Genome-wide identification, characterization and expression analysis of the DUF668 gene family in tomato
- Published
- Accepted
- Received
- Academic Editor
- Jiban Shrestha
- Subject Areas
- Agricultural Science, Bioinformatics, Molecular Biology, Plant Science
- Keywords
- Tomato, Genome-wide analysis, DUF668 gene family, Adverse stress, Gene expression pattern
- Copyright
- © 2024 Li et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Genome-wide identification, characterization and expression analysis of the DUF668 gene family in tomato. PeerJ 12:e17537 https://doi.org/10.7717/peerj.17537
Abstract
The domain of unknown function 668 (DUF668) is a gene family that may play a key role in plant growth and development as well as in responding to adversity coercion stresses. However, the DUF668 gene family has not yet been well identified and characterized in tomato. In this study, a total of nine putative SlDUF668 genes were identified in tomato, distributed on six chromosomes. Phylogenetic analyses revealed that SlDUF668 proteins were classified into two major groups. Members within the same group largely displayed analogous gene structure and conserved motif compositions. Several cis-elements were exhibited in the upstream sequences of the SlDUF668 genes, including elements implicated in plant growth and development processes, abiotic stress and hormone responses. Further, the study assessed the expression patterns of the SlDUF668 gene family in various tomato tissues, five plant hormones treatments, three abiotic stresses using qRT-PCR. The SlDUF668 genes expressed ubiquitously in various tissues, and five genes (SlDUF668-04, SlDUF668-06, SlDUF668-07, SlDUF668-08 and SlDUF668-09) showed tissue specificity. And SlDUF668 genes responded to abiotic stresses such as salt, drought and cold to varying degrees. Overall, our study provided a base for the tomato DUF668 gene family and laid a foundation for further understanding the functional characteristics of DUF668 genes in tomato plants.
Introduction
The Pfam database classifies and names the domains of unknown function (DUF) using a combination of “DUF” and numbers, such as DUF1 and DUF2 (Mistry et al., 2021; Bateman, Coggill & Finn, 2010; Schultz et al., 1998). Chris Ponting first proposed the naming scheme of DUF in 1998 by adding DUF1 and DUF2 to the SMART database. Subsequently, DUF1 and DUF2 were renamed based on their featured peptides as the GGDEF (PF00990) domain and EAL (PF00990) domain, respectively (Bateman, Coggill & Finn, 2010). These domains have two distinct characteristics: a relatively conservative amino acid sequence and a protein domain with an unidentified function (Bateman, Coggill & Finn, 2010). The number of DUF superfamily members has rapidly increased in recent years due to the sequencing of genomes from a large number of species. As of 2010, the entire family expanded to DUF2607 (DUF1-DUF2607). (Bateman, Coggill & Finn, 2010). Currently, the Pfam database (version 35.0) contains 19,632 families, with approximately 24% (4,795 out of 19,632) being composed of DUF families (Mistry et al., 2021).
Proteins containing the DUF domain are known to have a significant impact on the growth and development of plants. Arabidopsis thaliana, for instance, has several DUFs that regulate the biosynthesis of its plant cell wall, including DUF266, DUF231, DUF246, DUF1218, and DUF579 (Parsons et al., 2012; Yuan et al., 2013; Oikawa et al., 2010; Mewalal et al., 2016; Urbanowicz et al., 2012). Some DUFs, such as DUF640 and DUF827, control the development of chloroplasts and plant growth (Zhao et al., 2004; Kodama, Suetsugu & Wada, 2011). Additionally, DUFs like DUF828, DUF966, DUF668, DUF642, and DUF761 regulate the growth of roots (Shen et al., 2019; Zhao et al., 2021; Gao et al., 2012; Salazar-Iribe et al., 2016; Zhang, Zhang & Huang, 2019). The DUF640 family primarily contributes to the regulation of flower development (Li et al., 2012), while the DUF784 and DUF1216 families are involved in pollen development (Huang et al., 2008; Jones-Rhoades, Borevitz & Preuss, 2007).
In addition to controlling plant growth and development, DUFs have been discovered to play a role in plant stress responses. For instance, the AtRDUF1 gene (DUF1117) positively regulates responses to salt stress in Arabidopsis thaliana, and suppressing the expression of both AtRDUF1 and AtRDUF2 decreases tolerance to drought stress mediated by ABA in Arabidopsis (Kim, Ryu & Kim, 2012). ESK1 (AT3g55990), a member of the DUF231 gene family, acts as a negative regulator of cold acclimation (Xin et al., 2007). Overexpressing GmCBSDUF3 (DUF21) enhances tolerance to drought and salt stress in Arabidopsis (Hao et al., 2021). The AhDGR2 gene from Amaranthus hypochondriacus encodes a DUF642 protein that is involved in salt and ABA hypersensitivity in Arabidopsis (Palmeros-Suárez et al., 2017). The OsDSR2 gene, which encodes a DUF966 protein, negatively regulates salt stress, simulated drought stress, and ABA signaling in rice (Luo et al., 2013). OsSIDP361, a DUF1644 gene from rice, improves tolerance to drought and salinity (Li et al., 2016). Overexpressing the OsDUF946.4 and OsSIDP366 genes enhances tolerance to high salt and drought in rice (Li et al., 2017; Guo et al., 2016). OsSGL provides increased drought tolerance in transgenic rice and Arabidopsis (Cui et al., 2016). Other DUF genes that are related to abiotic stress in rice include OsDSR2 (DUF966) (Luo et al., 2013) and OsDUF810.7 (Li et al., 2018). Some TaDUF966 genes are induced by salt stress in wheat (Triticum aestivum L.), and the role of the TaDUF966-9B gene in salt stress has been confirmed (Zhou et al., 2020). Overexpressing the salt-inducing gene TaSRHP, a DUF581 gene from wheat, enhances resistance to salt and drought stress in Arabidopsis thaliana (Hou et al., 2013). Overexpressing AmDUF1517, a gene responsive to cold stress, significantly improves tolerance to various stresses in transgenic cotton (Hao et al., 2018). Some GmDUF668 genes significantly respond to salt stress in soybean (Zaynab et al., 2023). Some ZoDUF668 genes were upregulated under cold stress in ginger (Han et al., 2024). Four genes (IbDUF668-6, 7, 11 and 13) of sweet potato were significantly upregulated under ABA, drought and NaCl stress (Liu et al., 2023). Other DUF668 genes that are related to abiotic stress in cotton include Gh_DUF668-05, Gh_DUF668-08, Gh_DUF668-11, Gh_DUF668-23 and Gh_DUF668-28 (Zhao et al., 2021). All OsDUF668 genes respond to drought (Zhong et al., 2019).
The tomato is a widely cultivated vegetable crop that has significance in global agricultural production (Gruber, 2017). But, its growth and yield are greatly impacted by abiotic stressors (Solankey et al., 2004). The potential significance of DUF668 family genes in plant stress resistance has been demonstrated, but research on this gene family has been limited to Arabidopsis thaliana, rice, cotton, and sweet potato. However, the function, classification, and evolution of this gene family in tomatoes have not been thoroughly investigated (Zhong et al., 2019; Zhao et al., 2021; Liu et al., 2023; Zaynab et al., 2023; Han et al., 2024). In this study, DUF668 gene family was systematically identified, and bioinformatic analysis was performed based on the whole tomato genome. We comprehensively analyzed physicochemical properties, chromosomal location, evolutionary relationships, gene structure, protein motifs, cis-acting elements of promoter and expression pattern of nine DUF668 genes in tomato. The results of this study will provide a reference for further research on their possible functions in development and abiotic stress in the future.
Materials and Methods
Plant materials and treatments
Tomato plants (Solanum lycopersicum Mill. cv. Ailsa Craig) were grown in a growth chamber in soil under a 25 °C/16 h in light condition, 20 °C/8 h in dark condition and 60% relative humidity. For drought and salt stress, 4-week-old plants grown in soil were watered with 20% PEG6000 mass/volume fraction and 200 mM NaCl solution and grown at normal room temperature, respectively. For low-temperature treatment, plants were placed in a light incubator at a temperature of 4 °C for 24 h. For phytohormone treatments, solution of 100 µM ABA, 50 µM MeJA and 50 µM SA solutions were sprayed onto tomato plants. Tomato leaves of different treatments were collected randomly after 0, 1.5, 3, 9, 12 and 24 h time courses. Roots, stems, young leaves, shoot apexes, young flower buds, anthesis flowers, green fruits and ripening fruits were collected for the analysis of SlDUF668 expression levels. All samples were rapidly placed in liquid nitrogen and then stored in a refrigerator at −80 °C refrigerator. All samples were treated with three sets of replicates and four technical repetitions, and each replicate consisted of ten seedlings.
Identification of DUF668 genes in tomato genomes
In order to identify the tomato DUF668 genes, the whole-genome was downloaded from the tomato Genome Database (https://solgenomics.net/) (Fernandez-Pozo et al., 2015). The hidden Markov model (HMM) profile of the DUF668 protein (PF05003) was obtained from the Pfam database, and the potential gene family members of DUF668 was obtained via the HMMER search (https://www.ebi.ac.uk/Tools/hmmer/) (Wheeler & Eddy, 2013), with an E-value (≤1e−5). After removing redundant and incomplete sequences, the conserved domain architectures of the acquired sequences were further confirmed by the Simple Modular Architecture Research Tool (SMART) database (http://smart.embl-heidelberg.de) (Letunic, Khedkar & Bork, 2021) and Pfam database (http://pfam.xfam.org/) (Mistry et al., 2021). The number of amino acid residues, molecular weight (MW), and theoretical isoelectric point (pI) of each tomato SlDUF668 protein were predicted by ExPASy software (https://www.expasy.org/) (Mariethoz et al., 2018). The subcellular localizations of tomato SlDUF668s were predicted by the WoLF PSORT program (https://wolfpsort.hgc.jp/) (Horton et al., 2007).
Phylogenetic and collinearity analysis of the SlDUF668 gene family
The full-length amino acid sequences of six DUF668 proteins of Arabidopsis thaliana, twelve DUF668 proteins of rice, thirty-two DUF668 proteins of cotton, fourteen DUF668 proteins of sweet potato and nine DUF668 proteins of tomato were aligned by ClustalW with default settings (Larkin et al., 2007). Subsequently, a phylogenetic tree of DUF668 was constructed with MEGA 11.0 using the neighbor-joining (NJ) methods, and the validated bootstrap value was set to 1,000 using the proximity method (Kumar et al., 2008). The results were edited and beautified with the online tool Evolview (https://evolgenius.info/) (Subramanian et al., 2019). BlastP was performed between the different species, and MCScanX was used to search all collinearity gene pairs and finally visualized through Circos (http://circos.ca/) (Krzywinski et al., 2009; Lavigne et al., 2008; Wang et al., 2012). Non-synonymous (Ka) and synonymous (Ks) substitutions rates of the duplicated gene pair were obtained from PGDD to evaluate the evolutionary selection (Lee et al., 2013).
Chromosome localization, gene structure and protein motif analysis of SlDUF668 genes
Chromosome position information of the SlDUF668 gene family members was obtained from the annotation file of the ITAG 4.0 tomato genome and chromosome localization was visualized using Mapchart software (Voorrips, 2002). The exon/intron arrangement of SlDUF668 genes was analysed and visualized with TBtools (Chen et al., 2020). MEME software v5.0.5 (https://meme-suite.org/meme/tools/meme) was used to identify conserved motifs with the default parameters (Bailey et al., 2009). The conserved motifs were visualized using TBtools (Chen et al., 2020). All genes were renamed based on their positions across chromosomes.
Promoter cis-acting element analysis
Sequences 2,000 bp upstream of start codon of the SlDUF668 genes were downloaded from the tomato genomes and submitted to the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) for promoter cis-acting regulatory element screening. The possible cis-acting elements was visualized with TBtools after the statistical screening (Lescot et al., 2002).
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted using Plant RNA Extraction kit (Tiangen, Beijing, China) and the concentration of the isolated RNA was measured by a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). The cDNA was synthesized by SMART kit (Takara), with 2 µg of RNA from each sample according to the manufacturer’s protocol. The qRT-PCR assay was performed with a CFX96TM real-time fluorescent qPCR system (Bio-Rad, USA) using SYBR Green kit (Tiangen, Beijing, China) under the following conditions: 95 °C 2 min, followed by 35 cycles of 95 °C 15 s, 60 °C 30 s, and 72 °C 15 s. The EF1a gene (AY905538) was used as an internal reference (Aoki et al., 2010). Information about the primers for qRT-PCR were designed by Primer Premier 6.0 software and listed in Table S1. All the qRT-PCR analyses set three biological replicates and three technical repetitions for each treatment. The relative expression of the target gene was computed using the 2−ΔΔCt method (Livak & Schmittgen, 2001) and visualized as heatmaps by TBtools. SPSS 20.0 was used to analyze the relative expressions and Origin 9.0 was used to complete the histogram of relative expression.
Results
Identification and physicochemical properties of the DUF668 gene family members in tomato
A total of nine DUF668 protein sequences were characterized using the bioinformatics approach in tomato. We named the nine DUF668 genes as SlDUF668-01 to SlDUF668-09 according to the order in which genes are distributed on chromosomes in tomato (Table 1). The open reading frame (ORF) length of SlDUF668s ranged from 1,407 to 1,935 bp, with the amino acid length of SlDUF668 proteins ranged from 581 to 644. The protein molecular weights ranged from 53.20 to 71.89 kDa, and the theoretical isoelectric point (pI) ranged from 7.77 to 9.48. The prediction of subcellular localization results showed that most of the genes were distributed in the chloroplast, and a few genes were distributed in the nucleus and cytoplasm.
| Gene name | Gene ID | Chr | Open reading frame/bp | Protein length/aa | Relative Molecular weight (r)/KDa | Theoretical isoelectric point (pI) | Subcellular localization |
|---|---|---|---|---|---|---|---|
| SlDUF668-01 | Solyc03g007090.1 | Chr03 | 1437 | 478 | 54.29 | 9.48 | Chloroplast |
| SlDUF668-02 | Solyc04g025210.4 | Chr04 | 1890 | 629 | 70.90 | 9.06 | Cytoplasm |
| SlDUF668-03 | Solyc04g081510.4 | Chr04 | 1803 | 600 | 67.13 | 9.43 | Cytoplasm |
| SlDUF668-04 | Solyc06g065460.2 | Chr06 | 1935 | 644 | 71.89 | 9.14 | Chloroplast |
| SlDUF668-05 | Solyc09g008930.4 | Chr09 | 1746 | 581 | 65.86 | 9.10 | Chloroplast |
| SlDUF668-06 | Solyc09g089640.2 | Chr09 | 1764 | 587 | 65.91 | 8.38 | Nucleus |
| SlDUF668-07 | Solyc10g084880.3 | Chr10 | 1407 | 468 | 53.20 | 8.99 | Nucleus |
| SlDUF668-08 | Solyc11g007660.1 | Chr11 | 1812 | 603 | 67.29 | 9.17 | Chloroplast |
| SlDUF668-09 | Solyc11g017000.2 | Chr11 | 1935 | 644 | 71.42 | 7.77 | Chloroplast |
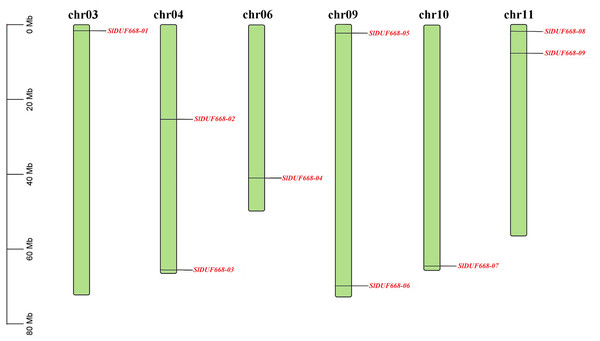
Chromosomal location indicated that nine SlDUF668 genes were distributed on six chromosomes (Chr03, Chr04, Chr06, Chr09, Chr10 and Chr11) of tomato (Fig. 1). Two putative SlDUF668 genes were located on Chr04, Chr09 and Chr11, and the rest of the chromosomes contain only one DUF668 gene. Although Chr09 has the longest length, it contains only one SlDUF668 gene. These results suggest that there is no significant positive correlation between chromosome length and gene number.
Figure 1: Chromosome distribution of the Sl DUF668 genes on tomato genome.
The chromosome number is indicated at the top of each chromosome, and red letters represent Sl DUF668 genes. The left scale indicates the size of each chromosome.Phylogenetic analysis, gene duplication and synteny analysis of DUF668 gene family in tomato
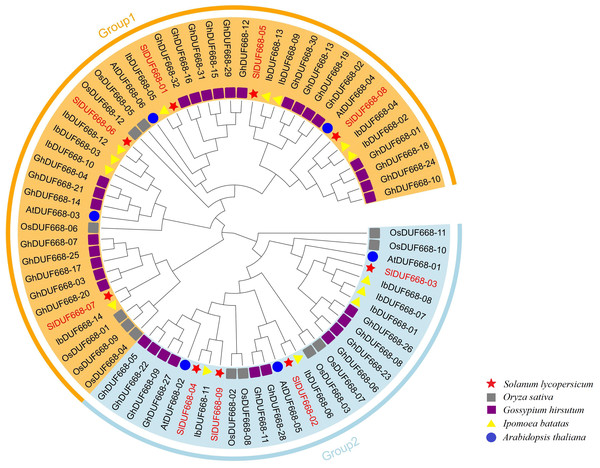
In order to extend our understanding of the relationship of evolution on SlDUF668, we constructed tree of the nine SlDUF668s, six Arabidopsis thaliana DUF668s, twelve rice DUF668s, thirty-two cotton DUF668s and fourteen sweet potato DUF668s by using the protein sequences (Table S2). The tree showed that these DUF668 proteins could be classified into two groups based on their groupings with Arabidopsis thaliana and rice DUF668 proteins (Fig. 2). Group 1 contained five tomato DUF668 proteins, three Arabidopsis DUF668 proteins, six rice DUF668 proteins, twenty-two cotton DUF668 proteins, and nine sweet potato DUF668 proteins. Group 2 contained four tomato DUF668 proteins, three Arabidopsis DUF668 proteins, six rice DUF668 proteins, ten cotton DUF668 proteins and five sweet potato DUF668 proteins.
Figure 2: Phylogenetic analysis of SlDUF668 in tomato, Arabidopsis thaliana, sweet potato, rice, cotton.
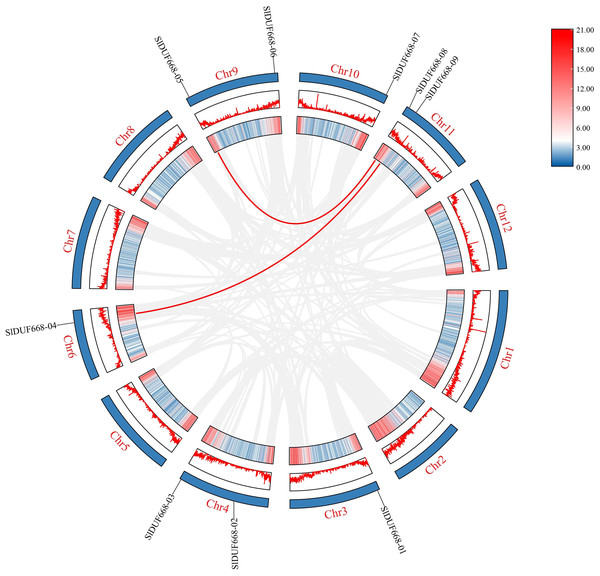
All SlDUF668 members were classified into two groups, and different color blocks represent one group. The stars, circles, triangles, gray squareres and purple squares presented the DUF668 proteins from tomato, Arabidopsis thaliana, sweet potato, rice and cotton, respectively.To better study the evolution of the SlDUF668 genes, we investigated the duplication events of SlDUF668 family genes. The results showed that two segmental duplication events involving nine SlDUF668 genes were identified, but no tandemly duplicated gene pairs were detected (Fig. 3, Table S3). In addition, the non-synonymous (Ka) and synonymous (Ks) substitution values and Ka/Ks ratios were conducted on these two identified duplicated SlDUF668 gene pairs. The results showed that all two gene pairs have Ka/Ks values of less than 1 (Table S3), which suggests that the duplicated SlDUF668 gene pairs underwent purifying selection in the course of evolution.
Figure 3: Interchromosomal relationships of tomato SlDUF668 genes.
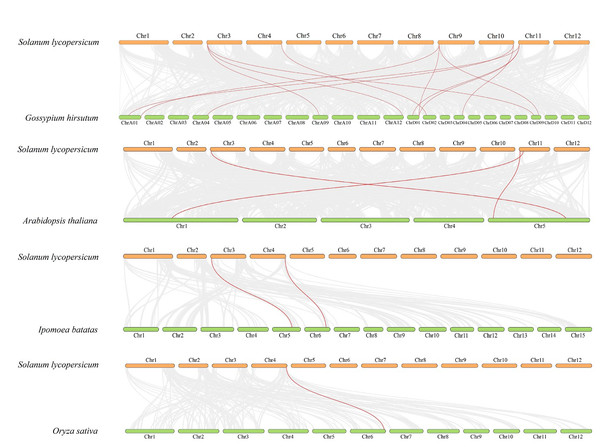
The red line indicates the segmental duplication and the gray lines in the background represent the collinearity between the same genome.To further explore the evolutionary relationship of the SlDUF668 gene family, we analyzed the duplication events among five representative species, including four dicots (tomato, cotton, sweet potato and Arabidopsis) and one monocot (rice) (Fig. 4). We found that 18 SlDUF668 genes were syntenic with the DUF668 genes of Arabidopsis thaliana (three), sweet potato (two), cotton (12) and rice (one) (Table S4). Taken together, the syntenic gene pairs of SlDUF668 were more presented in dicot than in monocot.
Figure 4: Interspecific collinearity relationship between SlDUF668 genes and DUF668s from Arabidopsis thaliana, sweet potato, rice, cotton.
The chromosomes of tomato are represented in orange, while those of Arabidopsis thaliana, sweet potato, rice and cotton are represented in green. The grey lines indicate the collinear blocks respective genomes, while the red lines represent the homologous gene pairs.Phylogenic tree, conserved motifs and gene structure of SlDUF6688 gene
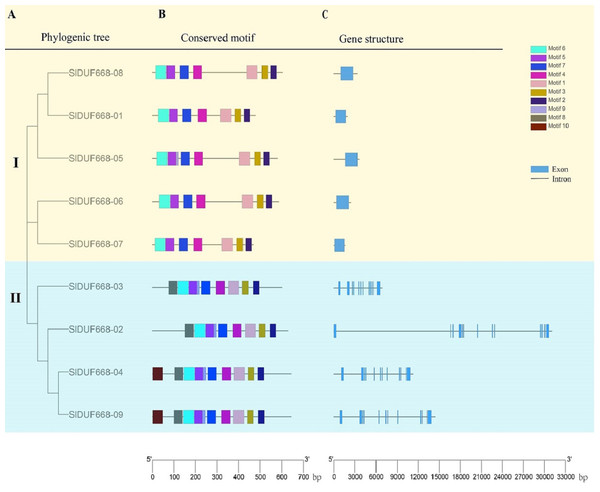
To explore further the phylogenetic relationship of SlDUF668 gene family, we constructed a neighbor-joining (NJ) phylogenetic tree based on the SlDUF668 protein sequences from tomato to fully analyze the conserved motif and gene structure (Fig. 5A). Ten putative conserved motifs were identified by MEME motif analysis, named motif 1–10 (Fig. 5B and Table S5). SlDUF668 protein in Group 2 had more motifs than Group 1. In addition, motif 1, 2, 3, 4, 5 and 7 were present in all the SlDUF668 proteins, suggesting that these motifs may be a conserved structure of the SlDUF668 family. Motif 8 and 10 were only found in Group 2, and motif 6 only existed in Group 1. In the investigation of the composition of the SlDUF668 gene, we found that the Group 1 subgroups all have only one exon, while the Group 2 all contain 12 exons except DUF668-02 (13 exons) (Fig. 5C). In conclusion, the motif constituent and exon-intron structures of the DUF668 genes were very closely related with their phylogenetic relationships.
Figure 5: Phylogenetic tree, gene structures and protein motif analysis of DUF668 gene family intomato.
(A) A phylogenetic tree of tomato DUF668 gene family. The blocks of different colors represented different groups. (B) Conserved domains of tomato DUF668 proteins. The colorful boxes represented different motifs. (C) Gene structures of tomato DUF668 genes. Green boxes represented exons and black lines represented introns. The clustering was performed according to the results of phylogenetic analysis.Cis-acting elements analysis of SlDUF668 genes in tomato
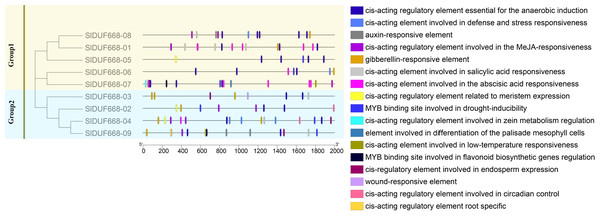
There is a significant positive correlation between the response gene and cis-acting elements in gene promoter regions (Walther, Brunnemann & Selbig, 2007; Abdullah et al., 2018). Therefore, we analyzed the potential cis-acting elements of SlDUF668 genes I promoter regions via the PlantCARE online website. The identified cis-acting elements could be mainly classified into three main categories: plant growth metabolism, plant hormones response and stress response (Fig. 6, Fig. S1). The predicted plant growth metabolism elements mainly included zein metabolism regulation (O2-site), meristem expression (CAT-box), endosperm-specific expression (AACA motif), root-specific expression (the motif I), circadian and palisade mesophyll cell differentiation (HD Zip1) and flavonoid biosynthesis (MBSI) elements. The predicted plant hormones response elements mainly included auxin (IAA), abscisic acid (ABA), gibberellin (GA), methyl jasmonate (MeJA) and salicylic acid (SA) elements. The predicted stress response elements mainly included WUN motif (wound-responsive), LTR (low-temperature response), MBS (drought-inducibility), ARE (anaerobic induction), and TC-rich repeat elements (related to defense and stress response). The presence of these cis-acting elements in SlDUF668 genes indicated their potential integral roles in different biological processes.
Figure 6: The cis-acting elements predication in the promoters of SlDUF668 gene family.
The different colors indicated the different cis-regulatory elements.Expression analysis of tomato SlDUF668 genes in different tissues
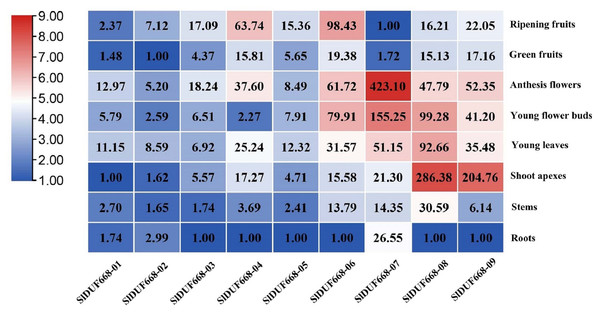
To better understand the function of SlDUF668 genes in tomato, we examined the expression patterns of the ten SlDUF668 genes in different tissues using qRT-PCR, including roots, stems, young leaves, shoot apexes, young flower buds, anthesis flowers, green fruits and ripening fruits. The SlDUF668 gene family members were expressed in different tissues (Fig. 7), indicating they have diverse functions. SlDUF668-04, SlDUF668-06, SlDUF668-07, SlDUF668-08 and SlDUF668-09 were mainly expressed in anthesis flowers, SlDUF668-06, SlDUF668-07, SlDUF668-08 and SlDUF668-09 were mainly preferentially high-expressed in flower buds. SlDUF668-08 and SlDUF668-09 were high-expressed in shoot apexes, while SlDUF668-04 and SlDUF668-06 were highly expressed in ripening fruits, suggesting that SlDUF668-04 and SlDUF668-06 might play important roles in fruit ripening. SlDUF668-08 were high-expressed in all tissues, indicating their diverse biological functions. In addition, all SlDUF668 genes except SlDUF668-07 were relatively low-expressed in roots.
Figure 7: Expression patterns of SlDUF668 genes in eight tissues.
The genes were displayed at the bottom of each column and the tissues were labeled on the right. The heat map of the expression levels of SlDUF668 genes in different tissues was calculated by the 2−ΔΔCT method and generated by TBtools. The color gradient (red/white/blue) indicates the gene expression level (high to low).Expression profiles of SlDUF668 genes under plant hormone
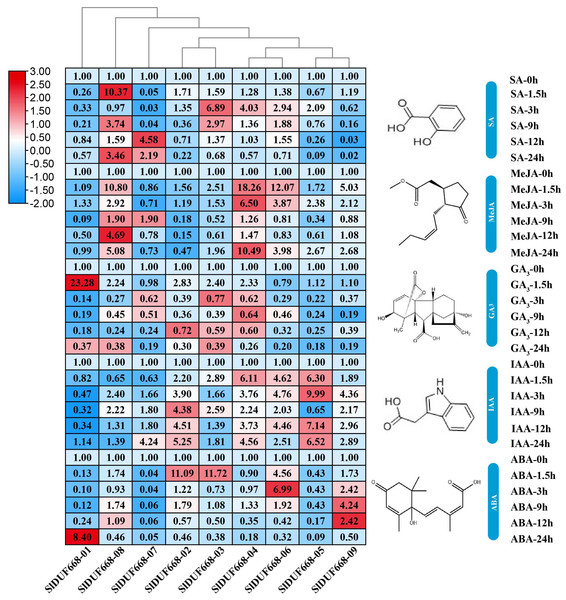
To explore the possible roles of SlDUF668 genes in response to different hormonal treatments, we examined their expression levels under SA, MeJA, GA3, IAA and ABA treatments by qRT-PCR (Fig. 8). During SA treatment, the expression levels of SlDUF668-03, SlDUF668-04 and SlDUF668-08 were up-regulated significantly at the 1.5 h and 3 h time point, respectively, and then showed a downward trend over time. After treatment with MeJA, the expression levels of SlDUF668-04, SlDUF668-06 and SlDUF668-08 was up-regulated considerably at 1.5 h and then showed a downward trend over time. Most of the SlDUF668 genes were either only slightly induced or not affected by GA3 treatment except SlDUF668-01, which was up-regulated at 1.5 h after GA3 treatment compared to control. Expression of SlDUF668-02, SlDUF668-03, SlDUF668-04, SlDUF668-06 and SlDUF668-09 were elevated when applying with IAA. During ABA treatment, SlDUF668-02 and SlDUF668-03 were both obviously increased at 1.5 h compared with 0 h, then decreased at 24 h. In addition, the expression levels of SlDUF668-06 and SlDUF668-09 were significantly up-regulated at 3 h and 9 h, respectively, then decreased at 24 h. The expression levels of SlDUF668-05 and SlDUF668-07 were down-regulated from 1.5 to 24 h after treatment in comparison with 0 h. SlDUF668-01 showed increasing expression only at 24 h after treatment compared to control.
Figure 8: Expression profiles of Sl DUF668s under SA, MeJA, GA, IAA and ABA treatments.
SA, salicylic acid; MeJA, methyl jasmonate; GA, gibberellic acid; IAA, indole-3-acetic acid and ABA, abscisic acid. The relative expressions of SlDUF668 genes were detected by qRT-PCR after treated at five-time points (1.5, 3, 9, 12 and 24 h) were normalized to 0 h treatment. The fold changes values were calculated by the 2−ΔΔCT method and log2 and visualized by heat map. Red and blue colors indicate up-regulation and down-regulated expression levels to the control respectively. Value for each time point represents the mean of three biological replicates.Expression of SlDUF668 genes under abiotic stresses
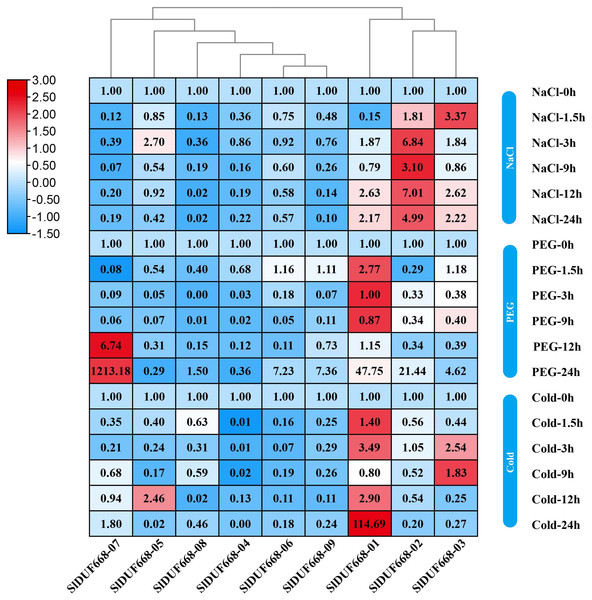
To investigate the potential responsiveness of SlDUF668 genes to abiotic stresses, the gene expression of SlDUF668 genes under different abiotic stresses was analyzed using qRT-PCR (Fig. 9). After 24 h of cold stress treatment, the expression of three genes (SlDUF668-01, -03 and -05,) significantly changed, suggesting that the expression of these three genes may be crucial in response to cold stress. The expression of four genes (SlDUF668-01, -02, -06, -07 and -09) was induced and reached a maximum at 24 h, meanwhile SlDUF668-07 showed strong up-regulation of over five times, indicating that these four genes might play a role in drought resistance in tomato. Under salt stress treatment, the expression of four genes (SlDUF668-01, -02, -03 and -05) significantly changed, suggesting that these three may play an important part in salt. Additionally, some genes showed a trend of gradual increase after stress under environmental stresses, such as SlDUF668-04, -06, -07, -08 and -09 for NaCl, SlDUF668-02, -04, -06, -08 and -09 for cold and SlDUF668-04 and -05 for PEG stress. In summary, these results indicated that the SlDUF668 family members may act as key roles in plant reactions to diverse abiotic challenges in tomato.
Figure 9: Expression changes of SlDUF668s under abiotic stress conditions.
The relative expressions of SlDUF668 genes were detected by qRT-PCR after treated at five-time points (1.5, 3, 9, 12 and 24 h) were normalized to 0 h treatment. The fold changes values were calculated by the 2−ΔΔCT method and log2 and visualized by heat map. Red and blue colors indicate up-regulation and down-regulated expression levels to the control respectively. Value for each time point represents the mean of three biological replicates.Discussion
The DUF668 gene family is significant in plant growth, development, and stress responses (Zhong et al., 2019; Zhao et al., 2021; Liu et al., 2023; Han et al., 2024; Zaynab et al., 2023). Several plant genomes, including Arabidopsis thaliana, rice, cotton, and sweet potato, have undergone genome-wide identification of the DUF668 gene family. The release of the high-quality tomato genome SL4.0 in 2019 (Hosmani et al., 2019) presented an opportunity to study the entire genome of the DUF668 gene family in tomatoes, which has remained unexplored to date. In this study, we discovered nine SlDUF668 genes in the tomato genome, distributed across six chromosomes (Table 1, Fig. 1). Interestingly, the number of SlDUF668 gene family members in tomatoes exceeded that of Arabidopsis but fell short of the numbers observed in rice, cotton, and sweet potato. These findings indicated that the number of DUF668 genes is not directly related to the genome size of a species. Furthermore, the subcellular localization prediction indicated that a majority of the SlDUF668 genes were situated in the chloroplast (Table 1), and similar results were observed in the investigations of SlDUF668 genes in rice (Zhong et al., 2019), implying that these genes may play a role in regulating the structure of the chloroplast. Analysis of the phylogenetic relationship demonstrated that the SlDUF668 proteins could be categorized into two groups (Fig. 2), and no significant tandem duplication phenomenon was observed (Figs. 3 and 4), suggesting that tandem duplications occurblue less frequently during the expansion and evolution of the DUF668 gene family. The SlDUF668 genes in the same group exhibited notable similarities in terms of their gene structure and motif composition (Fig. 5), indicating that the groupings of SlDUF668 genes were relatively reliable. Despite the smaller number of genes in Group 2, the DUF668 gene in this group was longer than that in Group 1 and contained more motifs and exon structures, suggesting that SlDUF668 in Group 2 may possess more intricate functions. These findings were highly congruent with the SlDUF668 genes discovered in other species (Zhong et al., 2019; Zhao et al., 2021; Liu et al., 2023; Han et al., 2024; Zaynab et al., 2023).
There is significant evidence indicating that differences in gene activities are often linked to variations in promoter regions (An, 1986; Lyu et al., 2015). Cis-elements play a crucial role in plant regulatory networks, contributing to a deeper understanding of transcriptional regulation and uncovering the functions of associated genes (Hernandez-Garcia & Finer, 2014). Predictive analysis of the SlDUF668 family gene promoter revealed the existence of multiple elements involved in plant growth metabolism, plant hormones response and stress response (Fig. 6). Among them, plant hormones response and stress response elements were most widely presented, such as ABREs, MBS, LTR, WUN-motif, TGACG, CGTCA, GARE motif, etc. The MBS element that participate in the plant response to drought stress (Zhang et al., 2012), the TGACG motif and CGTCA motif in the MeJA hormone response, the GARE motif in the gibberellin response and ABREs mainly in the ABA hormone response. These hormonal response processes can be indirectly participate in plant responses to abiotic stresses (Rouster et al., 1997; Imtiaz et al., 2015; Yoshida et al., 2010; Huang et al., 2017; Qi et al., 2018). These findings suggest that the SlDUF668 genes can enhance the tomato’s responses to abiotic stress, especially drought, salt and cold stress. This is consistent with the results of the expression analysis of SlDUF668 genes under drought, salt and cold stress in this study.
Gene expression is associated with gene function (Zhu et al., 2021). The expression levels of the SlDUF668 genes were observed in different tissues. The findings showed that the SlDUF668 genes were ubiquitously expressed in different tissues (Fig. 6), indicating that they may play necessary functions in different tissues. SlDUF668-04, -06, -07, -08 and -09 exhibited highly expressed in some specific tissues (Fig. 7), suggesting that the expression of these SlDUF668 genes in tomato is tissue specific. Abiotic stresses such as low temperature, salinity and drought play an important role in plant growth and yield. Many studies have also shown that hormones are required for plants to responses to abiotic and biotic stresses. Therefore, a comprehensive analysis of SlDUF668 genes under abiotic stresses and hormone treatments was performed in tomato. The results showed that some SlDUF668 genes were significantly up-regulated in SA, MeJA, IAA and ABA treatments (Fig. 8), implying that these genes may play a key role in the hormone signaling pathway. Additionally, the SlDUF668 genes were shown to have different expression levels under salt, drought and cold stresses, and the expression patterns of some genes distributed in the same group had similar expression trends (Fig. 9). For instance, SlDUF668-01, -03 and -05 positively responded to cold, indicating that they may enhance tomato resistance to cold. Similarly, SlDUF668-01, -02, -06, -07 and -09 were found to significantly up-regulated in response to drought stress, suggesting that these genes might may enhance tomato resistance to drought. In addition, SlDUF668-01, -02, -03 and -05 positively responded to NaCl, suggesting that these genes might involve in the regulation of salt-induced stress. Overall, these comprehensive results served as a starting point for further research on their roles in tomato growth and development as well as environmental stress responses.
Conclusions
In the current study, a total of nine SlDUF668 genes were identified genome-wide in the tomato whole genome. Gene family analyses were conducted to investigate their physicochemical properties, chromosomal locations, phylogenetic relationships, gene architectures, conserved motifs analysis, cis-acting elements and expression patterns. According to phylogenetic and collinearity analyses, the nine SlDUF668 genes were clustered into two groups. They were unevenly distributed on six chromosomes. Plant growth metabolism elements, plant hormones response elements and stress response elements were identified in the SlDUF668 promoter. Expression analysis showed that SlDUF668 genes had specific expression in different tissues and were widely involved in tolerance of abiotic stress in tomato. Taken together, we provide a valuable references to further understand the function of this gene family in tomato.
Supplemental Information
Cis-acting elements analysis of the SlDUF668 gene family in tomato
Numbers in the box are the number of cis-elements indicated by different intensity colors and numbers. The stacked graph on the right side represents the total number of promoter elements in each category.