Role of the transient receptor potential melastatin 4 in inhibition effect of arsenic trioxide on the tumor biological features of colorectal cancer cell
- Published
- Accepted
- Received
- Academic Editor
- Jiangjiang Qin
- Subject Areas
- Gastroenterology and Hepatology, Oncology, Pharmacology
- Keywords
- TRPM4 channel, Arsenic trioxide, HCT116 cells, Colorectal cancer
- Copyright
- © 2024 Gao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Role of the transient receptor potential melastatin 4 in inhibition effect of arsenic trioxide on the tumor biological features of colorectal cancer cell. PeerJ 12:e17559 https://doi.org/10.7717/peerj.17559
Abstract
Background
To investigate the effects of arsenic trioxide (ATO) on human colorectal cancer cells (HCT116) growth and the role of transient receptor potential melastatin 4 (TRPM4) channel in this process.
Methods
The viability of HCT116 cells was assessed using the CCK-8 assay. Western blot analysis was employed to examine the protein expression of TRPM4. The apoptosis of HCT116 cells was determined using TUNEL and Flow cytometry. Cell migration was assessed through the cell scratch recovery assay and Transwell cell migration assay. Additionally, Transwell cell invasion assay was performed to determine the invasion ability of HCT116 cells.
Results
ATO suppressed the viability of HCT116 cells in a dose-dependent manner, accompanied by a decline in cell migration and invasion, and an increase in apoptosis. 9-phenanthroline (9-Ph), a specific inhibitor of TRPM4, abrogated the ATO-induced upregulation of TRPM4 expression. Additionally, blocking TRPM4 reversed the effects of ATO on HCT116 cells proliferation, including restoration of cell viability, migration and invasion, as well as the inhibition of apoptosis.
Conclusion
ATO inhibits CRC cell growth by inducing TRPM4 expression, our findings indicate that ATO is a promising therapeutic strategy and TRPM4 may be a novel target for the treatment of CRC.
Introduction
Colorectal cancer (CRC) ranks as the third leading cause of death globally, following breast and lung cancer, with an incidence rate of 10% (Xi & Xu, 2021). Notably, the occurrence of early-onset CRC among individuals under 50 years old has seen a rise on a global scale (Patel et al., 2022). For most CRC patients, the standard treatment involves surgical resection of solid tumors, supplemented by adjuvant chemotherapy (Shi et al., 2023). Despite the therapeutic impact of surgery, the majority of CRC patients are diagnosed at an advanced stage or with metastasis, necessitating chemotherapy to improve their prognosis and survival rate (Tariq & Ghias, 2016). Nevertheless, existing chemotherapeutic drugs such as bevacizumab, afeliximab, and cetuximab are associated with significant toxicity and drug resistance (Labianca et al., 2010; Tonini et al., 2013; Biller & Schrag, 2021). Thus, a deeper understanding of the biological characteristics of colorectal cancer is imperative for identifying more potent and efficacious treatments.
Arsenic trioxide (ATO), a drug initially discovered by our group, has proven to be effective in treating acute promyelocytic leukemia (APL) and holds significant clinical value (Zhang et al., 2010). There is growing evidence indicating that ATO also demonstrates therapeutic effects on various solid tumors (Chen et al., 2023), including bladder cancer (Wang et al., 2009), pancreatic cancer (Xu et al., 2019), lung cancer (Huang & Zeng, 2019), and breast cancer (Nasrollahzadeh et al., 2020;). Additionally, prior studies have confirmed the effectiveness of ATO against CRC. For instance, Stevens et al. (2017) reported that ATO induces apoptosis via the up-regulation of Bcl2 Associated X Protein (Bax) in the human colon cancer cell line HT29 in vitro. Moreover, several investigations have shown that ATO inhibits CRC metastasis in mice by decreasing tumor invasion of regulatory T cells in vivo and enhancing cytokine-induced cytotoxic activity of killer cells (Wang et al., 2016). These findings suggest that ATO may exert direct or indirect tumor-suppressive effects. Furthermore, our previous study revealed that ATO affects angiogenesis by influencing the expression and activity of TRPM4 channels (Yu et al., 2019). Nevertheless, the specific effects and underlying mechanisms of ATO are still not entirely clear.
Ion channels play a pivotal role in cell proliferation and are potential therapeutic targets for various types of cancers (Schönherr, 2005). One such channel is TRPM4, a non-selective cation channel encoded by the TRPM4 gene, which is regulated by calcium activation and phosphatidylinositol diphosphate (Wang, Naruse & Takahashi, 2018). TRPM4 can modulate cell membrane potential and influence intracellular Ca2+ homeostasis (Dienes et al., 2021). It is widely expressed in various organs, with the highest expression observed in the prostate, colon, and heart (Launay et al., 2002; Nilius et al., 2003). Abnormal changes or mutations in TRPM4 have been linked to several pathological conditions, including heart disease (Guinamard et al., 2015), immune disorders (Smith & Nilius, 2013), and cancer (Borgström, Peinelt & Stokłosa, 2021). In recent years, there has been growing interest in the role of TRPM4 in cancer, particularly its involvement in key tumor functions such as cell migration, invasion, proliferation, and apoptosis inhibition. Studies have shown that TRPM4 mRNA and protein levels are significantly up-regulated in breast cancer tissues compared to normal tissues, correlating with a poor prognosis (Rivas et al., 2020). Conversely, in endometrial carcinoma, TRPM4 acts as a tumor suppressor gene, and its inhibition promotes proliferation and migration (Rivas et al., 2020). In CRC, the mRNA levels of TRPM4 were found to be decreased compared to normal colon tissue, indicating its involvement in the occurrence and development of CRC (Sozucan et al., 2015). Furthermore, a previous study has demonstrated that the expression of TRPM4 can be affected by ATO, impacting angiogenesis (Kappel et al., 2019). In addition, our previous study demonstrated that ATO could affect angiogenesis by affecting the expression of TRPM4 (Yu et al., 2019). This suggests that TRPM4 may be a potential candidate target for anticancer therapy, although this has not been verified. Up to now, 9-Ph is considered to be a specific blocker of TRPM4 (Grand et al., 2008; Guinamard, Hof & Del Negro, 2014). Therefore, this study aims to investigate the effect of ATO on CRC and explore the potential involvement of TRPM4 in this process using 9-Ph.
Materials and Methods
Cell cultures
The human CRC cell lines (HCT116) were obtained from the Department of Pharmacology, Harbin Medical University, Harbin and cultured in RPMI medium 1,640 basic (Thermo Fisher, Waltham, MA, USA) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) in 5% CO2 at 37 °C. HCT116 cells were grown until 70–80% confluence, and were used in passages 4–6 for all of the subsequent experiments, they were treated with 5 µM ATO or 1 µM 9-Ph for 24 h.
Western blot analysis
Total cellular proteins were extracted from pretreated HCT116 cells using RIPA lysis buffer (Beyotime, Suzhou, China) under ice bath conditions and subsequently separated on 10% SDS-PAGE gels (NCM Biotech, China) and transferred to nitrocellulose membranes (Millipore, Burlington, MA, USA). After blocking with 5% skim milk for 2 h, proteins were detected using rabbit TRPM4 antibody (1:200; ACC-004, Alomone Labs, Jerusalem, Israel) and mouse GAPDH antibody (1:1000; Prosun, China), and blots were detected using an Odyssey infrared imaging system (ODY-3149; LI-COR, Lincoln, NE, USA).
CCK-8
For Cell Counting Kit-8 (CCK-8) assays, HCT116 cells were seeded into 96-well plates with 200 µL of complete culture medium and they were treated with 5 mM ATO or 1 mM 9-Ph for 24 h. According to the protocol of CCK-8 solution (Beyotime, Suzhou, China), 20 µL of CCK-8 solution diluted in 200 µL of complete culture medium replaced the original medium of each group. After the cells were incubated in the dark at 37 °C for an additional 1 h, we detected viable cells by using absorbance at a 450 nm wavelength by CMAX PLUS Microplate Reader (Molecular Devices, China). The cell viability of HCT116 cells in each well was noted as a percentage of the control group.
Scratch wound assay
HCT116 cells were cultured in 6-well plates and treated with or without 5 µM ATO, 1 µM 9-Ph or both. Cells were scratched with a pipette tip to form a wound, and incubated at 37 °C with 5% CO2. Cells were imaged at 24 h post scratching. The images acquired were analyzed using ImageJ (NIH) software to measure the closure of the wounded area. The scratch healing area were analyzed to quantify cell migration.
Transwell assay
HCT116 cells (1–1.5 × 105) were seeded in the upper chamber of a 24-well Transwell chamber (Corning, NY, USA). After cell adhesion, 500 µL medium containing 10% FBS was added to the lower chamber to stimulate cell migration. Cells were treated with both 5 µM ATO and 1 µM 9-Ph for 24 h. The chambers were then removed, fixed with cold methanol, stained with 1% crystal violet, and allowed to dry before being photographed using a microscope. The transwell invasion assay was performed according to the same procedure as above. The upper chamber was coated with BD matrix glue (Corning, NY, USA) and serum-free medium at a 1:8 dilution.
Flow cytometry
Flow cytometry was conducted to assess apoptosis in HCT116 cells. The supernatant containing dead cells was collected in 15 mL centrifuge tubes. Following this, the cells were washed with PBS and trypsinized (without EDTA). T Subsequently, the trypsinized cells were collected, washed twice with ice-cold PBS, and centrifuged at 1,000 r/min for 5 min. The supernatant was then discarded, and the cells were collected. 1 ×Binding buffer was added and thoroughly mixed. A solution of fluorescein isothiocyanate (FITC) and propidium iodide (PI) was added to a 1.5 mL EP tube, followed by a 15-minute incubation at room temperature in the dark.
After staining and incubation, 1 ×Binding Buffer was added to each tube, and the mixture was detected by flow cytometry (CytoFLEX, Beckman Coulter, Indianapolis, IN, USA). All procedures were performed according to the manufacturer’s instructions of the Annexin V-FITC/PI apoptosis kit (Lianke Bio, Zhejiang, China).
TUNEL experiment
HCT116 cells were seeded in 12-well plates. After cell adhesion, the cells were treated with 5 µM ATO and 1 µM 9-Ph for 24 h, then fixed with 4% paraformaldehyde, penetrated with 0.1% Triton X-100 (Sigma) at room temperature, and incubated with TUNEL reaction solution (Roche, Indianapolis, IN, USA) for 1 h at 37 °C. Cells were washed with PBS and subsequently incubated with DAPI (sigma) at room temperature for 15 min. After completion of staining, images were taken by fluorescence microscopy (Zeiss, Jena, Germany).
Statistical analysis
The experimental results were treated as Mean ± SEM, and Student’s T-test was used for comparison between the two groups. ANOVA combined with Tukey’s test was used for statistical analysis of the comparison between multiple groups. The analysis program was GraphPad Prism 8. It was considered that the difference was statistically significant when P < 0.05.
Results
ATO inhibited the viability of HCT116 cells
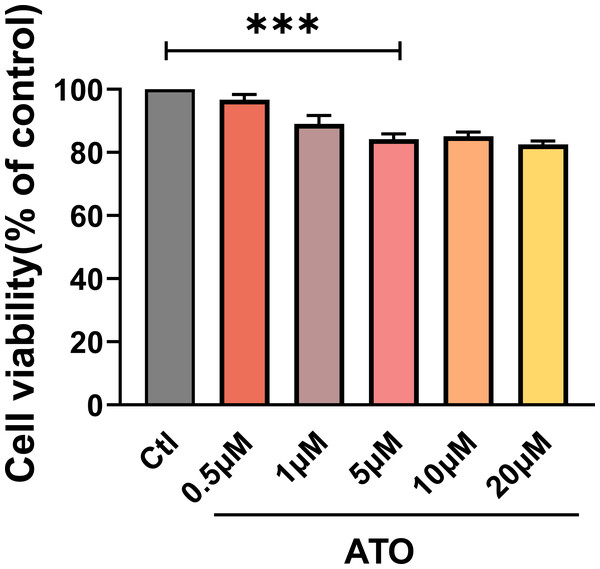
To evaluate the impact of ATO on HCT116 cells proliferation, cell viability was assessed using a CCK-8 assay in HCT116 cells treated with varying ATO concentrations (0, 0.5, 1, 5, 10, 20 µM) for 24 h. The findings indicated that HCT116 cell viability progressively declined as ATO concentrations increased (Fig. 1A), suggesting that ATO reproducibly inhibits HCT116 viability in a dose-dependent manner.
Figure 1: ATO inhibited the viability of HCT116 cells.
Quantitative analysis of cell viability after treatment with ATO at different concentrations. ∗∗∗P < 0.001 vs Ctl group; n = 3. Data are represented as the mean ± SD. One-way ANOVA followed by Tukey’s test for post-hoc comparisons was used. n, number of independent cells.ATO increased the expression of TRPM4 in HCT116 cells
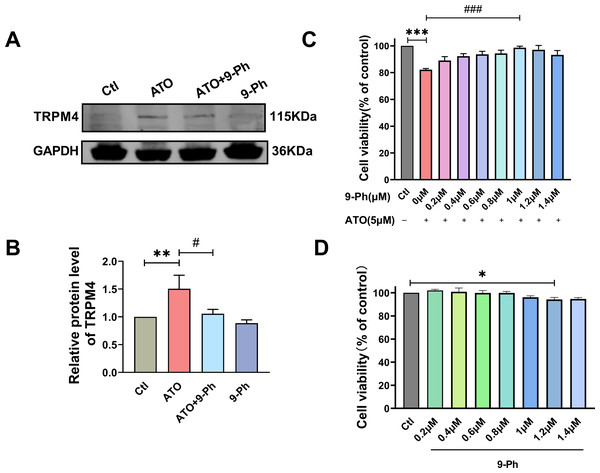
Prior research has indicated varying levels of TRPM4 expression across different types of cancers. Specifically, TRPM4 expression is elevated in prostate cancer, contributing to the activation of oncogenes (Berg et al., 2016). In endometrial carcinoma, the expression of TRPM4 is decreased and plays the role of tumor suppressor genes (Rivas et al., 2020). Given the link between reduced levels of TRPM4 and the progression of CRC, we investigated if ATO triggers cell death in HCT116 cells by modulating TRPM4 levels. Our findings demonstrated that ATO significantly boosted TRPM4 expression, which was suppressed upon administering 9-Ph. These results suggest that ATO exerts its anti-cancer effects by upregulating TRPM4, whereas 9-Ph mitigates the heightened TRPM4 expression caused by ATO. Subsequently, the efficacy of the TRPM4 specific inhibitor 9-Ph was examined with respect to the ATO-related decrease in HCT116 cell viability. HCT116 cells were pre-treated with graded concentrations of 9-Ph (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4 µM) before undergoing ATO treatment for 24 h. The study found that at doses of 1.0 and 1.4 µM, 9-Ph successfully reversed the inhibitory impact of ATO on HCT116 (Fig. 2C). However, concentrations of 9-Ph up to 1 µM had negligible effects on HCT116 cells (Fig. 2D). The outcomes demonstrated that 9-Ph exerts a significant counteractive effect on the ATO-induced reduction in HCT116 cell viability.
Figure 2: ATO increased the expression of TRPM4 protein in HCT116 cells.
(A-B) Representative images of western blot and quantification of TRPM4 in ATO treated HCT116 cells after 9-Ph stimulation. (C) Quantitative analysis of cell viability in ATO treated HCT116 cells after 9-Ph (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4 μM) stimulation. (D) Quantitative analysis of cell viability after treatment with 9-Ph at different concentrations. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs Ctl group, #P < 0.05, ### P < 0.001 vs ATO group; n = 3. Data are represented as the mean ± SD. One-way ANOVA followed by Tukey’s test for post-hoc comparisons was used. n, number of independent cells.TRPM4 inhibition abolished the ATO-induced decrease in migration and invasion of HCT116 cells
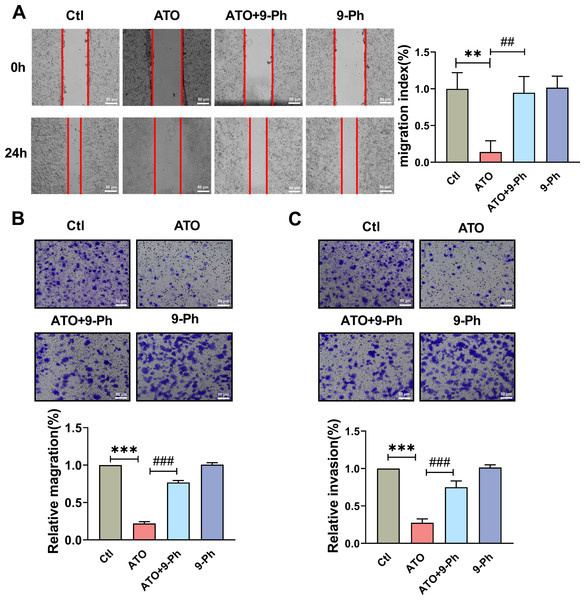
CRC is a diverse disease, with approximately 50% of patients presenting with distant metastasis upon diagnosis, often leading to unsuccessful treatments. This widespread metastasis primarily stems from changes in the migratory and invasive abilities of CRC cells. Therefore, impeding these abilities can potentially enhance therapy for CRC that tends to metastasize. To determine the influence of TRPM4 on HCT116 cells proliferation affected by ATO, wound healing/scratching and Transwell migration assays were carried out to assess cell migration recovery and capability. The wound healing/scratching test showed notable suppression of HCT116 cell migration recovery post ATO treatment (Fig. 3A).
Figure 3: TRPM4 inhibition abolished the ATO-induced decrease in migration and invasion of HCT116 cells.
(A) Representative images of wound healing assay and quantification of migration in ATO treated HCT116 cells after 9-Ph stimulation. scale bar = 50 µm. (B–C) Representative images of Transwell assay and quantification of migration and invasion in ATO treated HCT116 cells after 9-Ph stimulation. scale bar = 50 µm. ∗∗ P < 0.01, ∗∗∗ P < 0.001 vs. Ctl group, ##P < 0.01, ###P < 0.001 vs. ATO group; n = 5. Data are represented as the mean ± SD. One-way ANOVA followed by Tukey’s test for post-hoc comparisons was used. n, number of independent cells.The Transwell migration assay concurred, revealing a reduced count of HCT116 cells following ATO application compared to the control (Fig. 3B); this was in line with the scratch test findings. The migration impairment of HCT116 cells caused by ATO was counteracted by 9-Ph. Additionally, to investigate ATO’s impact on HCT116 cell invasiveness, a Transwell invasion assay was conducted. After ATO treatment, a significant reduction was observed in the quantity of invasive HCT116 cells relative to the control (Fig. 3C). Likewise, the diminished invasive capacity caused by ATO was negated by 9-Ph. Overall, these pieces of evidence imply that TRPM4 plays a crucial role in ATO’s suppression of HCT116 cell migration and invasion.
Blockade of TRPM4 reversed the potential effects of ATO on HCT116 cells apoptosis
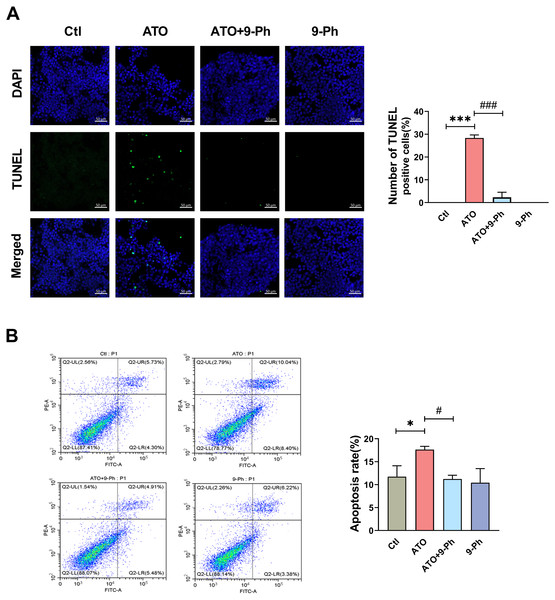
We further investigated the impact of ATO on various aspects of the growth process of HCT116 cells. Apoptosis, a form of programmed cell death, plays a vital role in eliminating damaged cells in an orderly and effective manner. The dysregulation of the apoptotic cell death mechanism is a hallmark of cancer development (Pistritto et al., 2016). TUNEL assay and flow cytometry were utilized to detect the apoptosis of HCT116 cells. TUNEL assay revealed that ATO increased the apoptosis in HCT116 cells, which was inhibited by 9-Ph treatment. 9-Ph alone did not alter cell apoptosis under basal conditions (Fig. 4A). Flow cytometry exhibited markedly increased apoptotic rate in HCT116 cells treated with ATO; this increase was significantly inhibited by 9-Ph (Fig. 4B). In summary, these results suggest that TRPM4 may at least partially contributes to the effects of ATO on HCT116 cells apoptosis.
Figure 4: ATO promotes apoptosis of HCT116 cells.
(A) Representative images of TUNEL staining assay and quantification in ATO treated HCT116 cells after 9-Ph stimulation. Scale bar = 50 µm . (B) Representative images of flow cytometry and quantification of positive cell in ATO treated HCT116 cells after 9-Ph stimulation. ∗P < 0.05, ∗∗∗ P < 0.001 vs. Ctl group, # P < 0.05, ###P < 0.001 vs ATO group; n = 3. Data are represented as the mean ± SD. One-way ANOVA followed by Tukey’s test for post-hoc comparisons was used. n, number of independent cells.Discussion
CRC is a complex and multifaceted disease, characterized by a broad spectrum of clinical manifestations, biological behaviors, and genetic mutations. This diversity, referred to as heterogeneity, complicates the diagnostic and treatment processes for CRC. A significant challenge in managing CRC is the high rate of distant metastasis; with studies indicating that up to 50% of patients already have metastatic disease at the time of their initial diagnosis (Paązek, Łukaszewicz-Zając & Mroczko, 2022). The therapeutic strategies of CRC include surgery, chemotherapy and radiotherapy, used in combination based on the location and progress of the disease. Although surgery can remove the tumor, complete elimination of all cancer cells is often impossible. For stage I and II colon cancer, most patients undergo colectomy without chemotherapy (84%), while about 2/3 of stage III colon cancer patients (and some patients with high-risk stage II diseases) receive adjuvant chemotherapy to reduce the risk of recurrence (Miller et al., 2022). Common chemotherapy side effects include neuropathy, resulting in pain and numbness, and intestinal dysfunction, leading to disruptions in bowel movements and digestion. These complications arise from the non-specific and cytotoxic nature of chemotherapy drugs, which can harm healthy cells alongside cancer cells. Moreover, the high rate of relapse associated with CRC treatments further complicates the management of the disease, emphasizing the urgent need for more effective and targeted therapeutic approaches (Garg et al., 2012). Thus, continuous research and innovation in CRC treatment are paramount to address the current limitations and improve the prognosis for patients with this complex disease.
ATO is currently utilized in the treatment of APL and recurrent/multiple myeloma (MM) patients (Munshi et al., 2002). While ATO demonstrates potential as an anticancer agent in solid tumors, such as bladder cancer, pancreatic cancer, and lung cancer (Wahiduzzaman, Ota & Hosokawa, 2020). The current study aims to investigate the effect of ATO on CRC cells growth. The results indicate that ATO significantly impedes the vitality of CRC cells. Furthermore, ATO triggers the expression of TRPM4, diminishes HCT116 cell migration and invasion ability, and augments cell apoptosis. While these studies demonstrate the effective inhibition of CRC cell growth by ATO, the specific mechanisms involved require further elucidation. TRPM4 is a voltage-dependent and non-selective cation channel, which can regulate intracellular calcium overload, maintain plasma membrane potential, regulate intracellular calcium oscillation and other cellular functions. Prior research has revealed that ATO induces apoptosis through generation of reactive oxygen species (ROS) (Medda, De & Maiti, 2021), and TRPM4 is identified as an ion channel involved in the intracellular ROS regulation of cell death (Simon et al., 2010). Thus, it is hypothesized that TRPM4 may be implicated in the action of ATO. Our previous studies also found that ATO up-regulated the expression of TRPM4 in endothelial cells, induced ROS accumulation, and eventually led to endothelial cell injury (Yu et al., 2019). In the context of CRC, our study found increased expression of TRPM4 during ATO-induced CRC cell death, yet the mechanisms underlying the elevated TRPM4 levels warrant further investigation.
9-ph is a specific inhibitor of TRPM4 (Guinamard, Hof & Del Negro, 2014). Grand et al. (2008) have demonstrated that 9-ph is sufficient to reduce the pharmacological effects of TRPM4, but does not inhibit TRPM5 channels. Our previous research has also confirmed 9-ph can disrupt the endothelium’s protective effect mediated by TRPM4 (Yu et al., 2019). In this investigation, we sought to determine whether the blockade of TRPM4 using 9-ph interfered with the anti-tumor effect of ATO. We observed that 9-ph diminished the anti-tumor effect of ATO, while demonstrating no independent effect. Additionally, 9-ph reduced the increase in TRPM4 expression induced by ATO, suggesting that the cell damage caused by ATO may stem from the upregulation of TRPM4. Moreover, reducing the expression of TRPM4 counters the effect of ATO.
Half of the patients diagnosed with CRC tumors have already experienced distal metastasis. Tumor metastasis is primarily caused by migration and invasion. Inhibiting tumor migration and invasion is suggested as an effective approach to reduce the incidence of tumor metastasis. Previous studies have shown that ATO can decrease the expression of matrix metalloproteinases and increase TIMP-1 expression, leading to the inhibition of migration and invasion in ovarian cancer cells (Zhang & Wang, 2006). Our experiment revealed that ATO reduced CRC migration and invasion by more than 50% (Fig. 3). Additionally, 9-ph impeded the reduction of ATO-induced metastasis. These findings suggest that TRPM4 plays a role in the process of tumor metastasis and has the ability to impede its occurrence.
Previous studies have demonstrated that apoptosis is proposed to associated with the effects of ATO on cancer cells, such gastric cancer cells and pancreatic cancer cells (Zheng et al., 2010). ATO is capable of triggering apoptosis in pancreatic cancer cells by modulating the expression of cyclic and activating the caspase pathway (Rivas et al., 2020). Our study reveals that ATO can also induce apoptosis in CRC cells and significantly elevate the apoptosis rate in CRC cells (Fig. 4A). Meanwhile, ATO notably raised the apoptosis rate of CRC cells as evidenced by flow cytometry analysis (Fig. 4B). Interestingly, these observed effects were reversed upon the blockade of TRPM4 using 9-Ph, indicating that TRPM4 may have a critical role in inducing late apoptosis in tumors.
Many studies have confirmed that TRPM4 inhibitors can act as an anti-tumor regimen, but in this study, we found that the TRPM4 inhibitors had no effect on tumor proliferation and migration under basal level, but weakened the inhibitory effects of ATO on HCT116 cells growth. This discrepancy may be attributed to the abnormally increased expression of TRPM4 in ATO, which is a predominant factor in tumor damage, while the expression of TRPM4 is insufficient to exert an effect on tumors in a normal physiological environment.
In conclusion, we demonstrate that ATO damages CRC cells, induces TRPM4 expression, reduces the cell viability, inhibits the migration and invasion ability and increases apoptosis of HCT116 cells. 9-Ph as a specific inhibitor of TRPM4, antagonizes the effect of ATO, indicating that ATO induces CRC cell injury via increasing TRPM4.
Supplemental Information
ATO inhibited the viability of HCT116 cells
Quantitative analysis of cell viability after treatment with ATO at different concentrations.
ATO increased the expression of TRPM4 protein in HCT116 cells
Representative images of western blot and quantification of TRPM4 in ATO treated HCT116 cells after 9-Ph stimulation
9-ph reversed the damage of HCT116 induced by ATO
Quantitative analysis of cell viability in ATO treated HCT116 cells after 9-Ph (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4 μM) stimulation.
Effect of 9-ph on the viability of hct116 cells
Quantitative analysis of cell viability after treatment with 9-Ph at different concentrations. ∗P < 0.05, ∗∗∗P < 0.001 vs Ctl group
ATO increased TRPM4 protein expression in HCT116 cells
Representative images of western blot and quantification of TRPM4 in ATO treated HCT116 cells after 9-Ph stimulation.
TRPM4 inhibition abolished the ATO-induced decrease in migration of HCT116 cells
Representative images of wound healing assay and quantification of migration in ATO treated HCT116 cells after 9-Ph stimulation.
TRPM4 inhibition abolished the ATO-induced decrease in migration of HCT116 cells
Representative images of Transwell assay and quantification of migration in ATO treated HCT116 cells after 9-Ph stimulation.
TRPM4 inhibition abolished the ATO-induced decrease in invasion of HCT116 cells
Representative images of Transwell assay and quantification of invasion in ATO treated HCT116 cells after 9-Ph stimulation.
ATO promotes apoptosis of HCT116 cells
Representative images of TUNEL staining assay and quantification in ATO treated HCT116 cells after 9-Ph stimulation.
ATO promotes apoptosis of HCT116 cells
Representative images of flow cytometry and quantification of positive cells in ATO-treated HCT116 cells after 9-Ph stimulation. n = 3.
ATO promotes apoptosis of HCT116 cells
Representative images of flow cytometry and quantification of positive cell in ATO treated HCT116 cells after 9-Ph stimulation.