Genome-wide identification and characterization of SPXdomain-containing genes family in eggplant
- Published
- Accepted
- Received
- Academic Editor
- Diaa Abd El-Moneim
- Subject Areas
- Agricultural Science, Bioinformatics, Genomics, Plant Science, Soil Science
- Keywords
- Eggplant, Phosphate deficiency stress, SPX domain-containing genes family, Expression profiling, Indoleacetic acid
- Copyright
- © 2024 Zhuomeng et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Genome-wide identification and characterization of SPXdomain-containing genes family in eggplant. PeerJ 12:e17341 https://doi.org/10.7717/peerj.17341
Abstract
Phosphorus is one of the lowest elements absorbed and utilized by plants in the soil. SPX domain-containing genes family play an important role in plant response to phosphate deficiency signaling pathway, and related to seed development, disease resistance, absorption and transport of other nutrients. However, there are no reports on the mechanism of SPX domain-containing genes in response to phosphorus deficiency in eggplant. In this study, the whole genome identification and functional analysis of SPX domain-containing genes family in eggplant were carried out. Sixteen eggplant SPX domain-containing genes were identified and divided into four categories. Subcellular localization showed that these proteins were located in different cell compartments, including nucleus and membrane system. The expression patterns of these genes in different tissues as well as under phosphate deficiency with auxin were explored. The results showed that SmSPX1, SmSPX5 and SmSPX12 were highest expressed in roots. SmSPX1, SmSPX4, SmSPX5 and SmSPX14 were significantly induced by phosphate deficiency and may be the key candidate genes in response to phosphate starvation in eggplant. Among them, SmSPX1 and SmSPX5 can be induced by auxin under phosphate deficiency. In conclusion, our study preliminary identified the SPX domain genes in eggplant, and the relationship between SPX domain-containing genes and auxin was first analyzed in response to phosphate deficiency, which will provide theoretical basis for improving the absorption of phosphorus in eggplants through molecular breeding technology.
Introduction
Phosphorus (P) is an essential nutrient for plant development and reproduction (Hou et al., 2020; Mng’ong’o et al., 2021; Ojeda-Rivera et al., 2022; Wang et al., 2022), which is involved in photosynthesis, respiration, energy storage and transfer, cell division and cell enlargement in plants. Low utilization of phosphate (P) in agricultural soils has seriously damaged crop production, resilience, seed germination and soil biological activity (Prathap et al., 2022; Suriyagoda et al., 2023; Wang et al., 2022). Large-scale application of phosphate fertilizer is the most common and expensive way to overcome the problem, which brings the waste of phosphate rock. Furthermore, plants could only absorb 10–15% of the total fertilizer applied, more than 80% of the fertilizer cannot be used because of soil absorption (Prathap et al., 2022). How to reduce fertilizer dosage, environmental pollution and improve plant productivity is a great challenge to be overcome in the future. Therefore, it is an important research direction for the development of agricultural industry to improve phosphorus utilization rate (Hou et al., 2020). Plants respond to phosphate deficiency stress mainly by changing root system architecture (RSA), controlling rhizosphere P activation, and participating in phosphorus recovery and conservation. Previous studies have reported that the response of root remodeling to phosphate deficiency was mainly regulated by genes and plant hormones (Katznelson, 1977; Satheesh et al., 2022).
The SPX domain-containing protein family was named by three members: SYG1, PHO81, and XPR1. They play a main function in maintaining P homeostasis at the cellular level through P transport and adaptation to P deficiency (Jung et al., 2018; Liu et al., 2018). SPX domain-containing family genes have been identified in many plant species, such as 20 in Arabidopsis (Duan et al., 2008), 33 in Zea mays (Xiao et al., 2021), 23 in Phyllostachys edulis, 46 in the Triticum, 69 in the Brassica napus (Luo et al., 2023; Yang et al., 2022). They could be divided into four different subfamilies in plants: SPX proteins subfamily only containing SPX domain, SPX–EXS proteins subfamily containing SPX and an EXS (ERD1, XPR1 and SYG1) domain, SPX–MFS proteins subfamily containing SPX and the major facility superfamily (MFS) domain, and SPX–RING proteins subfamily containing SPX and the RING-type zinc finger domain (Kant, Peng & Rothstein, 2011; Liu et al., 2018; Wang et al., 2012). The SPX subfamily functions primarily as phosphate-sensing signaling proteins. AtSPX1, AtSPX2, and AtSPX4 are functionally redundant in Arabidopsis and act as a negative regulator of the p-signaling center-regulated gene phosphate starvation response 1 (AtPHR1) in different P concentration (Zhou et al., 2015). AtSPX1 interacts with AtPHR1 to inhibit the phosphoric acid starvation induction (PSI) gene through P1BS, resulting in reducing expression of the phosphoric acid starvation response (PSR) gene. In the condition of P deficiency, the interaction between AtSPX1/AtPHR1 is weakened, which promotes the binding of AtPHR1 to P1BS and regulates the expression of PSR gene. Inhibition of AtSPX3 can aggravate the symptoms of phosphate deficiency, change P distribution and enhance the expression of PSRs (Puga et al., 2014). In maize, ZmSPX3, ZmSPX4.2, ZmSPX5, and ZmSPX6 can interact with ZmPHR1 respectively, to participate in the response to phosphate deficiency stress in a ZmPHR1-mediated manner (Xiao et al., 2021). SPX-MFS mainly transfers phosphate into vacuoles, one of the SPX-MSF members, VPT1 (vacuolar phosphate transporter 1), also known as PHT5; 1 (phosphate transporter 5; 1), plays a major role on P sequestration in Arabidopsis vacuoles. The vpt1 mutants showed sensitivity to P stress, which supported that VPT1 was essential for Arabidopsis thaliana to adapt to phosphate deficiency stress (Liu et al., 2016a; Młodzińska & Zboińska, 2016). The phosphate transporter PHT1 is responsible for taking up phosphate from the soil and further distributing it to aboveground plant organs, the expression of PHT1 was induced or strongly up-regulated during P deprivation (Młodzińska & Zboińska, 2016; Zhang et al., 2015). OsSPX-MFS1 is a key player in the maintenance of P homeostasis in leaves and may act as a P transporter (Lin et al., 2010). Both OsSPX-MFS1 and OsSPX-MFS2 are negatively regulated by osa-miR827 abundance in response to phosphate starvation (Zhang et al., 2015). In wheat, the TaSPX-MFS subfamily genes were targeted by nine different miRNAs, including Tae-miR1120A, Tae-miR1120b-3p, Tae-miR1120b-5p, Tae-miR1122c-3p, Tae-miR1122a, Tae-miR3b-1130p, Tae-miR1130a, and Tae-miR3b-1137p, and P starvation significantly induced the TaSPX gene expression (Kumar et al., 2019; Kumar et al., 2018). In Brassica napus, one SPX-MFS subfamily 11 genes were significantly induced by P starvation and recovered rapidly after P re-feeding (Du et al., 2017; Yang et al., 2022). SPX-EXS family plays an important role in phosphate acquisition, translocation, and distribution, mainly transfers phosphate into vascular cylinders in root, leaf, stem, or flower tissue (Liu et al., 2019; Wang et al., 2004). SlPHO1;1 is a gene in the SPX-EXS subfamily of tomatoes, which plays a major role in phosphate transport between roots and stems at seedling stage (Li, You & Zhao, 2021; Liu et al., 2019; Wang, Secco & Poirier, 2008). The SPX-RING subfamily recognizes PHR1 ubiquitination PHR1 complexes through the SPX domain. SYG1(NLA/BAH1) is the only one SPX-RING gene identified in Arabidopsis, which was first reported to have an E3 ubiquitin ligase and respond to nitrogen-restricted transcription (Kosarev, Mayer & Hardtke, 2002; Peng et al., 2007). It was later found that SYG1 was not only a target of microRNA 827, but also involved in the synthesis of salicylic acid and plant disease resistance (Kant, Peng & Rothstein, 2011; Val-Torregrosa et al., 2022; Yaeno & Iba, 2008). Recent studies have found that SYG1 act as a negative regulator of PHR1 by ubiquitylation, and inositol polyphosphate promotes the interactions between SYG1 and PHR1 resulting in the PHR1 destruction; overexpression of SYG1 seedlings showed accumulation of long root hairs and anthocyanins in the shoot under P deficiency (Park et al., 2023).
Auxin regulating root development relies heavily on the transcription of auxin response genes in (Aux/IAA)—auxin response factor (ARF) auxin signaling modules (Goh et al., 2012; Yang et al., 2021). P deprivation increases the expression of TIR1, which encoding an auxin receptor that mediates auxin-regulated transcription, in Arabidopsis seedlings and enhances auxin sensitivity in P-deficient plants, thereby accelerating the degradation of AUX/IAA proteins and unshackling ARF transcription factors to activate/repress genes involved in lateral root formation and emergence (Pérez-Torres et al., 2008). Mutations that disrupt auxin synthesis (taa1) and transport (aux1) can inhibit the generation of root hairs in Arabidopsis. The expression level of ARF19, RSL2, and RSL4 induced by auxin under phosphate deficiency conditions enhanced in root hair, and the lack of these genes can disrupt root hair generation (Satheesh et al., 2022). Additionally, the transcription factors ARF7 and ARF19 in Arabidopsis roots positively regulate the phosphorus deficiency response gene PHR1 (Huang et al., 2018), while the arf7 and arf19 double mutant plants show a significant reduction in the expression of certain PSI genes. It has been reported that OsPHR2 regulates the downstream gene OsSPX1 in rice, suggesting potential regulatory effects of auxin on some SPX genes (Huang et al., 2018; Wang et al., 2009). SPX domain-containing genes and auxin are both members that play a role under phosphate deficiency stress. Previous studies have reported that auxin could up-regulated AtPHO1;H1 and AtPHO1;H10 under phosphate deficiency (Ribot, Wang & Poirier, 2008). However, the relationship between other SPX domain-containing subfamilies genes and auxin is still unclear.
As a world’s cash crop with a vast area of cultivation, Eggplant, contains rich anthocyanins and is a vegetable with high nutritional value (Añibarro Ortega et al., 2022). The absorption of phosphorus by eggplants mainly occurs during the flowering and fruiting stages (Li et al., 2019), indicating that phosphorus is one of the important factors determining the formation of eggplant yield. Howerver, phosphorus is one of the lowest elements absorbed and utilized by plants in the soil (Katznelson, 1977). There is currently no report on the mechanism of eggplant response to phosphate deficiency stress. In this study, sixteen potential SPX domain-containing genes were identified and their phylogeny, gene structure and conserved motifs were analyzed. We observed the subcellular localization of eight SPX domain-containing genes and determined their gene expression patterns in different tissues as well as under phosphate deficiency with auxin. These results will be helpful for improving the absorption of phosphorus in eggplants through molecular breeding.
Methods
Identification of SPX domain-containing proteins in Solanum melongena
To identify proteins with SPX domains in the eggplant genome, “SPX” was used to search the TAIR (https://www.Arabidopsis.org/) database. Then, the SPX domain-containing biomolecular structure in Arabidopsis thaliana were screened and the SGN (https://solgenomics.net/) (Barchi et al., 2021) database was searched by BLASTP. Submit the detected biomolecular structure to the Pfam database (http://pfam.xfam.org/) (El-Gebali et al., 2019) and the SMART web site (http://SMART.embl-heidelberg.de/) (Letunic & Bork, 2018) to identify SPX domain. Solanum lycopersicum contains 19 SPX domain proteins (Table S2) (Li, You & Zhao, 2021).
SPX domain-containing protein properties, subcellular localization prediction, gene structure analysis
Each biomolecular structure containing the SPX domain was submitted to ExPaSy (http://expasy.org/) (Wilkins et al., 1999) for calculation of molecular weight (MW) and isoelectric point (pI). The gene number, coding region length (CDS) and amino acid length (ORF) of each SPX domain-containing protein were obtained from SGN (https://solgenomics.net/) (Barchi et al., 2021) database. For predicting subcellular localization of proteins, we used (TMHMM-2.0) to predict the transmembrane structure of 16 SmSPX proteins. The co-linear images between the untranslated regions of all SPX domain-containing genes and the coding sequences between the SPX domain-containing genes of multiple species were drawn using TBtools (software version 1.132) (Chen et al., 2020).
Chromosome information, phylogenetic tree construction and domain analysis of SPX domain-containing genes
The collinearity between the chromosome position information of SPX domain genes and the genes was generated by TBtools (software version 1.132) (Chen et al., 2020). The phylogenetic tree was built using MEGA 11.0 (Tamura, Stecher & Kumar, 2021) software and the neighbour-joining (NJ) method with 1,000 bootstrap replicates. The same method was applied to construct phylogenetic trees containing SPX domain-containing genes in different species. The high-quality phylogenetic tree graph of different species was optimized by online website ITOL (https://itol.embl.de/). SPX domain protein family domain information was identified using the online site Pfam (http://pfam-legacy.xfam.org/) (El-Gebali et al., 2019), and domain maps were generated using TBtools (software version 1.132) (Chen et al., 2020). Conserved motifs were identified using MEME software version 5.5.3 (https://meme-suite.org/tools/meme) with the following parameters: any number of repetitions, a maximum of 10 misfits, and an optimum motif width of 6–200 amino acid residues (Bailey et al., 2009).
Subcellular localization
The complete coding sequences of eight SPX domain-containing protein genes were obtained from the SGN (https://solgenomics.net/) database (Barchi et al., 2021). CaMV35S promoter vector pRI 201 (Takara, Beijing, China) with GFP tag was linearized with NDEI endonuclease. Sequences of eight SPX domain-containing protein genes were combined into the vector using in-fusion Snap Assembly cloning kits (Takara, Dalian, China) according to the manual. The fusion construct was transferred into Agrobacterium tumefaciens strain LBA4404, and subcellular localization assays were performed as previously reported (Sparkes et al., 2006). The protein localization was determined under 20× confocal microscopy (LSM880; Zeiss, Oberkochen, Germany). The primer sequences were listed in Table S1.
Growth conditions and treatments
All seeds (V8 and NO.41 Multigenerational inbred lines) were soaked in gibberellin 60 mg/L for 4 h and then bathed in 55 °C water for 30 min, the seeds were washed twice and placed in a glass culture dish containing wet filter paper to germinate at 28 °C in constant darkness for 2 days. Seeds were grown at 25 ± 2 °C under a light regimen of 16 h light and 8 h dark at a temperature of 16 ± 2 °C for 40 days. The hydroponic solution was fed with the classic Hoagland 1/2 concentration nutrient solution, and the seedlings were starved of phosphate and normal phosphate after 2 days. Samples were taken at the end of the two-day period as 0-day control samples, and the samples were taken at the 1st, 5th and 10th day after phosphate starvation treatment. The difference between normal P and low P treatment was that the concentration of P in normal Group was 500 µM. The concentration of P was 10 µM in low-phosphate treatment group. The deficient K element was supplemented with K2SO4. The other components are identical. The concentration of IAA in low phosphate treatment was 0.2 mg/L, and IAA was dissolved with DMSO and dissolved directly in nutrient solution. Samples were taken at 1 day, 5 days and 10 days after phosphate starvation treatment with IAA.
RNA extraction and qRT–PCR analysis
The process of collecting the sample data is similar to we conducted it previously (Chen et al., 2022). The tissue samples were flash-frozen in liquid nitrogen and subsequently stored at −80 °C for RNA extraction purposes. Total RNA was extracted from flowers, leaves, fruits, seeds, roots, and stems using RNA Isolation Kit (CWBIO) and then stored at −80 °C. The integrity of the extracted RNA was evaluated using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). First strand cDNA synthesis was performed on 1 µg of total RNA using the Prime Script RT Reagent Kit (Monad, Beijing, China). PCR amplification was carried out utilizing the ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, California, USA) and the ChemoHS Specificity Plus qPCR Mix Kit (Monad China). The amplification parameters were 95 °C for 10 min, followed by 40 cycles at 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 10 s. The mRNA expression levels were normalized to the level of SmActin expression using the 2−ΔΔCt method (Livak & Schmittgen, 2001). Each experiment included three biological replicates. The primer sequences are listed in Table S1.
Statistical analysis
The error bar in all charts represents the standard deviation, we used SPSS 23.0 (SPSS, Inc., Chicago, IL, USA) and used one–way ANOVA and Duncan’s New Multiple Range test (P < 0.05) to assess statistical significance.
Results
Identification of SPX-domain-containing proteins in S. melongena
Based on the SGN (https://solgenomics.net/) database, the Pfam database (http://pfam.xfam.org/), the TAIR (https://www.Arabidopsis.org/) database, and the SMART software (http://SMART.embl-heidelberg.de/), a total of 16 SPX domain-containing proteins were identified in the eggplant genome (Table 1). Sixteen SPX domain-containing genes are divided into four subfamilies, including SPX, SPX-EXS, SPX-MFS and SPX-RING. Based on the subfamily and the chromosomes where the genes are located, we named the identified 16 SPX domain-containing genes from SmSPX1 to SmSPX16. SmSPX1–SmSPX5 belong to the SPX subfamily, SmSPX6–SmSPX9 belong to the SPX-MFS subfamily, SmSPX10–SmSPX14 belong to the SPX-EXS subfamily, and SmSPX15–SmSPX16 belong to the SPX-RING subfamily. The coding sequences length of the 16 SPX domain-containing genes ranged from 780 bp to 4752 bp, and the amino acid sequences length ranged from 259 aa to 1583 aa. The predicted molecular weight ranged from 29.28KDa to 183.38KDa, and the isoelectric point ranged from 4.69 to 9.41.
| Rename | Gene ID | Description | ORF (aa) | CDS (bp) | MW (KDa) | pI |
|---|---|---|---|---|---|---|
| SmSPX1 | SMEL4.1_01g017740.1.01 | SPX | 259 | 780 | 29.28 | 7.64 |
| SmSPX2 | SMEL4.1_02g009290.1.01 | SPX | 346 | 1041 | 38.5 | 5.35 |
| SmSPX3 | SMEL4.1_02g028380.1.01 | SPX | 302 | 909 | 34.25 | 4.69 |
| SmSPX4 | SMEL4.1_03g024350.1.01 | SPX | 290 | 873 | 33.26 | 5.99 |
| SmSPX5 | SMEL4.1_06g020500.1.01 | SPX | 266 | 801 | 31.24 | 6.2 |
| SmSPX6 | SMEL4.1_01g016630.1.01 | SPX-MFS | 575 | 1728 | 65.15 | 6.3 |
| SmSPX7 | SMEL4.1_05g022980.1.01 | SPX-MFS | 696 | 2091 | 77.98 | 6.03 |
| SmSPX8 | SMEL4.1_08g001320.1.01 | SPX-MFS | 694 | 2085 | 77.77 | 6.15 |
| SmSPX9 | SMEL4.1_08g022180.1.01 | SPX-MFS | 697 | 2094 | 78.36 | 5.94 |
| SmSPX10 | SMEL4.1_02g028360.1.01 | SPX-EXS | 1583 | 4752 | 183.38 | 9.27 |
| SmSPX11 | SMEL4.1_02g028370.1.01 | SPX-EXS | 629 | 1890 | 72.22 | 9.41 |
| SmSPX12 | SMEL4.1_09g022670.1.01 | SPX-EXS | 790 | 2373 | 91.04 | 9.23 |
| SmSPX13 | SMEL4.1_10g015110.1.01 | SPX-EXS | 791 | 2376 | 92.48 | 9.35 |
| SmSPX14 | SMEL4.1_10g017040.1.01 | SPX-EXS | 702 | 2109 | 82.05 | 9.18 |
| SmSPX15 | SMEL4.1_09g018170.1.01 | SPX-RING | 332 | 999 | 37.9 | 8.9 |
| SmSPX16 | SMEL4.1_12g008960.1.01 | SPX-RING | 325 | 978 | 37.37 | 8.04 |
Chromosome location and collinearity analysis of SPX domain-containing genes in S. melongena
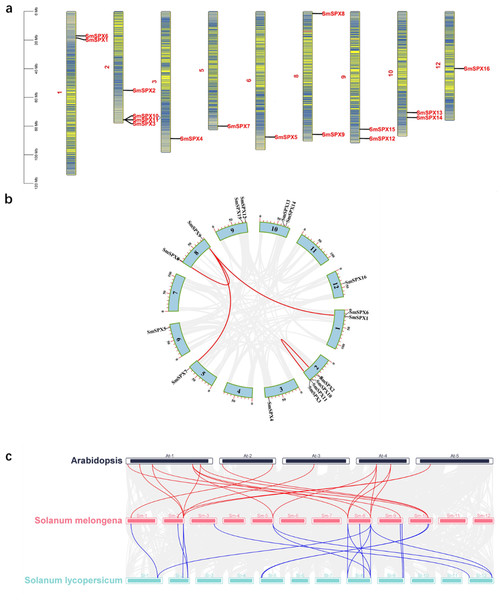
TBtools software was used to analyze all SPX domain-containing genes for chromosome localization and gene collinearity and mapping. There are 12 chromosomes in eggplant, and each chromosome contains SPX domain-containing genes. There are four SPX structural genes on chromosome 2. Chromosomes 1, 9, and 10 all contain two SPX structural genes, and each of the remaining chromosomes contains only one SPX structural gene. In addition, an intergenic collinearity analysis of these 16 genes revealed that 37.5% (six of 16) of the SPX domain-containing genes were derived from gene duplication (Fig. 1A). Intergenic collinearity was mainly located in the chromosome 2 and chromosome 8, accounting for 66.7% of tandem repeats. Of these, two gene tandem repeats belong to the SPX subfamily and four gene tandem repeats belong to the SPX-MFS subfamily (Fig. 1B). By analyzing the genetic collinearity between Arabidopsis, Solanum lycopersicum and S. melongena we found that tomato and eggplant have high homology (Fig. 1C). Analysis of the isograms among eggplant, Arabidopsis and tomato showed that 11 genes from Arabidopsis had gene tandem events with eight genes from eggplants, and 12 genes from eggplant had gene tandem events with 12 genes from tomato, there were seven gene cascades in the three species.
Figure 1: (A) Physical map of 16 SPX-domain-containing genes in 12 chromosomes. The blue arcs in the graph correspond to genes that undergo tandem duplication. (B) Segmental duplication of the 16 SPX genes in 12 chromosomes. Genes linked with a line show a pair of segmentally duplicated genes. (C) Three species SPX gene collinearity analysis, red line indicates Arabidopsis thaliana and tomato gene duplication, blue line indicates tomato and eggplant gene duplication.
Phylogenetic tree analysis and gene structure of SPX-domain-containing genes in S. melongena
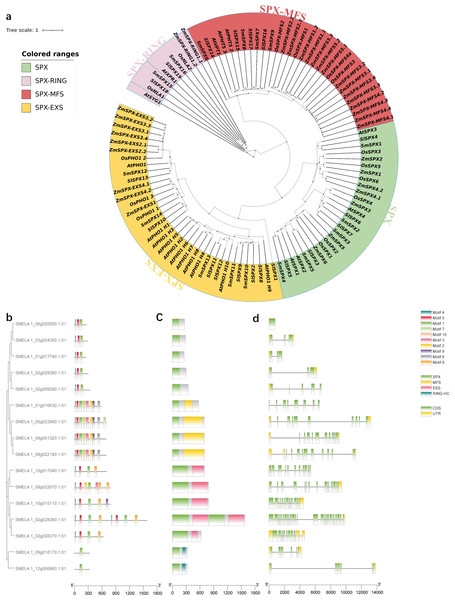
In order to analyze the evolutionary relationship of SPX domain-containing genes, a phylogenetic tree was constructed among Arabidopsis, Zea mays L, Oryza sativaL, Solanum lycopersicum and S. melongena (Fig. 2A). The phylogenetic tree divided into four subfamilies (SPX, SPX-MFS, SPX-EXS, SPX-RING). The first subfamily contains only SPX domains, including four AtSPXs, seven ZmSPXs, six OsSPXs, seven SlSPXs, and five SmSPXs. The second subfamily contains SPX and MFS domains, including three AtSPX-MFSs, 15ZmSPX-MFSs, three OsSPX-MFSs, four SlSPX-MFSs and four SmSPX-MFSs. The third subfamily of SPX and EXS domains consist of 11 AtSPX-EXSs, nine ZmSPX-EXSs, four OsSPX-EXSs, eight SlSPX-EXSs and five SmSPX-EXSs. The fourth subfamily contains SPX and RING domains, including two AtSPX-RINGs, two ZmSPX-RINGs, two OsSPX-RINGs, two SlSPX-RINGs and two SmSPX-RINGs. In order to determine the motif composition of the SPX-containing domain genes, the conserved motif was analyzed by MEME online software, and a total of 10 conserved motifs were identified (Figs. 2B–2C), named as motif 1∼10. SPX domain is located at the N-terminus, and SmSPX11 contains two sets of duplicate SPX-EXS domains. Analyzing the gene structure of 16 SPX-containing domain genes (Fig. 2C) found that the SPX-EXS subfamilies have more complex exons.
Figure 2: Phylogenetic tree analysis and gene structure of SPX-domain-containing genes in Solanum melongena.
(A) Phylogenetic analysis of SPX-domain-containing protein from Arabidopsis, maize, rice, tomato and eggplant. All genes are divided into four parts and labeled with different colors and text. Green background is SPX type, red is SPX-MFS type, yellow is SPX-EXS type, and pink is SPX-RING type. Phylogenetic relationships, conserved motifs, domains and gene structures of 16 SPX domain-containing genes. (B) The phylogenetic relationships of the 16 SPX domain-containing genes constructed with bootstrap values of 1000 replicates. Conserved motifs and domains of 16 SPX domain-containing genes were analyzed using MEME. (C) Rounded rectangles with different colors indicate different domains. (D) The gene structures of the 16 SPX domain-containing genes analyzed using TBtools. CDS and UTRs are colored with green and yellow boxes, respectively, while black lines represent introns.Subcellular localization of SPX domain-containing proteins
To better understand the function of the 16 SPX-containing domain genes, we conducted subcellular localization prediction. TMHMM-2.0 (https://services.healthtech.dtu.dk/services/TMHMM-2.0/) software was used to predict the transmembrane structure existed in the 16 SPX-containing domain genes. The results showed that SmSPX6-14 had a transmembrane domain, and SmSPX15-16 had no transmembrane domain, but it was expressed in the membrane system. Furtherly, the palmitoylation site of the SPX gene was predicted using (http://lipid.biocuckoo.org/index.php), and the signal appearing on the membrane may be due to the presence of palmitoylation sites in SmSPX15 and SmSPX16 (Table S3).
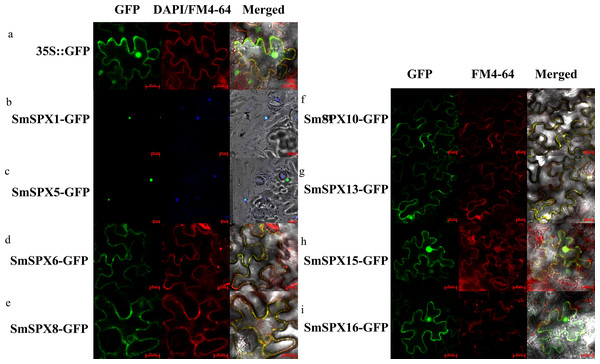
According to the analysis result of evolutionary relationship, the identified SPXs could be divided into four four subfamilies. Considering SPXs from the same subfamily might have similar function and localization, we selected randomly selected two members from each of the four subfamilies for subcellular localization. The selected genes were overexpressed using the CaMV35S promoter to form the target gene-GFP fusion protein. 35S::GFP was used as positive control. We used cell membrane and nucleus reference (FM4-64, DAPI) to ensure the accuracy of localization (Fig. S1A and Fig. 3). SmSPX1 and SmSPX5 were detected in nucleus, SmSPX6, SmSPX8, SmSPX10 and SmSPX13 were detected in membrane system, SmSPX15 and SmSPX16 were detected in nucleus and membrane system.
Figure 3: Subcellular localization of SPX domain-containing proteins (bars = 20 µm).
SmSPX-GFP fusion proteins were transiently expressed in tobacco leaves, and their localization was determined using confocal microscopy. (A) 35S:: GFP is located in the nucleus, cytoplasm and plasma membrane; (B–C) SmSPX1 and SmSPX5 are located in the nucleus; (D–E) SmSPX6 and SmSPX8 are located on the membrane system; (F–G) SmSPX10 and SmSPX13 are located on the cell membrane; (H–I) SmSPX15 and SmSPX16 are expressed in both the nucleus and the cell membrane. Red indicates the location of plasma membrane; green indicates the position of SmSPX proteins; dark blue represents the location of the nucleus; yellow merged represents the co-localization of plasma membrane and SmSPX proteins; Light blue represents the nucleus co-localized with SmSPX proteins in the nucleus.Tissue-specific expression of SPX domain-containing genes in S. melongena
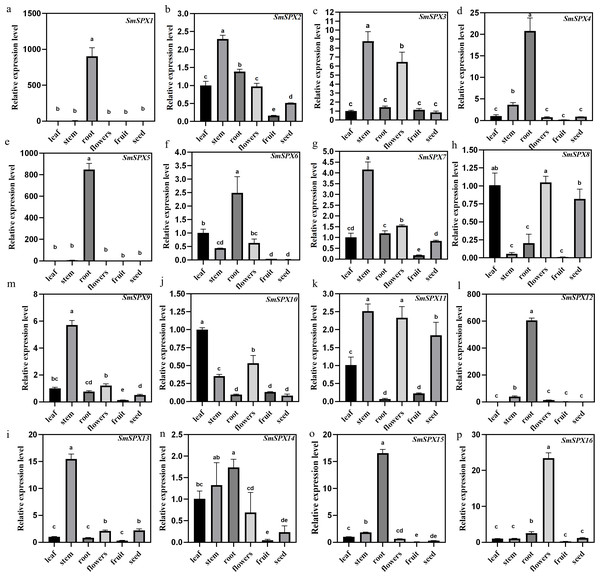
Under phosphate deficiency condition, the root of the plant first senses and transmits the stress signal to the whole plant through complex signal transduction modes. To explore the tissue expression patterns of the 16 SPX domain-containing genes in eggplant, the mRNA was extracted from leaves, stems, roots, flowers, fruits, and seeds for qRT-PCR analysis (Fig. 4). SmSPX1, SmSPX4, SmSPX5, SmSPX6, SmSPX12 and SmSPX15 are expressed in roots. SmSPX2, SmSPX3, SmSPX7, SmSPX9, SmSPX11 and SmSPX13 were more expressed in stems, while SmSPX8 and SmSPX10 were more expressed in leaves. SmSPX16 is specifically expressed in flowers, and all genes are little expressed in fruits, while SmSPX1, SmSPX8 and SmSPX11 are expressed in seeds.
Figure 4: The relative expression level of 16 SPX domain-containing genes in the leaves, stems, roots, flowers, fruits and seeds.
(A–P) The relative expression of SmSPX1-SmSPX16 in different plant tissues. The relative expression level was calculated using the method of 2−ΔΔCt. The relative mRNA levels of the leaves were used for the reference. The values are means ± SD (n = 3). An asterisk (*) represents significance at p < 0.05 comparing with reference.Expression patterns of 16 SPX domain-containing genes under phosphate deficiency stress
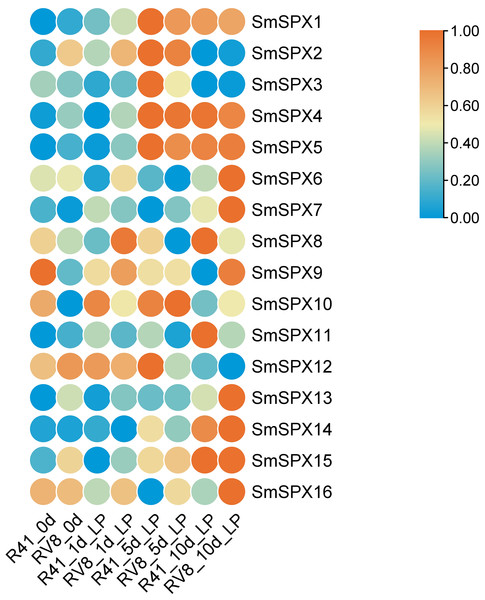
To identify the key genes under phosphate deficiency stress, we made root transcriptomes (PRJNA1030332, SAMN39278703 –SAMN39278741) of two varieties with different phosphate deficiency tolerance treating with low phosphate for 1 day, 5 days, and 10 days. The Log2FKPM (fragment per thousand base transcripts per million mapped fragments) method was used to estimate the expression level of the SPX domain-containing genes, and the zero to one method was used to normalize the data. The 16 SPX domain-containing genes expression levels were surveyed in the transcriptome data. According to RNA-seq data, the expression level of most SPX domain-containing genes increased at 1 day and changed significantly at 1∼5 days by low phosphate, and differed at 5∼10 days in the two varieties. The expression levels of SmSPX4, SmSPX5, SmSPX1, SmSPX3, and SmSPX14 between the two varieties were differences (Fig. 5). SmSPX4, SmSPX5, SmSPX1 and SmSPX14 expression level in V8 variety was higher than 41 variety at 1 day and 5 days, and there was no significant difference in expression level at 10 days, this difference may be key genes in response to phosphate deficiency stress.
Figure 5: Expression levels of 16 SPX domain-containing genes in two multi-generation self-bred varieties eggplant seedlings under low phosphorus treatment on different days.
Different colors indicate different expression level, the highest in red and the lowest in blue.Expression analysis of SPX domain-containing genes in response to IAA
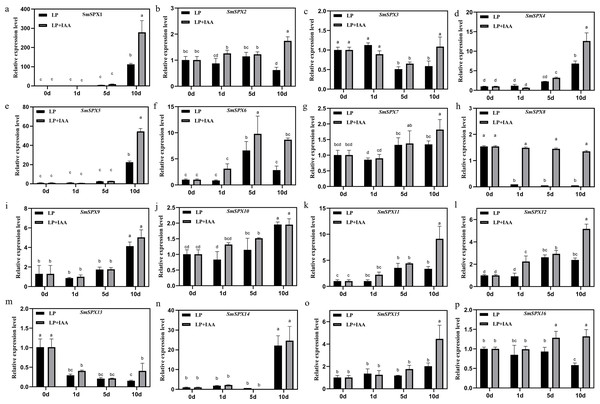
To further identify the biological reaction pathways in which SPX domain-containing genes involved, cis-elements in the promoters were analyzed (Fig. S4). Six main classes of cis-acting elements were found: including photo-responsive elements, coercion of the corresponding components, hormone-responsive elements, circadian response elements, seed developmental response elements, and MYB binding sites, etc. It is worth noting that SPX domain-containing genes have many hormone-responsive elements and photo-responsive elements, which may be a key way for its response to stress to regulate plant development. Considering SPX domain-containing genes and auxin are both members that play a main role under phosphate deficiency stress, we analyzed the relationship between SPX domain-containing genes and auxin under phosphate deficiency stress. We added 0.2 mg/L IAA to phosphate starvation treatment on 4-week-old eggplant seedlings in hydroponic culture, and detected the expression patterns of 16 SPX domain-containing genes at 1 day, 5 days, and 10 days of phosphate starvation, respectively (Fig. 6). The results showed that the expression of SPX domain-containing genes under phosphate starvation treatment combination with IAA was significantly different from that of phosphate starvation treatment alone. SmSPX1, SmSPX4, SmSPX5, and SmSPX15 were significantly induced by IAA under phosphate deficiency stress, especially at 10 days.
Figure 6: Gene expression levels of 16 kinds of SPX domain-containing genes in roots at 1 day, 5 days and 10 days after low phosphorus and low phosphorus treatment with IAA.
(A–P) The relative expression of SmSPX1-SmSPX16 in different treatment, black indicates phosphorus deficiency treatment, gray indicates phosphorus deficiency when adding IAA treatment. The relative expression level was calculated using the method of 2−ΔΔCt. The relative mRNA levels of the leaves were used for the reference. The values are means ± SD (n = 3). An asterisk (*) represents significance at p < 0.05 comparing with reference.Discussion and Conclusions
Phosphate deficiency has always been an important factor affecting plant development, and plants produce corresponding physiological and biochemical reactions to increase the way to obtain P from the soil or reduce their unnecessary nutrient consumption. Under phosphate deficiency condition, the stress response of plants is mainly regulated by the P signaling pathway, and the SPX domain-containing genes play an irreplaceable role as an important component of the P signaling response. Therefore, evaluating the biological information of SPX protein can better understand the signaling mechanism of eggplant under phosphate deficiency and contribute to future genetic breeding.
In our study, we identified 16 SPX domain-containing genes in eggplant, which are very similar to the SPX family members (19) in tomato (Li, You & Zhao, 2021). Three segments of repetitive gene pairs were found on four chromosomes of eggplant, indicating that these genes underwent strong selection during the evolution of eggplant (Fig. 1B). The function of SPX domain proteins could be determined by the evolutionary relationships among different species (Fig. 1C). The isograms of eggplant with Arabidopsis and tomato showed that most of these genes are conserved during evolution, similar to those reported in Arabidopsis (Duan et al., 2008). The majority of homologous genes between Arabidopsis and eggplant are found to be located on chromosomes 1 and 4 of Arabidopsis, as well as chromosomes 2 and 8 of eggplant. This suggests that SPX s changed throughout evolution; however, some SPX genes have remained relatively conserved. The induced expression of SmSPX genes under low phosphorus conditions also provides evidence for its role in resisting phosphorus deficiency in eggplant. We identified the subcellular localization of SmSPX1 and SmSPX5 and found that they are both located in the nucleus. SmSPX6 and SmSPX8 are located to the membrane system. SmSPX10 and SmSPX13 localized to the plasma membrane. SmSPX15 and SmSPX16 expressed both in nuclei and cell membranes (Fig. 3). SmSPX1, SmSPX4, and SmSPX5 belong to the same subfamily, and specifically expressed in roots. The expression trend of SmSPX1, SmSPX4, and SmSPX5 in different eggplant varieties is also consistent under phosphate deficiency situation, indicating that this subfamily may play an important role in eggplant phosphate deficiency stress (Figs. 4 and 6, Fig. S3). However, the expression level of SmSPX1 in anti-phosphate deficiency eggplants variety (V8) is higher than that in phosphate deficiency eggplants variety (41) at the first and fifth day under phosphorus deficiency. From an evolutionary perspective, SmSPX1, SmSPX4, and SmSPX5 are similar to AtSPX1 and AtSPX3. Previous study has reported that overexpression of AtSPX1 increases the transcriptional levels of ACP5, RNS1, and PAP2 under phosphorus sufficient and phosphorus deficient conditions in Arabidopsis, indicating that AtSPX1 has a potential transcriptional regulatory role in response to phosphorus starvation (Duan et al., 2008). OsSPX1 inhibits the formation of OsPHR2 dimer in rice, and its overexpression suppresses the expression of phosphate starvation-induced gene and disrupts its function in the phosphorus deficiency pathway (Liu et al., 2010; Wang et al., 2009). Nine GmSPX members have been identified in soybeans. Overexpression of GmSPX1 reduces the total P concentration in plants, alters root hair morphology, and inhibits both root hair elongation and quantity elongation (Zhang et al., 2016). Overexpression of GmSPX3 gene enhances P content in surface soil and root hairs, as well as upregulates transcription of seven genes involved in phosphorus hunger response in soybean root hairs (Yao, Tian & Liao, 2014). The expression levels of all the SPX subfamily identified in maize are increased under low phosphorus deficiency except ZmSPX3, among which ZmSPX4.1 and ZmSPX4.2 were the most obvious. Moreover, ZmSPX3 and ZmSPX4.2 can regulate phosphorus deficiency in a ZmPHR1-mediated manner (Xiao et al., 2021). Taken together the SPX subfamily members from different plant species seem to have a unified function. As shown in (Fig. 6 and Fig. S3), SmSPX1 was most significantly up-regulated after low phosphorus deficiency, we speculate that this expression difference of SmSPX1 between V8 and 41 variety is one of the reasons for the higher anti-phosphate deficiency stress of V8, SmSPX1 may play a positive regulatory role in eggplant adaptation to phosphorus starvation. SmSPX6 and SmSPX8 are members of SmSPX-MFS subfamily, mainly expressed in the membrane system, which were similar to AtPHT5;1 and AtPHT5;3 (Fig. 3). Under phosphate deficiency stress, the expression level of SmSPX6 was increased, while SmSPX8 was significantly decreased. A similar situation also exists in the root system of Arabidopsis, AtPHT5;1 was up-regulated in response to P deficiency, but AtPHT5;3 was down-regulated (Liu et al., 2016b). Overexpression of AtPHT5;3 results in mis-regulation and growth retardation of the PSR gene as a result of large amounts of P sequestered to the vacuole (Liu et al., 2016b). There are three vacuolar phosphorus transporters (OsSPX-MFS1-3) in rice, and they were mainly expressed in shoots. OsSPX-MFS1 and OsSPX-MFS3 were inhibited by P deficiency, while OsSPX-MFS2 was induced (Guo et al., 2023; Wang et al., 2012). The mutants of osspx-mfs1 and 3 showed lower vacuolar P concentration, while, OsSPX-MFSs overexpressed plants showed higher vacuolar P accumulation. From the evolutionary point, the function of SmSPX6 and SmSPX8 may be conserved with the homologous genes in Arabidopsis. SmSPX8 may be the most important vacuolar phosphorus transfer protein, because its expression is severely reduced in phosphorus deficiency. SmSPX10-14 and AtPHO1 belong to the SPX-EXS subfamily genes. The function of AtPHO1 is mainly phosphate transport in cells, and phosphoric acid deficiency induces AtPHO1 expression (Wang et al., 2004). AtPHO1 homologous gene OsPHO1;2 in rice plays a key role in the transfer of P from roots to shoots (Secco, Baumann & Poirier, 2010). In eggplant, SmSPX10 is abundant in leaves, SmSPX12 is abundant in roots, and SmSPX13 is abundant in stems (Fig. 3). The expression of these genes in specific locations may ensure the unhindered transport of phosphorus among organs. SmSPX11 is abundant in reproductive organs, which may be an important factor in determining the flowering and fruit setting of eggplant. However, SmSPX14 seems to be expressed in all sites except fruits, which may indicate that it is essential for its P transport function in plants. Although the expression pattern of SmSPX14 in two varieties of eggplant seedlings is resemblance, the expression level of SmSPX14 in V8 variety is twice than that of 41 under phosphate deficiency at one day (Fig. S3). We speculate that SmSPX14 might pay a role on the higher anti-phosphate deficiency stress of V8.
SmSPX15 and SmSPX16 have a RING domain, which is similar in structure to AtSYG1 reported in Arabidopsis. Previous studies have reported that AtSYG1 could polyubiquitination of PHR1 in vitro, resulting in SPX-PHR1 complex untangling and activation of downstream PSR gene expression (Park et al., 2023). Here, SmSPX15 was induced under phosphate deficiency stress, nevertheless, the expression level of SmSPX16 remained almost unchanged. This may be related to the expression specificity, of SmSPX16, which may play other functions in flowers organs (Fig. 3).
Many hormone-responsive elements were found in the promoters of SPX domain-containing genes, indicating that plant hormones may play an important role in regulating the expression of SPX domain-containing genes. Moreover, AtPHO1;H1 and AtPHO1;H10 have been found to be up-regulated by auxin under phosphate deficiency in Arabidopsis (Ribot, Wang & Poirier, 2008). Here, we found that the expression of SmSPX1, SmSPX4, SmSPX5 and SmSPX15 were also induced by auxin under phosphate deficiency (Fig. 6). These genes cover all subfamilies, fully demonstrating the close relationship between auxin and SPX domain-containing in eggplant responding to phosphate deficiency. These findings are worthy of further study and provide theoretical guidance for broadening the molecular mechanism of plant response to phosphate deficiency stress in the future.
Supplemental Information
16 SPX-domain-containing genes transmembrane structure prediction
(a)SmSPX1-5 proteins transmembrane structure prediction, no transmembrane domain. (b) SmSPX6-9 proteins transmembrane structure prediction. (c) SmSPX10-14 proteins transmembrane structure prediction. (d) SmSPX15-16 proteins transmembrane structure prediction no transmembrane domain.
PCA analysis of phosphate deficiency stress samples from two inbred lines of transcriptome at different days
Expression levels of 4 potential SPX domain-containing genes in two multi-generation self-bred varieties eggplant seedlings under low phosphorus treatment on different days
Different colors indicate different expression level, the highest in red and the lowest in green.
Cis-regulatory elements in the 2 kb upstream region of 16 SPX domain-containing genes coding sequences. Rounded rectangles with different colors indicate different cis-acting elements
Cis-regulatory elements in the 2 kb upstream region of 16 SPX domain-containing genes coding sequences. Rounded rectangles with different colors indicate different cis-acting elements.
Sixteen SPX-domain-containing genes palmitoylation prediction site
Sixteen SPX-domain-containing genes palmitoylation prediction site
SPX1-16 amount of gene expression original data
All the raw data about SPX gene expression after adding IAA under low phosphorus
SPX1-16 amount of gene expression original data
All the raw data about SPX gene expression in different tissues
MIQE checklist
Some details are in the manuscript and MIQE information files
The Minimum Information for Publication of Quantitative Real-Time PCR Experiments
MIQE essential information
To prove the validity of fluorescence quantitative test data and the quality of test materials