Polymorphism in the aggressive mimicry lure of the parasitic freshwater mussel Lampsilis fasciola
- Published
- Accepted
- Received
- Academic Editor
- Donald Kramer
- Subject Areas
- Animal Behavior, Aquaculture, Fisheries and Fish Science, Ecology, Zoology, Freshwater Biology
- Keywords
- Mimicry, Freshwater mussels, ddRAD, Behavior, Parasitism, Polymorphism, Captive brood
- Copyright
- © 2024 Hewitt et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Polymorphism in the aggressive mimicry lure of the parasitic freshwater mussel Lampsilis fasciola. PeerJ 12:e17359 https://doi.org/10.7717/peerj.17359
Abstract
Unionoid freshwater mussels (Bivalvia: Unionidae) are free-living apart from a brief, obligately parasitic, larval stage that infects fish hosts, and gravid female mussels have evolved a spectrum of strategies to infect fish hosts with their larvae. In many North American species, this involves displaying a mantle lure: a pigmented fleshy extension that acts as an aggressive mimic of a host fish prey, thereby eliciting a feeding response that results in host infection. The mantle lure of Lampsilis fasciola is of particular interest because it is apparently polymorphic, with two distinct primary lure phenotypes. One, described as “darter-like”, has “eyespots”, a mottled body coloration, prominent marginal extensions, and a distinct “tail”. The other, described as “worm-like”, lacks those features and has an orange and black coloration. We investigated this phenomenon using genomics, captive rearing, biogeographic, and behavioral analyses. Within-brood lure variation and within-population phylogenomic (ddRAD-seq) analyses of individuals bearing different lures confirmed that this phenomenon is a true polymorphism. The relative abundance of the two morphs appears stable over ecological timeframes: the ratio of the two lure phenotypes in a River Raisin (MI) population in 2017 was consistent with that of museum samples collected at the same site six decades earlier. Within the River Raisin, four main “darter-like” lure motifs visually approximated four co-occurring darter species (Etheostoma blennioides, E. exile, E. microperca, and Percina maculata), and the “worm-like” lure resembled a widespread common leech, Macrobdella decora. Darters and leeches are typical prey of Micropterus dolomieui (smallmouth bass), the primary fish host of L. fasciola. In situ field recordings of the L. fasciola “darter” and “leech” lure display behaviors, and the lure display of co-occurring congener L. cardium, were captured. Despite having putative models in distinct phyla, both L. fasciola lure morphs have largely similar display behaviors that differ significantly from that of sympatric L. cardium individuals. Some minor differences in the behavior between the two L. fasciola morphs were observed, but we found no clear evidence for a behavioral component of the polymorphism given the criteria measured. Discovery of discrete within-brood inheritance of the lure polymorphism implies potential control by a single genetic locus and identifies L. fasciola as a promising study system to identify regulatory genes controlling a key adaptive trait of freshwater mussels.
Introduction
In ecology, mimicry refers to a convergent adaptive trait prevalent in many biological communities: the deceptive resemblance of one organism to another (Pasteur, 1982; Schaefer & Ruxton, 2009; Maran, 2015). It involves three categories of interacting ecological players: mimic (organism displaying the deceptive resemblance), model (organism being mimicked), and receiver (organism being deceived) (Pasteur, 1982; Maran, 2015). Mimicry occurs across a wide variety of ecological contexts and sensory modalities, but conceptually (Jamie, 2017), individual cases can be categorized by the traits being mimicked (signals or cues), as well as by the degree of deceptiveness (aggressive, rewarding, Müllerian or Batesian mimicry). Mimicry is also ubiquitous throughout nature, with many prominent well studied examples including mantids (O’Hanlon, Holwell & Herberstein, 2014), spiders (Ceccarelli, 2013), fish (Randall, 2005), and many more.
Mimetic systems that are polymorphic (multiple within-species mimic morphs with discrete models) have been particularly influential in uncovering the genetic basis of complex adaptive traits in natural populations (Clarke, Sheppard & Thornton, 1968; Jay et al., 2018; Palmer & Kronforst, 2020). Such polymorphisms are rare in nature, with the most well studied examples occurring in papilionid butterflies (Clarke, Sheppard & Thornton, 1968; Clarke & Sheppard, 1971; Hazel, 1990; Joron & Mallet, 1998; Nijhout, 2003). For instance, polymorphisms in Heliconious species are determined by presence/absence of an introgressed chromosomal inversion ‘supergene’ (Jay et al., 2018), and alleles of a single ancestral gene (doublesex) control female-specific polymorphisms in Papilio species (Palmer & Kronforst, 2020).
In contrast to papilionid butterflies, the genetics of mimicry trait evolution among unionoid mussels is poorly understood. Unionoida comprise ~75% of the planet’s freshwater bivalve species and are free-living apart from a brief, obligately parasitic, larval stage that infects fish hosts (Bogan, 2007; Haag, 2012). Gravid female mussels have evolved a spectrum of strategies to infect hosts with their larvae (Zanatta & Murphy, 2006; Barnhart, Haag & Roston, 2008; Hewitt, Wood & Ó Foighil, 2019). Females in many species use a mantle lure (Welsh, 1933): a pigmented fleshy extension that provides a visual cue resembling the prey of host fish, eliciting a feeding response that results in host infection (Haag & Warren, 1999; Barnhart, Haag & Roston, 2008; Fig. 1A). Many species also have a behavioral component; usually in the form of lateral undulations that travel as a wave along the edges of each half (right and left) of the mantle lure (Ortmann, 1921; Barnhart, Haag & Roston, 2008). Although this behavior was observed and described in the early 20th century (Ortmann, 1921), it was not until much later that Haag & Warren (1999) observed how this behavior was used to attract strikes from host fish. The mantle lure presents itself as a reward to potential host fish but is deceptive in nature and leads to parasitization of the host fish. This mimetic system can therefore be classified as an example of aggressive mimicry following the definition by Jamie (2017). The variability in lure display behavior among species of unionid is not well understood. Mimetic mantle lures predominate in Lampsilini, a major clade of North American freshwater mussels recently identified as a cryptic adaptive radiation centered on larval ecologies and specialized host-infection behaviors (Hewitt, Haponski & Ó Foighil, 2021b). This interaction is referred to as ‘cryptic’ because the specific host-parasite interactions are transient and difficult to determine in-situ. Ortmann (1921) and Kramer (1970) reported the production of rudimentary mantle lures in juveniles and male lampsilines, but noted that formation of fully developed lures is restricted to sexually mature females, and that only gravid females engage in lure display behaviors. Surprisingly, neither Ortmann (1921) nor Kramer (1970) depicted male mussel lure rudiments, nor could we find any such depictions in the literature.
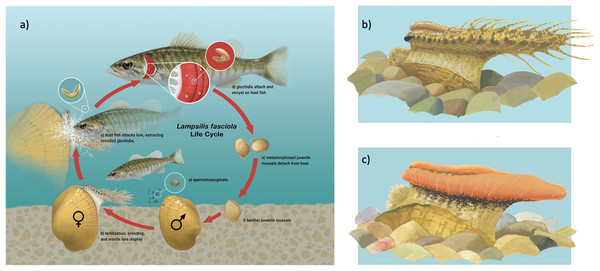
Figure 1: Illustration of Lampsilis fasciola life cycle.
(A) The life cycle of the freshwater mussel Lampsilis fasciola. A gravid female mussel displays a mantle lure, here a darter mimic, to the primary fish host, Micropterus dolomieu. This elicits an attack through which the host is infected by mussel parasitic larvae (glochidia). After a short infective period (~2 weeks), the parasitic larvae metamorphose into juvenile mussels that detach from the host and fall to the substrate. (B) (“darter-like”) and (C) (“worm-like”) depict the two primary phenotypes of lure observed in L. fasciola. The former (B) has “eyespots”, a mottled “main body” pigmentation composed of lateral and dorsal spots that can vary substantially in color, numerous and prominent marginal extensions, and a distinct “tail” region, whereas the latter lacks those features and has instead a uniform bright orange coloration underlain with a black basal stripe. Illustration by John Megahan.Although mimetic mantle lures are a key adaptive trait of freshwater mussel diversification, the genetic regulators underlying their formation (Kramer, 1970), variation (Haag, Warren & Shillingsford, 1999; Zanatta, Fraley & Murphy, 2007; Barnhart, Haag & Roston, 2008), and evolution (Zanatta & Murphy, 2006; Hewitt, Haponski & Ó Foighil, 2021b) remain completely unknown. This gap in our knowledge is exacerbated by the stark conservation status of North American freshwater mussels, with two thirds of species classified as threatened or near-threatened (Lopes-Lima et al., 2018).
As with papilionid butterflies (Jay et al., 2018; Palmer & Kronforst, 2020), targeting polymorphic lampsiline mantle lures for in-depth study may represent a tractable route to closing that gap between genes and phenotypes. Lampsilis fasciola, the Wavy-Rayed Lampmussel, is a promising candidate species in that it produces a number of distinct mantle lure phenotypes (Zanatta, Fraley & Murphy, 2007) across its Eastern North America distribution, extending from southern Ontario to northern Alabama (Parmalee & Bogan, 1998). Two range-wide lure phenotypes predominate in northern populations. The more common of the two, labeled “darter-like” by Zanatta, Fraley & Murphy (2007), has “eyespots”, a mottled “main body” pigmentation composed of lateral and dorsal spots that can vary substantially in color, numerous and prominent marginal extensions (AKA “appendages” or “tentacles”), and a distinct “tail” region (Kramer, 1970; Zanatta, Fraley & Murphy, 2007; Fig. 1B). A rarer lure phenotype, labeled “worm-like” by McNichols (2007), lacks the above features and has instead a uniform bright orange coloration underlain with a black basal stripe (Zanatta, Fraley & Murphy, 2007; Fig. 1C). The latter lure phenotype is highly distinctive within the genus Lampsilis where fish-like mantle lures are the norm (Kramer, 1970). Much work has been done in attempt to quantify similarity between models and mimics, and qualitatively assess most likely models (Kelly et al., 2021), but defining models for lampsiline lure mimics thus far has largely been based on visual similarities defined by expert opinion (Zanatta, Fraley & Murphy, 2007; Barnhart, Haag & Roston, 2008). Based on the results of laboratory larval infection experiments and on the degree of ecological overlap, Micropterus dolomieu (Smallmouth Bass), and to a lesser extent Micropterus salmoides (Largemouth Bass), have been identified as L. fasciola’s primary fish hosts (Zale & Neves, 1982; McNichols, 2007; Morris et al., 2008; McNichols, Mackie & Ackerman, 2011; VanTassel et al., 2021). Both host species are generalist predators of aquatic invertebrates and vertebrates (Clady, 1974).
Our study aimed to address outstanding, interrelated questions to develop L. fasciola into an integrated mantle lure polymorphism study system. First among them was residual uncertainty that the mantle lure morphs represent polymorphisms rather than cryptic species. Zanatta, Fraley & Murphy (2007), using microsatellite markers, did not detect evidence of cryptic species but qualified their conclusions due to small sample sizes, and their result requires corroboration (Fisheries & Oceans Canada, 2018). Secondly, we currently lack any data on the mantle lure phenotype ratios over time (or on a mechanism for its presumed maintenance). Thirdly, we attempt to define respective models of each L. fasciola mantle lure mimic in a natural population. Finally, mantle lure display behavior is an important component of effective mimicry in freshwater mussels (Welsh, 1933; Jansen, Bauer & Zahner-Meike, 2001; Haag & Warren, 2003; Barnhart, Haag & Roston, 2008), but it is unknown if morphologically divergent L. fasciola mantle lures, that presumably mimic very distinct host prey models, also differ in their display behaviors. We tested this by making and analyzing video recordings of lure movements of displaying polymorphic females in a natural population over 3 years. We used a combination of field-collection, captive breeding, museum specimens, and ecological surveys to collect genetic, phenotypic, and population data on this species. This publication was first released as a preprint (Hewitt et al., 2023; doi: https://doi.org/10.1101/2023.11.27.568842), however, the version presented here is the official peer-reviewed publication.
Materials and Methods
Tissue sample collection
L. fasciola mantle tissue samples were collected for genotyping purposes by taking non-lethal mantle clip biopsies (Berg et al., 1995) from wild population lure-displaying female mussels during the summers of 2017, 2018, and 2021 in three rivers (Fig. 2). Maps were made in ArcGIS (ESRI, 2022) using U.S. Geological Survey (2022) as a basemap layer. Two of the sampling locations were in southeastern Michigan: the River Raisin at Sharon Mills County Park (42.176723, −84.092453; N = 30; 24 “darter-like”, six “worm-like”, collectively sampled in 2017, 2018 & 2020), and the Huron River at Hudson Mills Metropark, MI (42.37552, −83.91650; N = 13; 7 “darter-like”, six “worm-like”, collectively sampled in 2017, 2018, and 2020 under the MI Threatened and endangered species collection permit TE149). Both rivers flow into Lake Erie and are part of the Saint Lawrence drainage. The third location was in North Carolina: the Little Tennessee River (N = 10; 35.32324, −83.52275; N = 10, all were “darter-like” and sampled in 2017); this river is a tributary of the Tennessee River and part of the Mississippi drainage. Prior to each biopsy, photographs of the intact, undisturbed, lure display were taken with an Olympus Tough TG-6 underwater camera (Fig. S1).
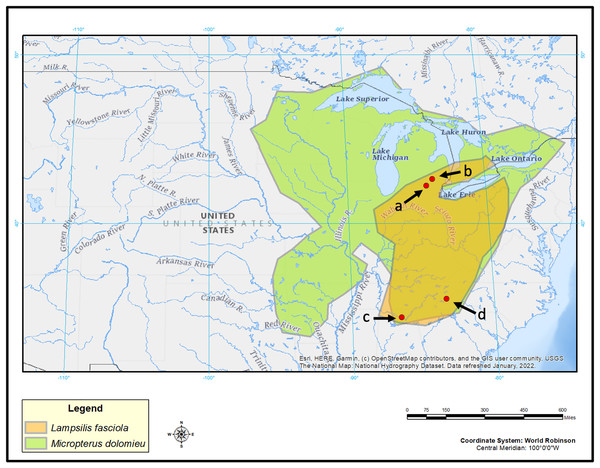
Figure 2: Map displaying geographic range of Lampsilis fasciola and its primary host, Micropterus dolomieu, as well as sampling locations.
Map of eastern North America showing the estimated ranges of Lampsilis fasciola (orange) and of its primary host fish Micropterus dolomieu (green). Red dots indicate sampling sites: Raisin River at Sharon Mils County Park (A), Huron River at Hudson Mills Park (B), Paint Rock River (C) and Little Tennessee River (D). Base map layer is from U.S. Geological Survey (2022).Captive brood tissue samples
We also obtained tissue samples from 50 captive-raised individuals of a single brood that had been ethanol-preserved. In 2009, the Alabama Aquatic Biodiversity Center (AABC) established a culture facility for endangered freshwater mussels. The Center’s inaugural culture attempt, by co-authors Paul Johnson and Michael Buntin, was a proof-of-concept trial involving a single gravid female L. fasciola sourced from the Paint Rock River (another Tennessee River tributary; N 34˚ 47.733′,W 86˚ 14.396′) in Jackson County, AL (Fig. 2) on June 11, 2009. This female L. fasciola had a “worm-like” lure: the AABC data sheet for the trial 2009 host infection (Fig. S2) records that it was “bright orange and black” and lacked the “eyespots”, mottled body coloration, marginal extensions, and “tail” of the “darter-like” lure phenotype (Buntin & Johnson, 2009, personal observations). On July 13 2009, about 31,000 glochidia larvae were extracted from the female’s marsupia and used to infect Micropterus coosae (Redeye Bass) hosts sourced from the Eastaboga Fish Hatchery (Calhoun County, AL, USA) using standard protocols (Barnhart, Haag & Roston, 2008). The female mussel was then returned live to the Paint Rock River. Following completion of larval development on the fish hosts, about 9,300 metamorphosed juvenile mussels were recovered and reared, initially for the first few weeks in mucket bucket systems (Barnhart, 2006), then in a suspended upwelling system (SUPSYS) for 2 years with about 2,200 surviving. In 2011, this proof-of-concept culture experiment was terminated, and the survivors were donated to several research groups, with the majority used for toxicology experiments (Leonard et al., 2014a, 2014b).
Prior to the brood’s termination, Johnson noticed that a few females had attained sexual maturity and were displaying polymorphic lures (Figs. 3B, 3C). To substantiate that 2011 observation, we examined 50 individuals that had been preserved in 95% ethanol and shipped to Nathan Johnson (USGS) in Gainsville, FL in 2011. Because Lampsilis spp. juveniles and males produce a rudimentary mantle lure (Ortmann, 1921; Kramer, 1970), we were able to determine the primary lure phenotype (darter-like” or “worm-like”) of all 50 preserved brood members. Using a Leica MZ16 dissecting microscope, individual photomicrographs were taken of the preserved rudimentary lure structures (Figs. 3D, 3E and S3), and their respective lure phenotypes were identified independently by both T. Hewitt and by D. Ó Foighil. Additionally, tissue samples were acquired from all 50 individuals and included for phylogenomic analyses.
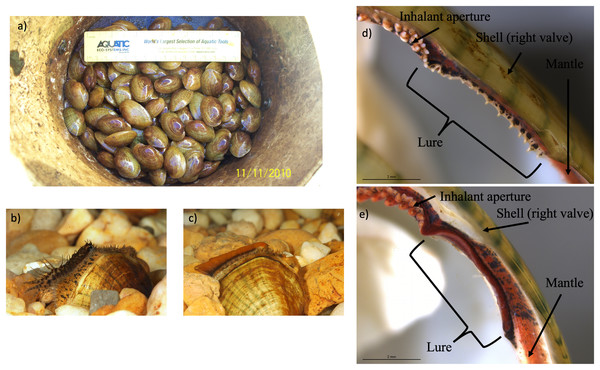
Figure 3: Photographs of Lampsilis fasciola brood raised at the Alabama Aquatic Biodiversity Center, as well as photographs of preserved lure rudiments.
The Lampsilis fasciola brood raised at the Alabama Aquatic Biodiversity Center from a wild, gravid female, with a “worm-like” mantle lure, sampled from the Paint Rock River in June 2009. (A) shows juvenile members of the brood after ~16 months in culture. (B and C) show single, sexually maturing females after ~2 years of culture. The young female in (B) displayed a developing “darter-like” mantle lure (with “eyespots”, mottled lateral coloration, marginal extensions, and a “tail”) whereas her full- or half-sibling in (C) displayed a “worm-like” mantle lure (lacking the “darter” characteristics and having orange pigmentation with a black underlay). (D and E) Respectively show photomicrographs, taken with a dissecting microscope, of 95% ethanol-preserved rudimentary “darter-like” and “worm-like” lures from two additional brood members, part of a 50-individual subsample preserved in 2011.Phylogenomic analyses
DNA sequencing and raw data processing were performed using the protocol outlined in Hewitt, Haponski & Ó Foighil (2021a, 2021b). Genomic DNA was extracted from tissue samples using E.Z.N.A. Mollusk DNA kit (Omega Bio-Tek, Norcross, GA, USA) according to manufacturer’s instructions and then stored at −80 °C. The quality and quantity of DNA extractions were assessed using a Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA) and ddRADseq libraries were prepared following the protocols of Peterson et al. (2012). We then used 200 ng of DNA for each library prep. This involved digestion with Eco-RI-HF and MseI (New England Biolabs, Ipswich, MA, USA) restriction enzymes, followed by isolating 294–394 bp fragments using a Pippen Prep (Sage Science, Beverly, MA, USA) following the manufacturer’s instructions. Prepared ddRADseq libraries then were submitted to the University of Michigan’s DNA sequencing core and run in four different lanes using 150 bp paired-end sequencing on either an Illumina HiSeq 2500 or Illumina novaseq shared flow cell. Two control individuals of L. fasciola were run in each lane and reads for both individuals clustered together in every analysis with 100% bootstrap support, indicating no lane effects on clustering among individuals. Raw demultiplexed data were deposited at GenBank under the bioproject ID PRJNA985631 with accession numbers SAMN35800743–SAMN35800847. Individuals included in phylogenomic analyses can be found in Table 1, and museum ID numbers can be found in Table S1.
| Sample name | Lure phenotype | Raw reads | Total clusters | Average clustering depth | Loci in assembly |
|---|---|---|---|---|---|
| L_fasciola_AL_brood_1 | Worm-like | 258,664 | 97,681 | 2.14 | 483 |
| L_fasciola_AL_brood_2 | Darter-like | 5,201,836 | 1,120,710 | 3.28 | 25,686 |
| L_fasciola_AL_brood_3 | Worm-like | 5,492,519 | 1,126,749 | 3.4 | 25,703 |
| L_fasciola_AL_brood_4 | Darter-like | 2,429,494 | 632,254 | 2.84 | 21,398 |
| L_fasciola_AL_brood_5 | Worm-like | 3,152,003 | 760,260 | 3.02 | 23,761 |
| L_fasciola_AL_brood_6 | Darter-like | 3,212,851 | 810,898 | 2.87 | 23,434 |
| L_fasciola_AL_brood_7 | Darter-like | 3,649,891 | 593,765 | 4.22 | 25363 |
| L_fasciola_AL_brood_8 | Darter-like | 4,869,307 | 1,462,723 | 2.29 | 19,089 |
| L_fasciola_AL_brood_9 | Worm-like | 3,158,818 | 718,169 | 3.08 | 23,033 |
| L_fasciola_AL_brood_10 | Darter-like | 4,000,321 | 915,881 | 3.12 | 24,916 |
| L_fasciola_AL_brood_11 | Worm-like | 5,679,854 | 1,171,842 | 3.35 | 25,770 |
| L_fasciola_AL_brood_12 | Darter-like | 4,212,783 | 979,265 | 3.04 | 24,693 |
| L_fasciola_AL_brood_13 | Worm-like | 1,300,563 | 399,134 | 2.51 | 12,145 |
| L_fasciola_AL_brood_14 | Darter-like | 4,100,372 | 1,043,360 | 2.79 | 23,521 |
| L_fasciola_AL_brood_15 | Darter-like | 5,804,293 | 1,412,102 | 2.91 | 25,570 |
| L_fasciola_AL_brood_16 | Worm-like | 1,555,906 | 427,061 | 2.7 | 14,099 |
| L_fasciola_AL_brood_17 | Darter-like | 2,073,968 | 598,680 | 2.59 | 13,668 |
| L_fasciola_AL_brood_18 | Worm-like | 6,919,783 | 1,574,429 | 3.08 | 25,811 |
| L_fasciola_AL_brood_19 | Darter-like | 3,434,210 | 829,507 | 2.94 | 23,708 |
| L_fasciola_AL_brood_20 | Darter-like | 4,778,853 | 994,416 | 3.35 | 25,500 |
| L_fasciola_AL_brood_21 | Worm-like | 2,462,560 | 590,095 | 2.91 | 20,588 |
| L_fasciola_AL_brood_22 | Worm-like | 6,600,876 | 1,406,451 | 3.26 | 26,080 |
| L_fasciola_AL_brood_23 | Darter-like | 7,090,859 | 1,628,965 | 3.06 | 25,932 |
| L_fasciola_AL_brood_24 | Worm-like | 4,546,435 | 1,061,394 | 3 | 24,174 |
| L_fasciola_AL_brood_25 | Worm-like | 5,379,577 | 1,135,906 | 3.35 | 25,703 |
| L_fasciola_AL_brood_26 | Worm-like | 5,592,652 | 1,501,130 | 2.67 | 23,965 |
| L_fasciola_AL_brood_27 | Worm-like | 4,893,957 | 825,855 | 4.09 | 25,924 |
| L_fasciola_AL_brood_28 | Darter-like | 2,596,873 | 519,103 | 3.59 | 22,103 |
| L_fasciola_AL_brood_29 | Darter-like | 3,401,334 | 883,485 | 2.87 | 21,377 |
| L_fasciola_AL_brood_30 | Worm-like | 3,876,395 | 1,014,133 | 2.8 | 22,072 |
| L_fasciola_AL_brood_31 | Worm-like | 5,391,442 | 1,246,528 | 3.07 | 25,009 |
| L_fasciola_AL_brood_32 | Darter-like | 4,365,005 | 1,084,596 | 2.85 | 23,030 |
| L_fasciola_AL_brood_33 | Darter-like | 5,116,507 | 1,117,916 | 3.16 | 24,667 |
| L_fasciola_AL_brood_34 | Darter-like | 7,480,755 | 1,601,100 | 3.19 | 26,163 |
| L_fasciola_AL_brood_35 | Darter-like | 8,121,426 | 1,825,135 | 3.02 | 25,972 |
| L_fasciola_AL_brood_36 | Darter-like | 5,521,997 | 1,414,238 | 2.78 | 24,163 |
| L_fasciola_AL_brood_37 | Darter-like | 6,562,641 | 1,579,514 | 2.88 | 25,476 |
| L_fasciola_AL_brood_38 | Darter-like | 6,303,766 | 1,596,624 | 2.76 | 24,448 |
| L_fasciola_AL_brood_39 | Darter-like | 6,206,795 | 1,488,925 | 2.91 | 24,648 |
| L_fasciola_AL_brood_40 | Darter-like | 8,630,897 | 1,891,164 | 3.11 | 26,176 |
| L_fasciola_AL_brood_41 | Darter-like | 7,293,683 | 1,716,571 | 2.95 | 25,604 |
| L_fasciola_AL_brood_42 | Darter-like | 4,896,252 | 1,193,262 | 2.88 | 22,829 |
| L_fasciola_AL_brood_43 | Darter-like | 6,098,052 | 1,471,714 | 2.9 | 25,074 |
| L_fasciola_AL_brood_44 | Darter-like | 7,495,994 | 1,698,871 | 3.04 | 25,701 |
| L_fasciola_AL_brood_45 | Darter-like | 3,937,758 | 670,698 | 4.06 | 24,947 |
| L_fasciola_AL_brood_46 | Darter-like | 6,370,942 | 1,343,655 | 3.26 | 25,855 |
| L_fasciola_AL_brood_47 | Darter-like | 5,542,864 | 1,318,463 | 2.96 | 24,550 |
| L_fasciola_AL_brood_48 | Darter-like | 6,313,913 | 1,469,606 | 2.98 | 24,983 |
| L_fasciola_AL_brood_49 | Darter-like | 3,163,000 | 789,239 | 2.9 | 24,776 |
| L_fasciola_AL_brood_50 | Darter-like | 1,728,370 | 548,529 | 2.35 | 17,837 |
| L_fasciola_Huron_5 | Darter-like | 953,302 | 259,898 | 2.8 | 10,996 |
| L_fasciola_Huron_6 | Worm-like | 1,682,931 | 362,706 | 3.31 | 16,809 |
| L_fasciola_Huron_7 | Worm-like | 746,944 | 157,212 | 3.29 | 10,644 |
| L_fasciola_Huron_8 | Worm-like | 1,899,689 | 402,515 | 3.25 | 16,584 |
| L_fasciola_Huron_9 | Darter-like | 1,213,655 | 293,090 | 2.97 | 11,818 |
| L_fasciola_Huron_10 | Darter-like | 7,775,910 | 1,275,602 | 3.87 | 22,035 |
| L_fasciola_Huron_11 | Darter-like | 1,533,281 | 295,767 | 3.55 | 15,386 |
| L_fasciola_NC_1 | Darter-like | 1,308,813 | 254,002 | 3.61 | 11,873 |
| L_fasciola_NC_2 | Darter-like | 4,862,573 | 852,380 | 3.77 | 18,321 |
| L_fasciola_NC_3 | Darter-like | 663,874 | 165,869 | 2.95 | 9,960 |
| L_fasciola_NC_4 | Darter-like | 2,610,453 | 465,228 | 3.76 | 13,790 |
| L_fasciola_NC_5 | Darter-like | 6,927,947 | 1,459,334 | 3.05 | 20,804 |
| L_fasciola_NC_6 | Darter-like | 1,051,195 | 202,171 | 3.27 | 12,415 |
| L_fasciola_NC_7 | Darter-like | 1,948,092 | 382,878 | 3.61 | 17,101 |
| L_fasciola_NC_8 | Darter-like | 3,475,751 | 669,278 | 3.69 | 20,683 |
| L_fasciola_NC_9 | Darter-like | 5,693,936 | 1,634,946 | 2.46 | 22,325 |
| L_fasciola_NC_10 | Darter-like | 2,175,381 | 464,794 | 3.38 | 17,094 |
| L_fasciola_NC_11 | Darter-like | 2,189,933 | 516,643 | 3.05 | 17,580 |
| L_fasciola_Redo_1 | Darter-like | 1,455,864 | 327,622 | 2.62 | 13,478 |
| L_fasciola_Redo_2 | Darter-like | 1,839,020 | 436,418 | 2.43 | 13,181 |
| L_fasciola_Raisin_2 | Darter-like | 8,235,827 | 1,716,137 | 3.29 | 25,555 |
| L_fasciola_Raisin_3 | Darter-like | 6,032,935 | 1,488,448 | 2.85 | 25,006 |
| L_fasciola_Raisin_4 | Darter-like | 12,947,164 | 3,587,458 | 2.45 | 25,245 |
| L_fasciola_Raisin_1 | Darter-like | 6,639,384 | 1,086,218 | 3.97 | 23,458 |
| L_fasciola_Raisin_5 | Darter-like | 10,059,843 | 1,997,619 | 3.41 | 25,363 |
| L_fasciola_Raisin_6 | Darter-like | 8,019,689 | 1,847,955 | 3.01 | 25,769 |
| L_fasciola_Raisin_7 | Darter-like | 3,816,242 | 681,697 | 3.95 | 24,606 |
| L_fasciola_Raisin_8 | Darter-like | 6,117,037 | 1,282,299 | 3.27 | 22,439 |
| L_fasciola_Raisin_9 | Worm-like | 5,170,380 | 775,979 | 4.64 | 25,798 |
| L_fasciola_Raisin_10 | Darter-like | 761,451 | 176,858 | 3.14 | 11,477 |
| L_fasciola_Raisin_11 | Worm-like | 7,140,657 | 1,670,143 | 2.97 | 25,519 |
| L_fasciola_Raisin_12 | Darter-like | 890,521 | 203,114 | 2.91 | 10,582 |
| L_fasciola_Raisin_13 | Darter-like | 1,071,361 | 225,030 | 3.47 | 13,512 |
| L_fasciola_Raisin_14 | Darter-like | 3,644,379 | 946,273 | 2.82 | 21,995 |
| L_fasciola_Raisin_15 | Darter-like | 3,578,043 | 482,446 | 5.04 | 17,514 |
| L_fasciola_Raisin_16 | Darter-like | 2,351,544 | 114,072 | 14.25 | 516 |
| L_fasciola_Raisin_17 | Darter-like | 5,272,816 | 1,304,726 | 2.87 | 23,305 |
| L_fasciola_Huron_1 | Worm-like | 13,366,692 | 4,050,829 | 2.26 | 17,555 |
| L_fasciola_Huron_2 | Darter-like | 2,819,896 | 928,226 | 2.24 | 20,205 |
| L_fasciola_Huron_3 | Darter-like | 662,275 | 186,602 | 2.66 | 7,653 |
| L_fasciola_Huron_4 | Darter-like | 4,792,093 | 855,457 | 3.88 | 24,512 |
| L_fasciola_AL_mom_1 | Darter-like | 8,095,030 | 1,840,917 | 2.95 | 25,420 |
| L_fasciola_AL_mom_2 | Darter-like | 10,329,331 | 3,504,027 | 2.03 | 24,488 |
| L_fasciola_AL_mom_3 | Darter-like | 10,384,477 | 2,987,559 | 2.34 | 25,056 |
| L_fasciola_Huron_12 | Worm-like | 6,906,349 | 1,672,394 | 2.87 | 25,281 |
| L_fasciola_Huron_13 | Worm-like | 6,955,496 | 1,670,627 | 2.88 | 25,593 |
| L_fasciola_Raisin_18 | Worm-like | 5,506,215 | 1,301,878 | 3 | 25,373 |
| L_fasciola_Raisin_19 | Worm-like | 6,611,596 | 1,524,682 | 3.03 | 25,604 |
| L_fasciola_Raisin_20 | Worm-like | 4,894,495 | 1,276,608 | 2.74 | 24,931 |
| L_fasciola_Raisin_21 | Worm-like | 8,396,562 | 1,736,736 | 3.26 | 25,490 |
| L_cardium_1 | 6,864,226 | 1,710,220 | 2.8 | 14,625 | |
| L_cardium_2 | 4,898,330 | 1,091,622 | 3.11 | 13,433 | |
| L_cardium_3 | 7,109,883 | 2,005,565 | 2.5 | 14,563 | |
| L_cardium_4 | 4,637,077 | 997,208 | 3.27 | 13,860 | |
| S_nasuta_1 | 4,544,989 | 1,169,260 | 2.55 | 10,441 |
The alignment-clustering algorithm in ipyrad v.0.7.17 (Eaton, 2014; Eaton & Overcast, 2020) was used to identify homologous ddRADseq tags. Ipyrad is capable of detecting insertions and deletions among homologous loci, which increases the number of loci recovered at deeper evolutionary scales compared to alternative methods of genomic clustering (Eaton, 2014). Demultiplexing was performed by sorting sequences by barcode, allowing for zero barcode mismatches (parameter 15 setting 0) and a maximum of five low-quality bases (parameter 9). Restriction sites, barcodes, and Illumina adapters were trimmed from the raw sequence reads (parameter 16 setting 2), and bases with low-quality scores (Phred-score < 20, parameter 10 setting 33) were replaced with an N designation. Sequences were discarded if they contained more than 5 N’s (parameter 19). Reads were clustered and aligned within each sample at an 85% similarity threshold, and clusters with a depth <6 were discarded (parameters 11 and 12). We also varied the number of individuals required to share a locus from ~50% to ~75%.
We analyzed the two concatenated ddRAD-seq alignment files (50% and 75% minimum samples per locus) using maximum likelihood in RAxML v8.2.8 (Stamatakis, 2014). A general time-reversible model (Lanave et al., 1984) was used for these analyses that included invariable sites and assumed a gamma distribution. Support was determined for each node using 100 fast parametric bootstrap replications. Lure phenotype information was recorded and mapped on to the phylogenetic tree. Phylogenetic signal of lure phenotype was tested using Pagel’s (1999) λ in R (R Core Team, 2018) with the ‘phylobase’ package (Hackathon et al., 2013).
River raisin mantle lure phenotype ratios over time
Mid-20th century L. fasciola specimens collected at the Sharon Mills County Park site (Raisin River, MI, USA; Fig. 2A) are preserved as part of the University of Michigan’s Museum of Zoology wet mollusk collection. They stem from eight different collecting events between 1954 and 1962 (Table S2), and their presence afforded an opportunity to assess the stability of the L. fasciola “darter/worm” mantle lure polymorphism in that population over a six-decade time interval. All of the museum specimens, males as well as females, were examined to determine whether their fully-formed (female) or rudimentary (male) mantle lures were “darter-like” or “worm-like”. For females, this could be achieved by simple visual examination, but male lure classification required a dissecting microscope. The percentages of mantle lure phenotypes observed in the Sharon Mills County Park population was compared among mid-20th century (UMMZ preserved females and males) and 2017 (field photographs and videos of displaying females) samples using a Fisher’s exact test, implemented in R.
Putative lure mimicry models
Population-specific putative model species for the L. fasciola mantle lure mimicry system were investigated at the River Raisin Sharon Mills County Park study site (Fig. 2), in part because of the availability of a comprehensive ecological survey of Raisin River fishes (Smith, Taylor & Grimshaw, 1981). “Darters”—members of the speciose North American subfamily Etheosomatinae— have been implictly identified as models for the predominant “darter-like” mantle lure phenotype (Zanatta, Fraley & Murphy, 2007), and they are preyed upon by Micropterus dolomieu (Surber, 1941; Robertson & Winemiller, 2001; Murphy et al., 2005), L. fasciola’s primary fish host (Zale & Neves, 1982; McNichols, 2007; Morris et al., 2008; McNichols, Mackie & Ackerman, 2011; VanTassel et al., 2021). Ten species of Etheosomatinae occur in the River Raisin, as does M. dolomieu (Smith, Taylor & Grimshaw, 1981).
River Raisin gravid female L. fasciola engage in mantle lure displays from May-August. During the summer of 2017, a total of 27 different displaying females were photographed along a 150-m stretch downstream of the dam at Sharon Mills County Park using an Olympus Tough TG-6 underwater camera. Individuals were located by carefully scanning the river bed with mask and snorkel to try and approximate the real ratios of phenotypes at this site. Additional lure photos were taken by coauthor Paul Johnson at the AABC of individuals from the Paint Rock River (AL). The lures were first categorized into broad groupings based on visual similarity, in terms of morphology and coloration. These groupings were then used to identify putative host prey fish model species from those present in the River Raisin drainage (Smith, Taylor & Grimshaw, 1981), based on similarities in size, shape, and coloration. Putative model species were further assessed based on their relative local abundance (Smith, Taylor & Grimshaw, 1981) and on their range overlap with both mimic and receiver. We also photograph and document the male rudimentary lures for both L. fasciola and L. cardium, taken from the River Raisin (Fig. S4). Geographic ranges of L. fasciola, the primary host M. dolomieu, and each prospective model species were produced by hand in Arcgis software (ESRI, 2022), and the overlap between L. fasciola, M. dolomieu, and each putative model species was assessed using Arcgis software.
Behavioral analyses
Standardized video recordings of 27 mantle lure-displaying female L. fasciola (15 “darter-like” and 12 “leech-like”) were recorded using a Go Pro Hero 6 camera in the summer of 2018 at the two different southeastern Michigan study sites: Sharon Mills County Park (River Raisin) and Hudson Mills Metropark (Huron River). All “darter-like” individuals were grouped together. An additional four video recordings of the lure behavior of sympatric Lampsilis cardium, a well studied congener lacking pronounced mantle lure polymorphisms (Kramer, 1970; Haag & Warren, 1999) were collected from the Sharon Mills site to assess interspecific variability in lure behavior. Recordings were captured from a top-down perspective during daylight hours using a standardized frame that included a metric ruler and a Casio TX watch to record date, time, and water temperature data within the video frame. For each displaying female, videos of the lure movements were recorded for 10 min at 120 frames-per-second. Setting up the camera occasionally disturbed the mussels, and video recordings began after waiting some time (usually 2–15 min) until the behavior qualitatively returned to its prior state. Analysis of the videos involved manually recording mantle lure movements for 20,000 frames (2.8 min), starting at 5,000 frames (42 s) to to avoid any camera shaking or hands accidentally blocking the view. The frame numbers when an individual movement began, defined as the first frame where contraction of mantle tissue was observed, and ended, defined as the time that mantle lure returns to it resting state, were noted. Movements of the left and the right mantle lure flaps were recorded seperately.
To quantitatively assess behavioral differences among samples, gait analysis diagrams were created in R for each displaying mussel. Because the lure is mimicking the swimming locomotion of fish, and fish locomotion has been characterized using gait analysis (Liao et al., 2003), we used gait analysis methods to characterize the non-locomotory motions that generate the luring behavior. Averages and standard deviations for the time intervals between lure undulations (the time between the start of one movement and the start of the next) were calculated for each side of each individual, as well as duration undulation (the time between the start of one movement and the end of that movement) and proportion of movements synchronized. Movements were defined as synchronized if the start of a movement on one side was within four frames of the start of a movement on the corresponding side. Proportion of movements synchronized were calculated by dividing the number of synchronized movements by the sum of left movements only, right movements only, and synchronized movements. A Kruskal-Wallis test was used to test for overall differences among lure groups (L. fasciola “darter-like”, L. fasciola “worm-like”, and L. cardium), and pairwise Wilcoxon Signed rank tests were used to compare groups directly with a Bonferroni p value adjustment to correct for multiple tests. A Spearman correlation was used to test for an effect of water temperature on time interval between lure undulations.
To further explore differences in lure behavior among groups, we used a general linear mixed model (GLMM), with sample ID as a random factor, to test for differences in lure movement intervals. The GLMM approach, unlike simple mean comparisons, allows the inclusion of all lure movements for all individuals in the model. Because displaying mussels all varied in the number of lure movements recorded over 20,000 frames analyzed, a dataset of 1,000 random bootstrap values was constructed for each individual by randomly sampling values, with replacement. Models were fitted using the ‘lmerTest’ package in R, and Satterthwaite’s (1946) Method (Kuznetsova, Brockhoff & Christensen, 2017) was used to test for significance of fixed effects of lure phenotype on the interval between lure undulations.
Results
Captive brood
Two independent classifiers concurred that the 50 preserved specimens from the same maternal brood included 33 “darter-like” (66%) and 17 “worm-like” (34%) individuals (Figs. 3D, 3E and S4).
ddRAD-seq and phylogenomic analyses
Genomic sequencing returned raw reads ranging from 258,664 to 13,366,692 per individual across the 108 unionid specimens included in the analyses comprising samples of the ingroup L. fasciola, sourced from four different populations, along with outgroups L. cardium and Sagittunio nasuta. Mean coverage depth for the 85% clustering threshold ranged from 2.03 (L_fasciola_AL_mom_2) to 14.25 (L_fasciola_Raisin_16; Table 1). Between 28,725 and 16,161 homologous loci were identified across the two best ddrad datasets (85–50% and 85–75% respectively) and the number of loci recovered was generally consistent among all samples.
The maximum likelihood tree produced by RAxML (Fig. S4) recovered the following ingroup/outgroup topology: (S. nasuta (L. cardium, L. fasciola)) with outgroup branch lengths greatly exceeding those of the ingroup. To optimize the legibility of ingroup relationships, a compressed, color-coded graphic excluding S. nasuta was constructed (Fig. 4). A nested series of phylogenetic relationships was recovered for the four L. fasciola fluvial populations with the two Michigan drainages being paraphyletic: (Little Tennessee River (Paint Rock River (River Raisin (River Raisin, Huron River))). The ingroup topology also showed evidence of within-population genealogical relationships with all Paint Rock River brood members forming an exclusive clade (Fig. 4).
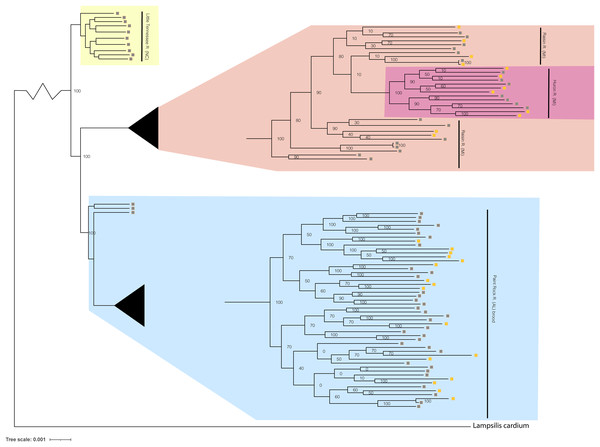
Figure 4: Phylogenomic tree displaying Lampsilis fasciola from two MI populations, one NC population, and the Paint Rock River brood raised at the Alabama Aquatic Biodiversity Center.
Phylogenomic tree of 96 Lampsilis fasciola individuals created in RAxML using 28,735 concatenated ddRAD-seq loci. Gravid, lure-displaying females sampled from two Michigan drainages, River Raisin and Huron River, are respectively highlighted in peach and pink. Specimens sampled from the Paint Rock River, Alabama are highlighted in blue and consisted of three gravid, lure-displaying females, in addition to 50 larval brood members raised at the Alabama Aquatic Biodiversity Center in the zoomed-in tip clade. Gravid, lure-displaying females sampled from the Little Tennessee River in North Carolina are highlighted in yellow. Boxes indicate primary mantle lure phenotypes—“darter-like” (gray) or “worm-like” (orange)—of all L. fasciola individuals.The respective primary mantle lure phenotypes—“darter-like” or “worm-like”—of all 92 L. fasciola ingroup individuals are indicated in Fig. 4. Note that three of the four population samples—Little Tennessee River, River Raisin and Huron River—were exclusively composed of mantle-lure displaying wild females, and the latter two samples were polymorphic in mantle lure composition. Regarding the Paint Rock River sample, polymorphic lures were restricted to the 50 captive-raised AABC brood members sourced from a gravid, wild female in 2009 (not included in the analyses). The ingroup phylogeny (Fig. 4) contained two polymorphic mantle lure clades, one composed of both Michigan populations (River Raisin and Huron River), the other consisting only of the AABC brood, and both clades had individuals of either lure phenotype interspersed across their respective topologies. Little phylogenetic signal associated with either primary mantle lure phenotype (λ = 0.21; P = 0.13).
Phenotypic ratios over time
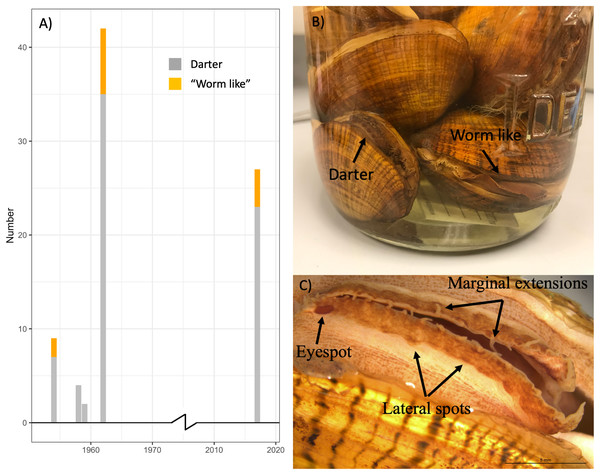
Table S2 summarizes the sex and primary lure phenotypes of 57 L. fasciola specimens collected from 1954–1962 at the River Raisin Sharon Mills County Park study site (Fig. 2A) and preserved in the University of Michigan Museum of Zoology’s wet mollusk collection (Figs. 5B and 5C). These historical samples had a collective “darter-like” to “worm-like” ratio of 48:9, with 84.2% of individuals having the more common “darter-like” mantle lure phenotype and 15.8% having the “leech-like” phenotype. Figure 5A contrasts the mid-20th century lure phenotype ratios with a contemporary (2017) estimate in that same population, based on photographic recordings of 27 displaying females. The contemporary ratio was 23:4, with 85.2% of individuals having the more common “darter-like” mantle lure phenotype and 14.8% having the “leech-like” phenotype. The contemporary ratio was not significantly different from the historical ratio (Fisher Exact Test, Χ2 = 0.01, P = 0.91).
Figure 5: The ratio of “worm-like” and “darter-like” Lampsilis fasciola lures over time in the River Raisin, MI, using historical and contemporary samples.
The observed frequency of River Raisin Lampsilis fasciola primary mantle lure phenotypes (“darter-like”; gray vs. “worm-like”; orange) at the Sharon Mills County Park study site during two different time periods. The 1954–1962 data were obtained from the University of Michigan Museum of Zoology (UMMZ) collection specimens, both female and male. The 2017 data were based on field observations of displaying females. (B) A jar of preserved UMMZ Sharon Mills specimens showing a “darter-like” and a “worm-like” mantle lure. (C) A “eyespot”, lateral pigmented blotches, and marginal extensions in a “darter-like” lure of a preserved specimen.Putative raisin river lure mimicry models
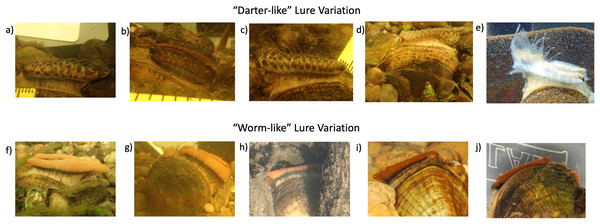
The field photographs of 27 displaying female L. fasciola mantle lures in the Raisin River Sharon Mills County Park population in 2017 (Fig. S1) were categorized into either “darter-like” (Zanatta, Fraley & Murphy, 2007) or “worm-like” (McNichols, 2007), as summarized in the Materials & Methods section. In addition to the specific features that separate these two primary mantle lure phenotypes (presence/absence of “eyespots” mottled pigmentation, marginal extensions and a “tail”), “darter-like” lures exhibited a much higher degree of variation than did “worm-like” lures, both within populations and across the species range. The latter lure phenotype exhibited a relatively simple, uniform morphology combined with a bright orange coloration underlain with a black basal stripe phenotype in Michigan (Figs. 6F–6H), in Alabama (Figs. 6I and 6J), and in North Carolina populations (Fig. 2A in Zanatta, Fraley & Murphy, 2007). In contrast, Raisin River “darter-like” mantle lures exhibited individual-level variation that was sometime quite marked, especially in details of their pigmentation, and to a more limited degree in their marginal extensions (Figs. 6A–6D and S1). Among individual variation was most pronounced for inter-population camparisons, e.g., see the much larger “tail” in the lure displaying Paint Rock River, Alabama specimen shown in Fig. 6E, and also the wider range of phenotypes present in North Carolina populations (Figs. 2B–2D in Zanatta, Fraley & Murphy’s (2007). Male mantle lure rudiment photos are found in Fig. S5.
Figure 6: Panel displaying variability in the two primary lure phenotypes of Lampsilis fasciola.
Variability in lure phenotype, both within a population and across the range of Lampsilis fasciola. (A–D) are “darter-like” Raisin River (MI) lures photographed in the field at Sharon Mills County Park. (E) Depicts a “darter-like” lure displayed by a Paint Rock River (AL) female. (F–H) show field photographs of “worm-like” lures displayed by three Sharon Mills females, with specimen H being a younger adult. (I and J) are photographs of two captive AABC specimens, with “worm-like” lures, sourced from the Paint Rock River. The former photo (I), taken in 2011, shows a young (2-year old) female, a member of the captive brood, displaying her lure, and the latter photo (J) is of a female field-sampled in 2022, and showing a partially retracted mantle lure.Despite the considerable individual variation among the 24 photographed Raisin River “darter-like” mantle lures (Fig. S1), it was possible to identify some shared phenotypic motifs, especially in pigmentation pattern, and to informally categorize 23/24 mantle lures with those shared motifs into four general groupings. Group 1 “darter-like” mantle lures were characterized by prominent, chevron-like, darker pigmented blotches, spaced regularily along the flanks of the lure, over a lighter background coloration (Fig. 6A). This general pattern occurred in 7/24 Raisin River “darter-like” lures examined. Group 2 was rarer (3/24 individuals) and consisted of a darker background coloration with large orange blotches spaced regularily along the lure flanks, some divided into “dorsal” and “ventral” elements (Fig. 6B). Group 3 (9/24 individuals) lures were characterized by prominent dark lateral maculation spatially divided into a “ventral” pattern of larger, regularly spaced blotches and a “dorsal” pattern of more numerous, irregular blotches of different sizes (Fig. 6C). Finally, Group 4 (3/24 individuals) lures were characterized by an evenly-dispersed, fine grained freckling of numerous pigmented spots over a lighter background (Fig. 6F).
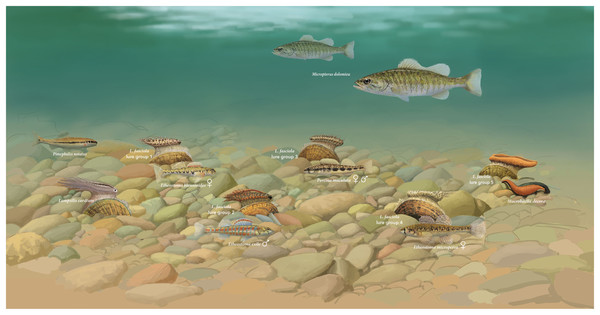
To explore putative model species for the four L. fasciola Raisin River “darter-like” mantle lure groupings (Figs. 6A–6D and 6F), potential matches (in terms of size, shape and coloration) were sought among the 10 species of Etheosomatidae that occur in the River Raisin (Smith, Taylor & Grimshaw, 1981), many of which display pronounced sexual dimorphism in body coloration (Kuehne & Barbour, 2014). The best apparent matches, depicted in Fig. 7, are as follows: Group 1 (Fig. 6A)-Etheostoma blennioides (female coloration), Group 2 (Fig. 6B)-Etheostoma exile (male coloration), Group 3 (Fig. 6C)-Percina maculata (male and female coloration) and Group 4 (Fig. 6D)-Etheostoma microperca (female coloration).
Figure 7: Illustration of hypothetical benthic assemblage of main Lampsilis fasciola lure groups, and proposed models.
A hypothetical Raisin River (Michigan) benthic assemblage showing displaying exemplars of the putative five main Lampsilis fasciola mimetic mantle lure groups (Figs. 6A–6D and 6F) present at the Sharon Mills County Park study site, together with their respective model species, and their primary receiver/fish host, Micropterus dolomieu. Also shown is a displaying Lampsilis cardium with a “small minnow” mimetic mantle lure (Patterson et al., 2018) and its putative model, Pimephales notatus, the most common fish species in the River Raisin (Smith, Taylor & Grimshaw, 1981). Illustration by John Megahan.The distinctive color combination of the L. fasciola “worm-like” lure-solid orange with a black underlay (Figs. 6F–6J) does not match that of any Raisin River darter, or other Raisin River fishes (Smith, Taylor & Grimshaw, 1981). It does, however, match the coloration and size/shape, of the common North American leech, Macrobdella decora, which is widespread and abundant in eastern North America watersheds and typically feeds on aquatic vertebrates (Klemm, 1982; Munro et al., 1992). M. dolomieu, L. fasciola’s primary host fish, is a generalist predator with a diet of aquatic invertebrates, including leeches, in addition to small fishes (Clady, 1974), and recreational fishers frequently use live and/or artifical leeches as bait to catch this species (Cooke et al., 2022). Based on the available data, it seems that Macrobdella decora may be the best model species candidate for the “worm-like” (McNichols, 2007) L. fasciola mantle lure phenotype, and will hereafter be referred to as the leech phenotype.
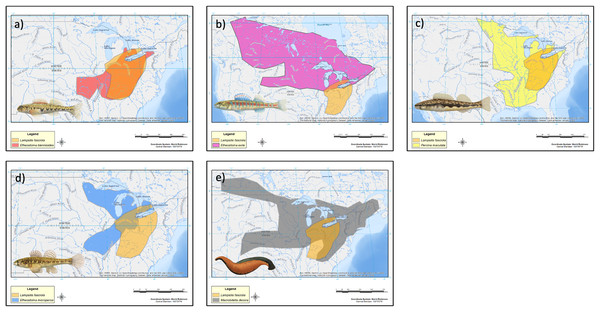
The geographic range of the mimic, L. fasciola, is a subset of that of its receiver/host M. dolomieu (Fig. 2), and the extent of range overlap with all five putative River Raisin mantle lure models were calculated using Arcgis (Table 2) and are shown in Fig. 8. Three of the five putative models-Etheostoma blenniodes, Percina maculata and Macrobdella decorata have extensive overlap with L. fasciola’s range, but E. exile and E. microperca are restricted to northern portions.
| Type | Proposed model | Range overlap (km2) |
|---|---|---|
| Group a | Etheostoma blennioides | 480,731 |
| Group b | Etheostoma exile | 87,796 |
| Group c | Percina maculata | 525,772 |
| Group d | Etheostoma microperca | 164,539 |
| Group e | Macrobella decora | 419,259 |
Figure 8: Estimated range maps for proposed models of Lampsilis fasciola lures.
Estimated range maps for five proposed models for Lampsilis fasciola lures compared to the estimated geographic range of Lampsilis fasciola (orange). (A) Etheostoma blennoides (red), (B) Etheostoma exile (mauve), (C) Percina maculata (yellow), (D) Etheostoma microperca (blue), and (E) Macrobdella decora (gray). Note the differences in spatial scales in the panels. Model Illustrations by John Megahan. Base map layers are from U.S. Geological Survey (2022).Behavioral analyses
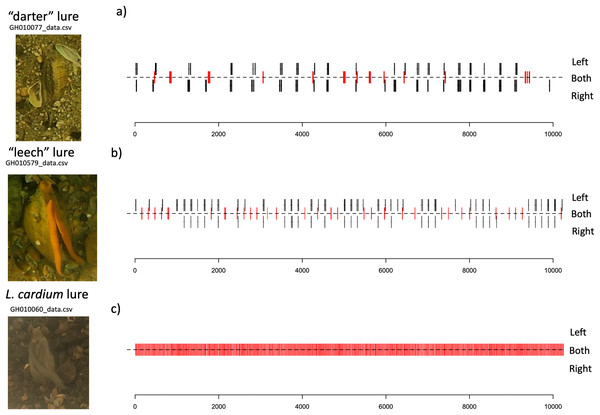
Lure movements for both species consist of small undulations along the length of the mantle lure, beginning about two thirds of the way towards the “tail” side of the lure, and travelling towards the “head” of the lure. The L. cardium lure movements always occur on both left and right sides of the mantle lure simultaneosly, while both L. fasciola lure phenotyopes exhibit independent movement of the left and right sides of the lure. Qualitatively, L. fasciola and L. cardium have very different mantle lure display behaviors. Gait diagrams show a clear distinction between L. cardium and both primary L. fasciola lure phenotypes (“darter” and “leech”). L. cardium consistently exhibited a synchronized lure undulation of both mantle lure flaps, whereas L. fasciola samples frequently moved left and right mantle flaps independently (Fig. 9 and S6). Gait diagrams also qualitatively showed that L. fasciola is charicterized by a high level of variability in undulation interval, L. cardium is much more regular in undulation interval with a steady beat frequency.
Figure 9: Gait diagrams for three exemplar mussel displays; a “darter-like” L. fasciola, a “leech-like” L. fasciola, and a Lampsilis cardium.
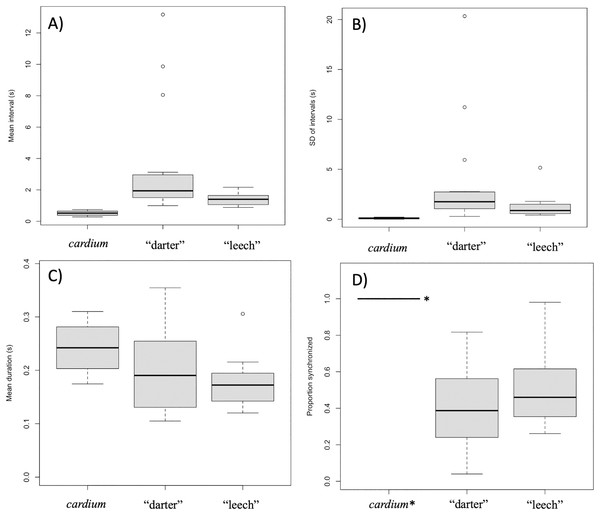
Mantle lure gait diagrams for three representative individuals sampled. (A) shows a Lampsilis fasciola “darter” lure sample (https://figshare.com/articles/media/GH010077_cropped_mp4Polymorphism_in_the_aggressive_mimicry_lure_of_the_parasitic_freshwater_mussel_Lampsilis_fasciola/24850899), (B) displays a Lampsilis fasciola “leech” lure sample (https://figshare.com/articles/media/GH010579_cropped_mp4Polymorphism_in_the_aggressive_mimicry_lure_of_the_parasitic_freshwater_mussel_Lampsilis_fasciola/24850902), and (C) shows a Lampsilis cardium sample (https://figshare.com/articles/media/GH010060_cropped_mp4Polymorphism_in_the_aggressive_mimicry_lure_of_the_parasitic_freshwater_mussel_Lampsilis_fasciola/24847932). Red center lines indicate synchronized lure movement for both left and right mantle flaps, and black lines above and below the center line indicate independent left and right movements, respectively. The x-axis denotes time in seconds and frame number (120 fps).Intervals between movements in L. cardium were shorter (Wilcoxon test, W = 0, N = 4 L. cardium, 15 darter lure L. fasciola, 13 leech lure L. fasciola, p < 0.01 for both comparisons), less variable (Wilcoxon test, W = 0, p < 0.01 for both comparisons) and more synchronized (Wilcoxon test, W = 60, 48, p < 0.01 for comparisons with darter and leech lures, respectively) than in L. fasciola (Fig. 10). There was no difference in duration of lure undulations between L. cardium and both L. fasciola lure phenotypes (Wilcoxon test, W = 42,40, p = 0.26,0.06 for comparisons with darter and leech lures, respectively). Differences between the lure types of L. fasciola were smaller, with inter-movement intervals in the darter phenotype that were longer (Wilcoxon test, W = 142, p = 0.01) and marginally non-significantly more variable (W = 128, p = 0.07) but similar in duration (Wilcoxon test, W = 97, p = 0.76) and degree of synchronization (Wilcoxon test, W = 64, p = 0.22, Fig. 10). Table S3 details the time, date, location, temperature and summary statistics of all 34 lure display field recordings.
Figure 10: Summary plots for behavioral analysis of the two primary Lampsilis fasciola lure phenotypes and Lampsilis cardium.
Boxplots from behavioral analyses of the two primary Lampsilis fasciola mantle lure phenotypes (“darter” vs. “leech”, N = 15,12 respectively) and of Lampsilis cardium (N = 4). The middle line in the represents the median, the gray rectangle represents the interquartile range, the whiskers represent the minimum and maximum, excluding outliers, which are defined by 1.5* the interquartile range. (A) Comparison of the mean interval between movements of left mantle flap (s). (B) The standard deviation of lure movement interval (s) as a proxy for variability. (C) The average duration of each mantle lure movement. (D) The proportion of movements that are left-right synchronized. Note that the value for L. cardium is 1.0 (all lure movements for all individuals were synchronized) and indicated with *. (A–C) Show means for left mantle flap movements only.GLMM were used as an alternative analytical approach that included a large, bootstrapped dataset of lure movements. GLMM results were similar to those of the mean comparisons, with L. cardium individuals having shorter movement intervals than either L. fasciola lure morphs (an estimated 0.21 s for L. cardium vs. 3.2 and 1.0 s, respectively for L. fasciola “darter” and “leech” lures). However, these fixed effects are not statistically significant.
Discussion
Two new pieces of evidence, phylogenomic and genetic, corroborated (Zanatta, Fraley & Murphy’s, 2007) preliminary finding that the primary mantle lure morphs in L. fasciola (Figs. 1B and 1C) represent a within-population polymorphism rather than cryptic taxa. In phylogenomic analyses, all three polymorphic population samples (Huron, Raisin, and Paint Rock Rivers), collectively spanning the species range (Figs. 2A–2C), produced tip clades that were comprehensively polyphyletic regarding lure morph type (Fig. 4), and the “darter vs. leech” dichotomy yielded a low estimate of phylogenetic signal (λ = 0.21). However, the phylogenomic data did reveal clear evidence of geographic structuring (Fig. 4), with each geographic population forming discrete clades, even among regional populations with a continuous freshwater connection. For example, the Huron and Raisin drainages empty in Western Lake Erie and the Little Tennesse and Paint Rock drainages empty into the Tennessee River (see also VanTassel et al. (2021)). The Paint Rock River (AL) population was sister to the Michigan populations (Fig. 4), a result consistent with phylogeographic associations of multiple other North American species, including unionid mussels and Micropterus dolomieu, attributed to hypothesized glacial refugia in the southern Appalachian mountains (Soltis et al., 2006; Borden & Krebs, 2009; Zanatta & Harris, 2013; Hewitt et al., 2018).
Discovery of within-brood mantle lure heterogeneity (Fig. 3), apparently the first such record for unionids, confirms that the L. fasciola “darter-like” and “leech-like” mantle lures are polymorphisms rather then cryptic species, corroborating (Zanatta, Fraley & Murphy, 2007), and provides initial, although limited, genetic insights into lure phenotype inheritance. Of the 50 available offspring, the maternal “leech” phenotype was inherited by 17; the remaining 33 had the “darter” phenotype, but none exhibited a recombinant phenotype, e.g., “leech” coloration with “darter” marginal extensions or “darter” coloration without marginal extensions. Evidence of discrete, within-brood segregation of the mantle lure polymorphism implies potential control by a single genetic locus and expression of the maternal phenotype in about one third of the offspring is inconsistent with a hypothetical dominant “leech” allele. Additional pedigree insights are currently inhibited by not knowing the number of sires that contributed to the brood: the dam was a wild-mated Paint Rock River individual. Freshwater mussel broods frequently have multiple paternity (Ferguson et al., 2013; Wacker et al., 2018). However, additional analyses of the RADseq dataset are needed to resolve that issue (Thrasher et al., 2018).
There are well-known cases of a single genetic locus controlling a mimic polymorphism in other systems. In butterflies, polymorphic mimicry in wing pigmentation is controlled by an introgressed mimicry supergene in Heliconius species (Sheppard et al., 1985; Jay et al., 2018) and by mimicry alleles of the transcription factor doublesex (dsx) in some Papilio species (Palmer & Kronforst, 2020). Note, however, that the L. fasciola mantle lure mimicry polymorphism differs in important ways from these butterfly systems. It is more complex because it involves putative models (darters and leeches) from disparate phyla rather than from similar morphospecies (other butterflies), thereby requiring polymorphic trait differentiation in pigmentation and in morphology (Figs. 1B and 1C). It is also a case of aggressive mimicry (Jamie, 2017), different from the Müllerian mimicry of Heliconius (Kronforst & Papa, 2015) or the Batesian mimicry of Papilio (Kunte, 2009).
Persistence of L. fasciola mantle lure polymorphism across a broad geographic scale (Fig. 2) is notable, although the mechanism responsible for widespread maintenace is unclear. One hypothesized mechanism for the persistence of polymorphisms in a species or population is frequency-dependent selection, where fitness is inversly proportional to frequency of a trait (Clarke, 1964; Ayala & Campbell, 1974). Frequency-dependent selection has been observed in other polymorphic mimicry systems (Shine, Brown & Goiran, 2022), and it has been suggested as a possible mechanism for persistence of the L. fasciola polymorphism (Zanatta, Fraley & Murphy, 2007; Barnhart, Haag & Roston, 2008; Hewitt, Haponski & Ó Foighil, 2021b). One criterion for frequency-dependent selection is that phenotype ratios oscillate over time as initially rare phenotypes become more successful. However, the historical (1954–1962) and contemporary (2017) data from Sharon Mills County Park (Fig. 5) did not show evidence of such oscillation: the frequencies of the lures (darter lure = 84.2% vs. 85.2%, leech lure = 15.8% vs. 14.8%) remained essentially the same for both time windows, although we lack data for the intervening years. Theoretically, there are other mechanisms for balancing selection to maintain polymorphisms over long time-scales, including heterozygote advantage or opposing selection pressures favoring different alleles at polymorphic loci (Ford, 1963; Prout, 2000; Mérot et al., 2020), but underlying genetics of the L. fasciola polymorphism is unknown at this time, and more data are clearly needed.
The relative uniformity of the “leech” mantle lure phenotype in the River Raisin and throughout the L. fasciola range (Figs. 6F–6J) stands in sharp contrast with much higher local and range-wide variation shown by “darter” lures (Figs. 6A–6E). The four putative River Raisin darter model species–Etheostoma blennioides, E. exile, E. microperca and Percina maculata–are all common and widespread members of the drainage’s ichthyofauna with 300–900 specimens of each species recovered from 30–100 sampling locations (out of 160 total) by the Smith, Taylor & Grimshaw (1981) ecological survey. That phenotypic lure disparity mirrors the collective phenotypic variability of darters vs. Macrobdella decora; darters are the second-most diverse fish clade in North America, with ~170 species (Warren & Burr, 1994; Stein & Morse, 2000). Another possibility is that at least some L. fasciola “darter-like” lures across the mussel’s range are composite mimics of visual elements from more than one member of their local darter fauna. However, that remains to be established, as does the underlying nature of L. fasciola darter lure variation, i.e., the degree to which it stems from a continuous spectrum of phenotypes or from the presence of additional discrete polymorphisms. The variability in “darter” lure phenotype does not seem to be associated with any environmental factors, which suggests this variability is not due to ecophenotypic plasticity, although more subtle factors, such as chemical cues, were not measured. Irrespective of the factors promoting variation among L. fasciola “darter” lure morphs, maintenance of close phenotypic tracking by lures of their respective models is expected, given host fishes’ strongly adversive reactions to becoming infected (Haag & Warren, 1999).
While the behavior of mantle lures in L. mussels has been documented and studied for many decades (Ortmann, 1921; Kramer, 1970; Haag & Warren, 1999), detailed analysis of lure undulation behavior is currently lacking, and the relative importance of behavior vs. coloration and morphology is not well understood. The lure undulation for both L. cardium and L. fasciola starts about two thirds of the way to the “posterior” (“tailed”) side of the lure, and then travels “forward” toward the “eyespot”-bearing “anterior”. This is quite different from the oscilatory “S” shaped anterior-to-posterior swimming movements used by many fishes (Liao, 2007; Smits, 2019). However, it shares some resemblence to the “C” start behavior that many fishes use as an escape mechanism (Witt, Wen & Lauder, 2015). The unusual motion of the mantle lures may therefore be mimicking an escape behavior to some extent, but this remains to be established.
Although the L. fasciola behaviors differ significantly from those exhibited by L. cardium, there appears to be smaller behavioral polymorphism that distinguish the darter from leech lure phenotypes. Our putative model for River Raisin L. cardium mantle lures is a species of pelagic minnow, Pimephales notatus (Fig. 7), whose swimming behavior and ecology differs markedly from that of darters (Burress et al., 2017). Darters have lost or greatly reduced their swim bladder and are primarily benthic in habit, spending much of their time resting on the stream bed with slight body movements caused by ambient water flow (Demski, Gerald & Popper, 1973; Zeyl et al., 2016). They intermittently swim by “hopping” across the substrate using pectoral fins and caudal undulations in a manner that is much more erratic than the midwater swimming behavior of most minnows (Winn, 1958; personal observations). This matches a general difference observed between L. cardium and L. fasciola lures: L. cardium lures move faster and more regularly in a highly synchronized way, in contrast with the erratic, often left-right-unsynchronized movements of L. fasciola lures, apart from slight passive undulations caused by the ambient river currents. Unfortunately, the sample size of L. cardium was low (N = 4), despite a great deal of effort, trying to locate gravid female L. cardium that were actively displaying.
The only major difference in lure behavior between the “darter” and “leech” lure behvior of L. fasciola is a slightly slower rate exhibited by the “darter” lures, and marginally non-significant differences in variability between lure undulations. Both L. fasciola morphs have a similar erratic motion, despite the polymorphism putatively modeling taxa from disparate phyla. Leeches swim by a dorsoventeral bending wave moving from head to tail (Jordan, 1998). This swimming behavior is very different from the lure undulations observed in the leech-like L. fasciola lures. It is possible that leech behavior differs when moving along the substrate, where displaying L. fasciola are located, but we currently lack data on leech swimming behavior in different environments. The ecological importance of the minor, but statistically significant, differences in overall lure beat frequency observed between “darter” and “leech” mimics (Fig. 10) is difficult to evaluate at present, and it remains to be established if it, like the lure morphological differences, is also under genetic control. One additional caveat is that we focused primarily on differences in the timing of mantle lure displays, which were the most practical to measure in-situ with the ambient river flow. We also did not have any data on possible chemosensory cues that could potentially be involved.
Our discovery of discrete within-brood inheritance of the L. fasciola lure polymorphism is of particular interest because it implies potential control by a single genetic locus. There are a number of parallel cases in the recent literature, e.g., in butterflies, the regulation of polymorphic mimicry in wing pigmentation also involves single genetic loci (Jay et al., 2018; Palmer & Kronforst, 2020). Timmermans et al. (2020) used SNP data from Papilio dardanus to discover a genomic inversion associated with its mimetic polymorphism, and this approach is likely also tractable for L. fasciola given the occurance of polymorphic brood. We are currently raising an additional polymorphic brood at the AABC. Mantle lures are a key adaptive trait in Lampsiline evolution and diversification (Hewitt, Haponski & Ó Foighil, 2021b), and L. fasciola is a promising and highly tractable model system to uncover the genetics of lure development and variation in a unionoid mussel.
Supplemental Information
R code used to create diagrams, manipulate raw behavioral data, and run statistical models.
Average behavioral measurements used in statistical analyses.
Summary data on individual mantle lure display field recordings. Video recordings taken during the summer of 2018 at Sharon Mills (Fig. 2a) and Hudson Mills (Fig. 2b). Average movement length and interval were calculated from frame number using a 120fps video recording taken with a Go Pro Hero 6.