 7 | 8 | 70 | 100 | 65.7598 |
| 8 | 8 | 80 | 125 | 56.9552 |
| 9 | 8 | 70 | 100 | 65.7612 |
| 10 | 8 | 70 | 100 | 66.5578 |
| 11 | 8 | 70 | 100 | 65.2032 |
| 12 | 11 | 70 | 75 | 32.7527 |
| 13 | 5 | 60 | 100 | 66.1607 |
| 14 | 11 | 80 | 100 | 43.0082 |
| 15 | 8 | 60 | 75 | 51.5302 |
| 16 | 8 | 80 | 75 | 34.3047 |
| 17 | 5 | 70 | 75 | 42.7653 |
The regression equation for IPS content (Y) was calculated as:
The variance analysis of the RSM analysis model is shown in Table 8. The default term had a p-value of 0.0845, which exceeds 0.05, indicating the model’s soundness. The determination coefficient R2 and adjusted coefficient Adj R2 were 0.9970 and 0.9932, respectively, demonstrating an excellent correlation between the theoretical and actual values of IPS content. The F-values of factors A, B, and C were 581.67, 49.24, and 181.67, respectively, indicating the precedence of their effects on IPS content as A > C > B. Factors A, B, C, and the interaction terms AC, BC, A2, B2, and C2 exhibited statistically significant effects on IPS content (p < 0.05).
| Source | Sum of squares | df | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 2,495.17 | 9 | 277.24 | 260.9 | <0.0001** |
| A: Yeast extract concentration | 618.11 | 1 | 618.11 | 581.67 | <0.0001** |
| B: ST inoculum ratio | 52.32 | 1 | 52.32 | 49.24 | 0.0002** |
| C: Liquid loading volume | 193.05 | 1 | 193.05 | 181.67 | <0.0001** |
| AB | 1.33 | 1 | 1.33 | 1.25 | 0.3006 |
| AC | 71.2 | 1 | 71.2 | 67 | <0.0001 |
| BC | 180.61 | 1 | 180.61 | 169.96 | <0.0001 |
| A2 | 249.45 | 1 | 249.45 | 234.74 | <0.0001 |
| B2 | 41.82 | 1 | 41.82 | 39.36 | 0.0004 |
| C2 | 990.06 | 1 | 990.06 | 931.7 | <0.0001 |
| Residual | 7.44 | 7 | 1.06 | ||
| Lack of fit | 5.8 | 3 | 1.93 | 4.7 | 0.0845 |
| Pure error | 1.64 | 4 | 0.41 | ||
| Cor total | 2,502.61 | 16 | |||
| R2 | 0.9970 | ||||
| Adj R2 | 0.9932 |
Note:
RSM analysis
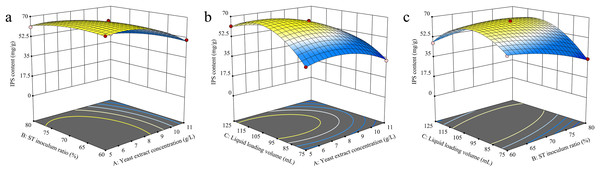
The RSM analysis results demonstrated the interaction effects between yeast extract concentration, S. vaninii inoculum ratio, and liquid loading volume on IPS content (Fig. 4). The contour plot visually represents the transition from the blue region to the yellow area, with a steeper gradient indicating more significant results. The surfaces of yeast extract concentration, S. vaninii inoculum ratio, and liquid loading volume exhibited a high degree of curvature, suggesting a highly significant impact of these three factors on IPS content.
Figure 4: (A–C) Response surface diagram of the interaction of two factors on IPS content.
Design-Expert 13 software was used to optimize the cultivation conditions for IPS content, and the optimal culture conditions were determined to be a yeast extract concentration of 6.12 g/L, S. vaninii inoculum ratio of 68.87%, and a liquid loading volume of 105.55 mL. Under these conditions, the projected IPS content was calculated to be 69.4559 mg/g. However, due to practical constraints, the yeast extract concentration was adjusted to 6 g/L, the S. vaninii inoculum ratio was recalibrated to 69%, and the liquid loading volume was adjusted to 106 mL.
Regression model validation
The results obtained from the optimization design experiments of the RSM showed that the IPS content was 69.4559 mg/g under optimal cultivation conditions, which was 16.19% higher than the IPS content under the pre-optimized culture conditions. Furthermore, when the optimum conditions determined by the RSM optimization method were applied in the actual culture, the IPS content reached 69.9626 mg/g, with a deviation of only 0.73% from the projected value (Table 9). These findings highlight the accuracy and reliability of the projected results obtained through RSM optimization.
| Indicator | Unoptimized | Optimized | Optimized to increase the percentage |
|---|---|---|---|
| Biomass (g/100 mL) | 0.7651 ± 0.0069 | 0.7628 ± 0.0381 | −0.3% |
| IPS content (mg/g) | 59.7789 ± 0.3635 | 69.9626 ± 2.1491 | 17.04% |
Antioxidant capacity analysis
Analysis of DPPH free radical scavenging capacity
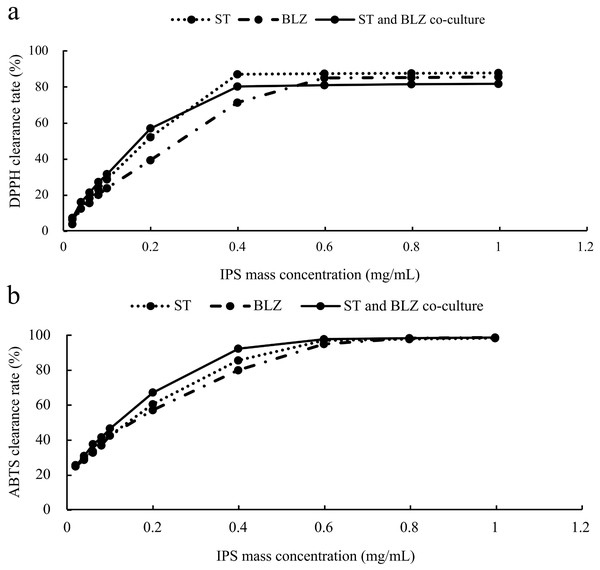
The scavenging rate of DPPH radicals by S. vaninii, P. sapidus, and the S. vaninii and P. sapidus co-culture increased with the higher mass concentration of IPS. At a mass concentration of 0.02 mg/mL, the scavenging rates were 3.71%, 6.26%, and 7.20% for S. vaninii, P. sapidus, and the S. vaninii and P. sapidus co-culture, respectively. As the mass concentration increased to 0.4 mg/mL, the scavenging rates for S. vaninii and the S. vaninii and P. sapidus co-culture rapidly rose to 87.10% and 80.23%, stabilizing around 87% and 81%, respectively. When the mass concentration reached 0.6 mg/mL, the P. sapidus scavenging rate peaked at 84.99% and remained stable at around 85% (Fig. 5A). The mass concentration of antioxidants (IC50) at a scavenging rate of 50% was used as the evaluation criterion to assess the antioxidant performance and free radical scavenging ability of antioxidants. A lower IC50 value indicates a higher ability of antioxidants to scavenge free radicals (Tang et al., 2010). The IC50 values of IPS from S. vaninii, P. sapidus, and the S. vaninii and P. sapidus co-culture were calculated as 0.1875, 0.2581, and 0.1687 mg/mL, respectively. This suggests that IPS from S. vaninii and P. sapidus co-cultures exhibit a stronger ability to scavenge DPPH free radicals compared to pure cultures of S. vaninii and P. sapidus.
Figure 5: Antioxidant capacity of IPS samples.
(A) Ability to scavenge DPPH radicals; (B) ability to scavenge ABTS radicals.Analysis of ABTS free radical scavenging capacity
The scavenging rate of ABTS free radicals by S. vaninii, P. sapidus, and the S. vaninii and P. sapidus co-culture increased as the mass concentration of IPS increased. At a mass concentration of 0.02 mg/mL, the scavenging rates were 25.62%, 24.86%, and 25.19% for S. vaninii, P. sapidus, and the S. vaninii and P. sapidus co-culture, respectively. As the mass concentration of IPS increased to 0.6 mg/mL, the scavenging rates rapidly rose to 97%, 95.05%, and 97.86% for S. vaninii, P. sapidus, and the S. vaninii and P. sapidus co-culture. With further increases in mass concentration, the clearance remained around 98% (Fig. 5B). The IC50 of IPS on ABTS radical scavenging for S. vaninii, P. sapidus, and the S. vaninii and P. sapidus co-culture were calculated to be 0.1397, 0.1515, and 0.1136 mg/mL, respectively. This indicates that the IPS of S. vaninii and P. sapidus co-cultures exhibit a stronger scavenging ability of ABTS radicals compared to the IPS of S. vaninii and P. sapidus pure cultures.
Discussion
This study aimed to investigate the impact of co-culture conditions on IPS content by creating an artificial symbiosis platform to mimic natural fungal interactions. Initially, liquid co-cultures were conducted with seven different fungi and S. vaninii. Results indicated that the co-culture of S. vaninii and P. sapidus exhibited the most significant increase in IPS content, reaching 59.8302 mg/g. It is important to note that not all fungal co-cultures led to an increase in polysaccharide content, highlighting the importance of considering strain interaction, culture medium selection, and growth rate. Other researchers have also explored co-cultures of various fungi (Peng et al., 2023). For instance, Yao et al. (2016) established 136 fungus-fungus symbiosis systems by co-culturing 17 basidiomycete species, identifying optimal interactions. The co-culture of Trametes versicolor and Ganoderma applanatum demonstrated the most favorable outcome. To achieve an effective co-culture of S. vaninii and P. sapidus, different co-cultivation combinations were tested, with combination 8 proving most effective. This combination involved pre-culturing S. vaninii and P. sapidus seeds separately for 2 days and 0 days, respectively, followed by liquid co-culturing. Guo et al. (2021) conducted experiments on six co-culture models of Sanghuangporus lonicericola and Cordyceps militaris, determining that pre-fermentation of S. lonicericola and C. militaris for 3 days and 1 day, respectively, before co-fermentation was optimal. These findings emphasize the variability in co-culture effects among different fungi, highlighting the importance of selecting appropriate culture combinations based on specific production requirements. As shown in Fig. 2 of this study, selecting combination 1 for subsequent experiments is recommended when considering only the biomass of mycelial balls. Fungi, as a significant source of natural pigments (Meruvu & dos Santos, 2021), have garnered attention for their versatile applications in various industries including food and beverage, textile, and medical fields (Abel et al., 2023). The 12 culture combinations depicted in Fig. 2 exhibit significant differences in the color of the fermentation broth, suggesting potential research directions for true pigments.
Medium components and culture conditions play a crucial role in the growth of microorganisms and in the production of secondary metabolites (Sun, 2022). Therefore, it is essential to optimize both the medium components for microbial growth and the culture conditions necessary to produce secondary metabolites. RSM can elucidate the relationship between different variables and is an effective approach for optimizing culture medium components and co-culture conditions. In this study, single-factor tests, PB tests, and RSM showed that IPS content is significantly influenced by yeast extract powder concentration, liquid filling volume, and S. vaninii inoculation volume proportion. When using optimal co-culture conditions, the IPS content was 69.9626 mg/g, with a deviation of only 0.73% from the predicted value of 69.4559 mg/g. Thus, the findings of this study suggest that RSM is suitable for optimizing the co-culture conditions of S. vaninii and P. sapidus.
Sanghuangporus vaninii and P. sapidus are two fungi known for their significant medicinal properties. They have been found to enhance the body’s immune system, regulate blood lipids, exhibit antibacterial and anti-tumor effects, and produce active polysaccharides (Li, Bau & Yang, 2022; Yang et al., 2023). Free radicals, constantly generated and removed in the body, can lead to cell damage, accelerated aging, and various diseases, including cancer, if accumulated in excess (Wang et al., 2014). Therefore, the antioxidant and free radical scavenging properties of fungal polysaccharides play a crucial role in maintaining health. Results from this study indicate that the IC50 values for IPS co-cultured with S. vaninii, P. sapidus, and the S. vaninii and P. sapidus co-culture on DPPH and ABTS free radical scavenging rates show stronger antioxidant capacity when co-cultured with both S. vaninii and P. sapidus compared to single cultures with either S. vaninii or P. sapidus. Antioxidants currently in use are typically synthetic, such as butylhydroxyanisole and 2,6-bis(1,1-dimethylethyl)-4-methylphenol. While these antioxidants can enhance product stability and maintain quality, they also come with various drawbacks and potential risks to human health (Cai et al., 2019). Thus, the search for new and safe natural antioxidants is of utmost importance. The conventional pure culture method has limitations in enhancing the antioxidant activity of fungal polysaccharides. However, the co-culture method employed in this study resulted in fungal polysaccharides with stronger antioxidant capabilities compared to those from single cultures of each strain, suggesting a promising avenue for the development and utilization of antioxidants.
Conclusions
This study screened P. sapidus strains through liquid co-culturing to identify those suitable for co-culturing with S. vaninii and those capable of increasing IPS content. After evaluating 10 co-culture combinations of S. vaninii and P. sapidus, differences were observed in terms of IPS content, biomass, color of the fermentation broth, and the size, shape, and color of the bacterial balls. Combination 8 demonstrated the most favorable outcomes, where the seed pre-culture solutions of S. vaninii and P. sapidus were initially cultured for 2 days and 0 days, respectively, before co-culturing. Through single factor experiments, PB, and RSM, the co-culture conditions of S. vaninii and P. sapidus were optimized to enhance IPS production in a shorter timeframe. The IPS content achieved under the optimized conditions was 69.9626 mg/g, representing a 447.16% increase compared to the single culture of S. vaninii (12.7866 mg/g) and a 34.69% increase compared to the single culture of P. sapidus (51.9439 mg/g). These results indicate that the method proposed in this study effectively boosts IPS content in co-cultured S. vaninii and P. sapidus, showcasing promising feasibility. The analysis of antioxidant activity indicated that IPS co-cultured with multiple strains exhibited stronger scavenging abilities against DPPH and ABTS free radicals compared to polysaccharides cultured with a single strain. These findings offer valuable insights for the selection of fungal co-culture strains and combinations, as well as lay a foundation for the future development and utilization of fungal polysaccharides. However, this is a preliminary study, and investigating potential changes in product components or generating new substances will require further research using metabolomics. Additionally, understanding the molecular mechanisms of co-culturing through transcriptomics is essential for advancing this area of research.
Supplemental Information
ST and BLZ seed pre-culture liquid culture time.
Note: Combinations 1-10 are ST and BLZ co-culture, combination 11 is BLZ single culture, and combination 12 is ST single culture.
Factors and levels of the Placket-Burman experimental design.
Biomass and IPS content of ST in pure culture and co-culture with each strain to be screened.
Note: Different letters indicate significant differences in means (p < 0.05).
First-order polynomial model analysis of Placket-Burman test results.
Note: *, p<0.05.
ANOVA of the fitted quadratic polynomial model.
Note: **, p<0.01.