Genome-wide identification and expression analysis of growth-regulating factors in Dendrobium officinale and Dendrobium chrysotoxum
- Published
- Accepted
- Received
- Academic Editor
- Lin Zhang
- Subject Areas
- Bioinformatics, Molecular Biology, Plant Science
- Keywords
- Growth-regulating factor, Dendrobium officinale, Dendrobium chrysotoxum, Gene family, Expression profiles
- Copyright
- © 2023 Zhu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Genome-wide identification and expression analysis of growth-regulating factors in Dendrobium officinale and Dendrobium chrysotoxum. PeerJ 11:e16644 https://doi.org/10.7717/peerj.16644
Abstract
Background
Dendrobium, one of the largest genera in Orchidaceae, is popular not only for its aesthetic appeal but for its significant medicinal value. Growth-regulating factors (GRFs) play an essential role in plant growth and development. However, there is still a lack of information about the evolution and biological function analysis of the GRF gene family among Dendrobiumspecies.
Methods
Growth-regulating factors from Dendrobium officinale Kimura et Migo and Dendrobium chrysotoxum Lindl. were identified by HMMER and BLAST. Detailed bioinformatics analysis was conducted to explore the evolution and function of GRF gene family in D. officinale and D. chrysotoxum using genomic data, transcriptome data and qRT-PCR technology.
Results
Here, we evaluated the evolution of the GRF gene family based on the genome sequences of D. officinale and D. chrysotoxum. Inferred from phylogenetic trees, the GRF genes were classified into two clades, and each clade contains three subclades. Sequence comparison analysis revealed relatively conserved gene structures and motifs among members of the same subfamily, indicating a conserved evolution of GRF genes within Dendrobiumspecies. However, considering the distribution of orthologous DoGRFs and DcGRFs, and the differences in the number of GRFs among species, we suggest that the GRF gene family has undergone different evolutionary processes. A total of 361 cis-elements were detected, with 33, 141, and 187 related to plant growth and development, stress, and hormones, respectively. The tissue-specific expression of GRFs showed that DoGRF8 may have a significant function in the stem elongation of D. officinale. Moreover, four genes were up-regulated under Methyl-jasmonic acid/methyl jasmonate (MeJA) treatment, showing that DoGRFs and DcGRFs play a crucial role in stress response. These findings provide valuable information for further investigations into the evolution and function of GRF genes in D. officinale and D. chrysotoxum.
Introduction
To adapt to changes in the growing environment, almost all plants have developed a variety of mechanisms and complex signal networks to ensure their growth and development during long-term evolution, and transcriptional regulation of gene expression is an important component. Transcription factors (TFs), which act as master regulators of gene expression, have an impact on the development of land plants, including the establishment of metabolism, species differentiation, and plant reproduction (Shi et al., 2019). The majority of TFs in plants are related to gene families such as MYB, WRKY, and TCP. Among them, growth-regulating factors (GRFs) play an important role in plants. It has been proven to be involved in the growth and development of multiple plant organs, particularly in stems and leaves. Initially, studies on GRFs mainly focused on their function in the development of plant leaves and stems (Van der Knaap, Kim & Kende, 2000; Kim, Choi & Kende, 2003; Horiguchi, Kim & Tsukaya, 2005; Kim & Lee, 2006). However, recent research has discovered their involvement in other aspects of plant growth and development, including seed and root development (Bao et al., 2014; Debernardi et al., 2014), growth control under stress conditions (Pajoro et al., 2014; Liu et al., 2014), and regulation of plant longevity (Liang et al., 2014; Kim et al., 2012; Hewezi et al., 2012). Therefore, GRFs play a crucial role in the growth and development of plants.
Previous research has identified two conserved domains located in the N-terminal portion of GRF genes: QLQ and WRC (Van der Knaap, Kim & Kende, 2000; Omidbakhshfard et al., 2015). The WRC domain, unique to plants, is expected to be involved in DNA binding and TF targeting to the nucleus. It can bind with the cis-acting region to regulate gene expression (Choi, Kim & Kende, 2004; Zhang et al., 2008). On the other hand, the QLQ domain serves as a protein-protein interaction domain and can interact with the GRF-interacting factor (GIF) family to form the GRF-GIF complex. This complex activates transcription and regulates plant growth and development. For instance, AtGRF5 and AtGIF1 cooperate to promote the development of leaf primordia (Horiguchi, Kim & Tsukaya, 2005).
With an increasing number of high-quality genome sequences of plant species being published, the GRF gene family has become popular in molecular evolution analyses. The GRF family has been identified in various species, including Arabidopsis thaliana (L.) Heynh. (Kim, Choi & Kende, 2003), Brassica rapa var. glabra Regel (Wang et al., 2014), Zea mays L. (Zhang et al., 2008), and Oryza sativa L. (Choi, Kim & Kende, 2004). Dendrobiums, as an endangered orchid, grows in adverse conditions, e.g., epiphytic on cliffs or tree trunks, and distributed at high altitudes. Most of them have significant horticultural and medicinal values, such as Dendrobium officinale Kimura et Migo and Dendrobium chrysotoxum Lindl. (Zhu et al., 2018; Li et al., 2020; Niu et al., 2018). The stem of D. officinale, in particular, is a rare Chinese medicinal material with high market demand. OsGRF1, the first reported member of the GRF family, has been shown to regulate gibberellic acid-induced stem elongation and transcriptional activity (Van der Knaap, Kim & Kende, 2000). Therefore, it is crucial to understand the functions of GRFs in flowering, stem and leaf growth, seed formation, and root development in Dendrobiumspecies. However, the evolution of the GRF family among Dendrobiumspecies is still unknown. With the recent availability of chromosome-level genome sequences for D. officinale and D. chrysotoxum (Niu et al., 2021; Zhang et al., 2021), it is now possible to conduct a comprehensive study of the GRF gene family in these species.
Therefore, in this study, we employed bioinformatics techniques to search for GRF genes using the genome sequences of D. officinale and D. chrysotoxum as references. We characterized their sequence attributes, chromosomal locations, evolutionary relationships, and conducted syntenic and gene duplication analyses. Additionally, we predicted cis-elements, expression patterns, 3D protein structures, and protein-protein interaction networks of the GRF genes to uncover their potential biological functions. These findings will provide valuable insights into the GRF gene family in both Dendrobiumspecies and may pave the way for future research in this field.
Materials & Methods
Plant materials
The D. officinale and D. chrysotoxum used in this study were all from the well-growing rooting stage tissue culture seedlings in the Dendrobiums tissue culture Room, Institute of Plant and Environmental Resources, College of Life Sciences, Nanjing Normal University. D. officinale and D. chrysotoxum seedlings treated with 100 µM MeJA were used as the treatment group, and the seedlings with normal growth were used as the control group. After the treatment, the D. officinale and D. chrysotoxum seedlings were removed from the culture bottle, washed with water 2-3 times, and then absorbed with absorbent paper, and frozen in liquid nitrogen, and stored in an ultra-low temperature refrigerator at −80 °C for use.
Identification of GRFs in D. officinale and D. chrysotoxum genome
First, we downloaded the HMM profiles of the GRF gene family (PF00244) from the Pfam protein family database (http://pfam-legacy.xfam.org/). Using these profiles, we conducted a search for candidate GRF proteins in the two Dendrobiumspecies, with a parameter setting of E-value = 1e−5. Additionally, we obtained the GRFsequences of A. thaliana from the NCBI (https://www.ncbi.nlm.nih.gov/). These sequences were used in a BLASTP search to identify proteins in the two Dendrobiumspecies. The protein sequences obtained from both methods were integrated to obtain putative DoGRFs and DcGRFs. To ensure the presence of conserved domains, these sequences were submitted to the SMART (http://smart.embl-heidelberg.de/), NCBI-CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), and Pfam websites. Finally, we utilized the ExPASy software online (https://web.expasy.org/protparam/, Wilkins et al., 1999) to analyze the features of DoGRFs and DcGRFs, including molecular weight, gene distribution, theoretical isoelectric point, and length. The subcellular localization was predicted using Cell-PLoc v2.0 software online (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/).
Phylogenetic trees, gene motifs and structures
First, the GRF amino acid sequences of D. officinale, D. chrysotoxum, Phalaenopsis equestris (Schauer) Rchb. (Cai et al., 2015), Cymbidium ensifolium (L.) Sw. (Ai et al., 2021), and A. thaliana from NCBI (https://www.ncbi.nlm.nih.gov/) and NGDC (https://ngdc.cncb.ac.cn/) were aligned using MEGA7 (Kumar, Stecher & Tamura, 2016). The phylogenetic trees were constructed using the Neighbor-Joining method, with a bootstrap value of 1000, using the MEGA7 software. Next, we identified conserved motifs using the online MEME website (https://meme-suite.org/meme/, Bailey & Elkan, 1994), with a motif number of 10 and other parameters set to default. Additionally, we used the GSDS software online (http://gsds.gao-lab.org/index.php, Hu et al., 2015) to visualize the exon-intron structures of each sequence.
Evolution analysis of gene duplications and collinearity within Dendrobiums
To start, we aligned the DoGRFs and DcGRFs using BLASTN with a parameter setting of E-value threshold = 1e−20 against the genome sequence of the two Dendrobiumspecies. Next, based on the BLASTN results, we identified gene duplication events using MCScanX. The duplication events of DoGRFs and DcGRFs were visualized using the TBtools v1.6 software (Wang et al., 2012; Chen et al., 2020). Additionally, we determined the syntenic blocks between the two analyzed Dendrobiumspecies and other plants using the MCScanX software, with the parameter of cscore ≥ 0.7.
The calculation analysis of Ka and Ks
We used the software KaKs_Calculator v2.0 (Wang et al., 2010) to calculate the synonymous (Ks) value and non-synonymous (Ka) value. Additionally, we estimated the comparative ratio of Ka and Ks.
Promoter analysis
The upstream 1,500 bp genomic DNA sequences of GRF genes were extracted as putative promoters. These promoters were then submitted to the PlantCare database (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/, Lescot et al., 2002) for searching and analyzing the putative cis-elements. The total cis-elements were visualized using the TBtools software.
Expression profiles
To investigate the different expression patterns of the DoGRFs, we conducted a search in the online database of NCBI SRA for RNA-sequence data from root, stem, leaf, and flower. The login IDs for the expression data are SRR2014476, SRR2014396, SRR2014325, SRR2014297, SRR2014230, SRR2014227, SRR1917043, SRR1917042, SRR1917041, and SRR1917040 (Chen et al., 2017). Firstly, the download RNA-sequence data were converted to fastq format via fastq-dump of SRA toolkit.3.0.0. Then the clean reads were aligned and mapped to the D. officinale genome by Hisat2 v2.2.1. The sam data was converted to bam by SAMtools v1.14. The FPKM value of DoGRFs were calculated by StringTie v2.2.0 (Pertea et al., 2015) to estimate the transcript abundances. To visualize the expression patterns, we constructed a heat map using the heatmap package in RStudio v1.4.1717 software (RStudio Team, 2021).
Quantitative real-time PCR analysis of DoGRFs and DcGRFs
The extracted materials of RNA were reverse-transcripted by PrimeScript 1-strand cDNA synthesis kit (TaKaRa). Each reaction had a total volume of 20 µL, including SYBR Green I fluorescent dye 10 µL, primer (10 µM) 0.4 µL, cDNA 2 µL and ddH2O 7.2 µL. The reaction conditions were predenaturation at 95 °C for 30s, 40 cycles (95 °C 5 s, 60 °C 30s), and dissolution curve (95 °C 15 s, 60 °C 60 s, 95 °C 15 s) (Tables S2–S4). We designed the primers using SnapGene v6.0 software (http://www.snapgene.com), and calculated the expression data using the method inferred from Livak & Schmittgen (2002).
The prediction of 3D protein structure and interaction network analysis
We predicted the 3D structures of GRF proteins from D. officinale and D. chrysotoxum using the online software SWISS-MODEL (https://swissmodel.expasy.org/, Waterhouse et al., 2018). First, we aligned the GRF protein sequences using the STRING v11.0 database online (https://cn.string-db.org/cgi/input?sessionId=bMUfhtTbeC2f&input_page_show_search=on) to predict their relationships, and the regulatory networks were visualized using the Gephi v0.9.6 software (Von Mering et al., 2003).
Results
Identification and distribution of GRFs in D. chrysotoxum and D. officinale
A total of 37 GRF genes were identified from the genomes of D. officinale and D. chrysotoxum, with 19 and 18 GRFs identified using the methods of HMMER and BLASTP, respectively. There were differences in the characteristics of GRF genes between D. officinale and D. chrysotoxum. For example, the DoGRF proteins had a higher number of variable amino acids (ranging from 106 in DoGRF16 to 392 in DoGRF6) compared to DcGRF proteins (ranging from 86 in DcGRF8 to 321 in DcGRF3). The molecular weight of DoGRF proteins ranged from 11.7 kDa (DoGRF16) to 42.9 kDa (DoGRF6), which was higher than that of DcGRF proteins (ranging from 9.8 kDa in DcGRF8 to 37.1 kDa in DcGRF6). Additionally, the isoelectric point of DoGRF proteins (ranging from 4.19 in DoGRF16 to 10.07 in DoGRF12) was higher than that of DcGRF proteins (ranging from 4.02 in DcGRF13 to 9.28 in DcGRF10).
The DoGRFs and DcGRFs were distributed on seven and eight chromosomes, respectively, among the 19 assembled chromosomes of D. officinale and D. chrysotoxum. As shown in Tables 1–2 below, most DoGRFs and DcGRFs were evenly distributed among the chromosomes mentioned. Notably, Chromosome 10 (Chr10) exhibited the highest number of DcGRF genes (Table 2). In addition, almost all the GRF genes from D. officinale and D. chrysotoxum were predicted to be distributed in nucleus and cytoplasm, which were probably the main working region for GRF genes.
| No. | Gene name | Gene ID | Chr | Genomic location | Protein | Molecular weight (kDa) | Theoretical pI | Subcellular location |
|---|---|---|---|---|---|---|---|---|
| 1 | DoGRF1 | Dof000773 | 1 | 25271270-25289574 | 246 | 27.782 | 4.81 | N. |
| 2 | DoGRF2 | Dof001775 | 1 | 87182419-87197524 | 275 | 31.231 | 4.59 | N. |
| 3 | DoGRF3 | Dof007872 | 5 | 2696374-2767924 | 258 | 28.952 | 4.43 | N. |
| 4 | DoGRF4 | Dof007881 | 5 | 2922050-2950591 | 251 | 28.255 | 4.73 | N. |
| 5 | DoGRF5 | Dof011242 | 7 | 3937557-3941920 | 262 | 29.622 | 4.6 | N. |
| 6 | DoGRF6 | Dof011366 | 7 | 8699540-8742219 | 392 | 42.960 | 4.59 | C. |
| 7 | DoGRF7 | Dof011962 | 7 | 63779975-63798763 | 258 | 29.101 | 4.53 | N. |
| 8 | DoGRF8 | Dof014759 | 10 | 7700573-7705243 | 258 | 29.057 | 4.48 | N. |
| 9 | DoGRF9 | Dof014810 | 10 | 9466121-9476099 | 290 | 32.711 | 4.46 | N. |
| 10 | DoGRF10 | Dof016970 | 12 | 19189885-19205441 | 355 | 38.987 | 9.96 | C. N. |
| 11 | DoGRF11 | Dof016971 | 12 | 19206077-19241077 | 372 | 40.313 | 8.57 | C. |
| 12 | DoGRF12 | Dof016973 | 12 | 19402650-19434919 | 301 | 33.371 | 10.07 | C. N. |
| 13 | DoGRF13 | Dof021876 | 16 | 1828252-1846553 | 258 | 28.950 | 4.55 | N. |
| 14 | DoGRF14 | Dof021877 | 16 | 1848488-1849460 | 243 | 27.259 | 6.92 | C. N. |
| 15 | DoGRF15 | Dof022251 | 16 | 11590340-11593140 | 256 | 28.766 | 4.48 | N. |
| 16 | DoGRF16 | Dof023056 | 17 | 7309376-7318548 | 106 | 11.719 | 4.19 | N. |
| 17 | DoGRF17 | Dof023057 | 17 | 7318691-7321768 | 117 | 13.039 | 4.3 | N. |
| 18 | DoGRF18 | Dof023549 | 17 | 34990936-35016843 | 254 | 28.434 | 6.26 | C. N. |
| 19 | DoGRF19 | Dof026766 | UN | 158494-162784 | 257 | 29.258 | 5.1 | N. |
Notes:
- N
-
nucleus
- C
-
cytoplasm
| No. | Gene name | Gene ID | Chr | Genomic location | Protein | Molecular weight (kDa) | Theoretical pI | Subcellular location |
|---|---|---|---|---|---|---|---|---|
| 1 | DcGRF1 | KAH0449449 | 18 | 27696253-27735081 | 271 | 30.820 | 4.72 | N. |
| 2 | DcGRF2 | KAH0449492 | 18 | 90325059-90331686 | 260 | 29.437 | 4.46 | N. |
| 3 | DcGRF3 | KAH0453121 | 16 | 4317217-4350471 | 321 | 36.097 | 5.49 | N. |
| 4 | DcGRF4 | KAH0453427 | 16 | 4024734-4056002 | 257 | 28.954 | 4.43 | N. |
| 5 | DcGRF5 | KAH0453534 | 16 | 13763326-13768257 | 263 | 29.980 | 4.9 | N. |
| 6 | DcGRF6 | KAH0455432 | 14 | 36418564-36435531 | 320 | 37.198 | 8.05 | C. N. |
| 7 | DcGRF7 | KAH0455781 | 14 | 5996726-6009621 | 258 | 29.122 | 4.24 | N. |
| 8 | DcGRF8 | KAH0458321 | 12 | 1245507-1245858 | 86 | 9.887 | 4.41 | C. M. N. |
| 9 | DcGRF9 | KAH0458964 | 11 | 18249753-18250544 | 263 | 28.647 | 8.81 | C. N. |
| 10 | DcGRF10 | KAH0459081 | 11 | 18342320-18343057 | 245 | 27.582 | 9.28 | C. N. |
| 11 | DcGRF11 | KAH0459817 | 10 | 14861543-14863463 | 255 | 28.766 | 4.48 | N. |
| 12 | DcGRF12 | KAH0459925 | 10 | 2214094-2217553 | 264 | 29.288 | 4.9 | C. N. |
| 13 | DcGRF13 | KAH0460355 | 10 | 2202923-2212464 | 142 | 16.021 | 4.02 | N. |
| 14 | DcGRF14 | KAH0460481 | 10 | 2187485-2192245 | 257 | 29.302 | 5.32 | N. |
| 15 | DcGRF15 | KAH0464062 | 7 | 48667464-48672171 | 257 | 29.057 | 4.48 | N. |
| 16 | DcGRF16 | KAH0464557 | 7 | 46549349-46565636 | 265 | 30.168 | 4.74 | N. |
| 17 | DcGRF17 | KAH0468224 | 4 | 70550448-70567786 | 257 | 29.118 | 4.53 | N. |
| 18 | DcGRF18 | KAH0468498 | 4 | 4229841-4241919 | 261 | 29.606 | 4.6 | N. |
Notes:
- M
-
microbody
- N
-
nucleus
- C
-
cytoplasm
Phylogenetic analysis of DoGRFs and DcGRFs
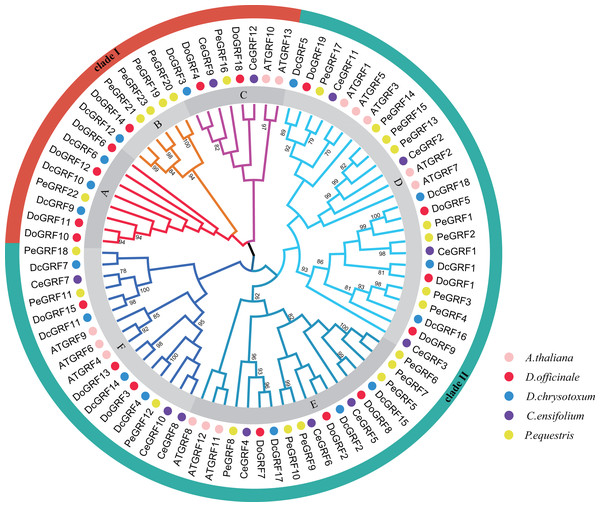
The phylogenetic relationships are crucial for understanding the possible evolution of DoGRFs and DcGRFs. Using the Neighbor-Joining method, a phylogenetic tree was constructed with a total of 81 GRF genes from five species: A. thaliana (13), D. officinale (17), D. chrysotoxum (16), Cymbidium ensifolium (L.) Sw. (12), and Phalaenopsis equestris (Schauer) Rchb. (23) using MEGA software. The results showed that the 81 GRFs were divided into two major clades, designated as clade I and clade II (Fig. 1). Clade I was further subdivided into three subclades, labeled as A, B, and C, containing 8, 6, and 8 GRF genes, respectively. Clade II was also divided into three subclades, labeled as D, E, and F, containing 25, 18, and 16 GRF genes, respectively. Within different subclades, most of the GRF genes from the two Dendrobiumspecies clustered together. Notably, we identified 8 pairs of orthologous genes with a close relationship (Bootstrap value >90), such as DoGRF14 and DcGRF12. This finding suggests a close relationship between the GRFs of Dendrobiumspecies. Furthermore, subfamilies A and B did not contain any AtGRF s or CeGRF s, whereas each of the other subfamilies included GRF genes from all five species. The number of GRF genes in different branches of closely related species was relatively consistent. Clade I included seven GRF genes from D. officinale and five GRF genes from D. chrysotoxum, respectively. Clade II included 10 GRF genes from D. officinale and 11 GRF genes from D. chrysotoxum, respectively. Considering the distribution of orthologous genes between D. officinale and D. chrysotoxum and the differences in the number of GRF genes among Dendrobiumspecies, we suggest that while the GRF gene family has undergone different evolutionary processes (gene loss or gain), the evolution of GRF genes remains conservative in closely related species.
Figure 1: Phylogenetic relationships of GRF genes in D. officinale, D. chrysotoxum, A. thaliana, C. ensifolium and P. equestris.
Neighbor-Joining phylogenetic tree was constructed by MEGA7 with 1000 bootstraps. Pink, red, blue, purple and yellow colors represent GRF protein sequences from A. thaliana (AT), D. officinale (Do), D. chrysotoxum (Dc), C. ensifolium (Ce) and P. equestris (Pe), respectively. Different subfamilies are shaded with different colors.Gene motifs and structures of DoGRFs and DcGRFs
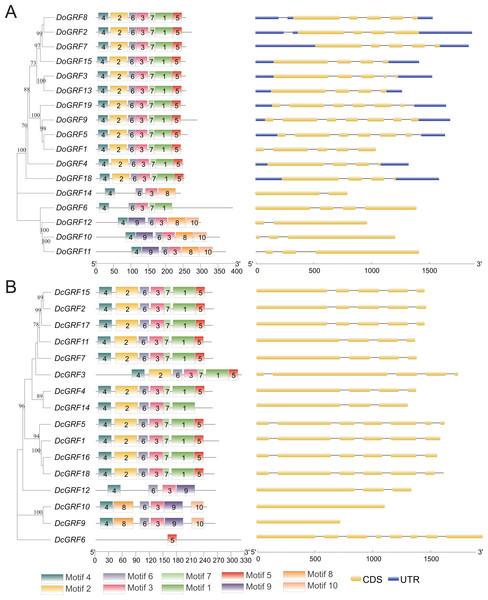
The amino acid sequences of 17 DoGRFs and 16 DcGRFs were used to construct phylogenetic trees. To further analyze their motif compositions, these sequences were submitted to the MEME website. The results revealed that both D. officinale and D. chrysotoxum exhibited 10 motifs within a length range of 14aa-50aa. However, upon examining the detailed sequence information, differences in motifs between the two species were observed. Figure 2 shows that motifs 1-7 were widely distributed in the majority of DoGRFs, while motifs 8-10 were found in only three genes. Similar distribution patterns were observed in D. chrysotoxum, with motifs 1-7 being relatively conserved and widespread among most DcGRFs, except for DcGRF6, which exhibited a unique distribution pattern with only 1 motif (Fig. 2 and Fig. S1).
Figure 2: Phylogenetic relationships, conserved motifs and exon-intron structures of GRF genes in D. officinale (A) and D. chrysotoxum (B).
The conserved motifs were identified using MEME and visualized by TBtools. Different colors represent 10 different motifs. Yellow and blue boxes are respectively indicating CDS and UTR.By referring to published genomic information, the structure of GRFs was further elucidated through exon-intron structure analysis. The results demonstrated that GRFs within the same species shared a highly similar structure. The lengths and numbers of exons clustered together in the phylogenetic tree were nearly identical, and the lengths of introns were also highly similar. This indicates that GRFs in both species have been evolutionarily conserved. However, compared to D. officinale, D. chrysotoxum had slightly fewer introns in its GRFs.
Gene duplication of DoGRFs and DcGRFs
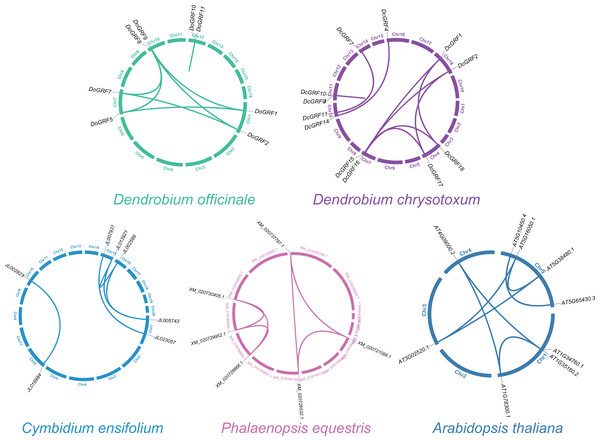
To investigate GRF gene duplication events and uncover potential evolutionary histories in D. officinale and D. chrysotoxum, BLASTN and MCScanX were employed for synteny analysis of GRF genes between the two species. The results revealed the presence of similar homologous gene pairs (eight pairs in D. officinale and nine pairs in D. chrysotoxum, as shown in Fig. 3) and approximate replication patterns. Detailed examination showed that segmental duplications were widely distributed in both species, with a clear predominance, while tandem duplications were also observed in one gene pair in both D. officinale and D. chrysotoxum. Therefore, segmental duplication was the main mechanism contributing to the expansion of DoGRFs and DcGRFs.
Figure 3: Schematic representations of the gene duplications of GRF genes from five different plants.
The Ka/Ks ratio, which measures the frequency of non-synonymous (Ka) and synonymous (Ks) substitutions in homologous pairs of DoGRFs and DcGRFs, was used to assess the presence of selection pressure. Among the eight pairs of D. officinale GRF genes, seven pairs exhibited purifying selection effects, while one pair had a Ka/Ks ratio greater than one, indicating positive selection effects (Table 3).
| Seq_1 | Seq_2 | Ka | Ks | Ka/Ks | Duplication type |
|---|---|---|---|---|---|
| DoGRF1 | DoGRF5 | 0.04546 | 0.959153 | 0.047396 | Segmental duplication |
| DoGRF1 | DoGRF9 | 0.062838 | 2.52223 | 0.024914 | Segmental duplication |
| DoGRF2 | DoGRF7 | 0.987025 | 1.03655 | 0.952224 | Segmental duplication |
| DoGRF2 | DoGRF8 | 0.059869 | 0.928183 | 0.064502 | Segmental duplication |
| DoGRF3 | DoGRF13 | 0.061013 | 1.01233 | 0.06027 | Segmental duplication |
| DoGRF5 | DoGRF9 | 0.978744 | 1.07199 | 0.913013 | Segmental duplication |
| DoGRF7 | DoGRF8 | 0.052936 | 2.86335 | 0.018487 | Segmental duplication |
| DoGRF10 | DoGRF11 | 0.108897 | 0.102434 | 1.06309 | Tandem duplication |
| DcGRF11 | DcGRF7 | 0.0924322 | 1.43463 | 0.0644291 | Segmental duplication |
| DcGRF18 | DcGRF1 | 0.0781159 | 1.08391 | 0.0720686 | Segmental duplication |
| DcGRF18 | DcGRF16 | 0.0735203 | 3.65543 | 0.0201126 | Segmental duplication |
| DcGRF9 | DcGRF10 | 0.994268 | 1.0153 | 0.979283 | Tandem duplication |
| DcGRF15 | DcGRF2 | 0.0285821 | 0.935346 | 0.0305577 | Segmental duplication |
| DcGRF16 | DcGRF1 | 0.0743845 | 2.03182 | 0.0366098 | Segmental duplication |
| DcGRF14 | DcGRF4 | 0.217967 | 1.44347 | 0.151002 | Segmental duplication |
| DcGRF17 | DcGRF15 | 0.0560215 | 2.47396 | 0.0226445 | Segmental duplication |
| DcGRF17 | DcGRF2 | 0.0501976 | 1.67991 | 0.0298811 | Segmental duplication |
Notes:
Synonymous (Ks) and nonsynonymous (Ka) substitution rates of duplicate gene pairs (Ka/Ks ratios).
In addition, we compared the replication events between two Dendrobiums and other three species (A. thaliana, C. ensifolium, and P. equestris) to further understand the replication event of GRFs. A total of five, six and seven paralogous genes were detected among C. ensifolium, P. equestris and A. thaliana, respectively. Among these paralogs, all the gene pairs experienced a negative selection (Ka/Ks <1), which were conserved (Fig. 3 and Table S5).
Syntenic analysis of DoGRFs and DcGRFs
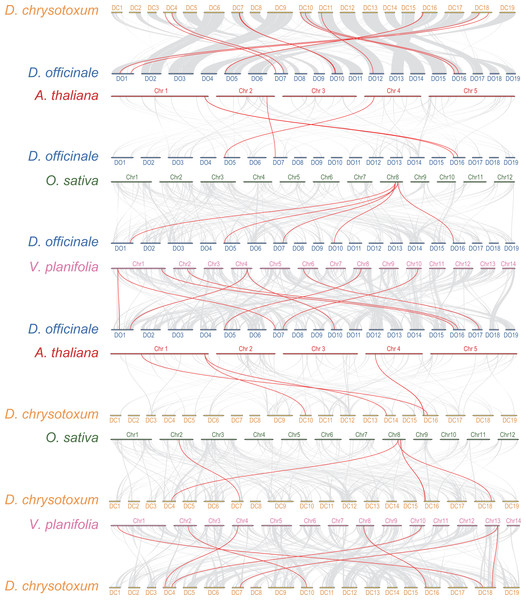
Interspecific collinearity analysis provides valuable insights into the evolution of gene families. We conducted collinearity analysis between DoGRFs and DcGRFs, and further examined their collinear relationships with A. thaliana, O. sativa, and Vanilla planifolia Andrews, as depicted in Fig. 4. (i) Collinear analysis revealed that D. officinale and D. chrysotoxum exhibited the highest number of homologous genes, with a total of 12 gene pairs. Specifically, there were five pairs of homology genes between D. officinale and O. sativa, nine pairs of homology genes between D. officinale and V. planifolia, and only four pairs of homology genes between D. officinale and A. thaliana. Similarly, there were five pairs of homology genes between D. chrysotoxum and O. sativa, seven pairs of homology genes between D. chrysotoxum and V. planifolia, and five pairs of homology genes between D. chrysotoxum and A. thaliana. The results indicate that the GRF gene families of monocots and dicots, such as O. sativa and A. thaliana, show relatively fewer differences, while more collinear relationships are observed among orchids. (ii) DoGRF13 in D. officinale and DcGRF3 in D. chrysotoxum exhibited homologous genes with the other four plants, suggesting a common ancestor predating the divergence of monocots and dicots and indicating functional conservation and importance. Excluding the influence of the dicot A. thaliana, it was observed that DoGRF7, DoGRF2, and DoGRF8 in D. officinale, as well as DcGRF17, DcGRF2, and DcGRF15 in D. chrysotoxum, displayed homologous genes in the other three monocots, suggesting relative conservation in monocot evolution. Furthermore, these six genes corresponded to collinear results between D. officinale and D. chrysotoxum (DcGRF17-DoGRF7, DcGRF2-DoGRF2, DcGRF15-DoGRF8). (iii) Compared to O. sativa and A. thaliana, orchids, including D. officinale and D. chrysotoxum, exhibited a significant doubling in the number of GRF genes. For instance, the gene LOC_Os08g33370 in O. sativa displayed collinearity with three genes (DoGRF7, DoGRF2, DoGRF8) in D. officinale and two genes (DcGRF17, DcGRF2) in D. chrysotoxum, and numerous similar cases were observed. Additionally, even within the orchid family, D. officinale and D. chrysotoxum exhibited a doubling compared to vanilla orchid. For example, the gene Vpl04Ag09642 in V. planifolia displayed collinearity with two genes (DoGRF8, DoGRF2) in D. officinale.
Figure 4: Collinearity analysis of GRF genes in D. officinale, D. chrysotoxum and three other plants, including A. thaliana, O. sativa and V. planifolia.
Grey lines indicate the collinear blocks. Red lines indicate the collinear blocks of GRF genes.Analysis of DoGRFs and DcGRFs promoter
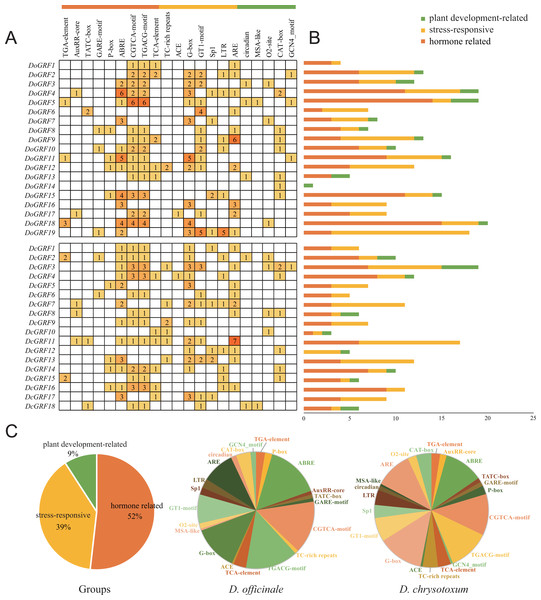
To gain a better understanding of the potential functions of DoGRFs and DcGRFs, we identified cis-elements within the 1,500 bp upstream regions of the initiation codon (ATG). After excluding non-functional terms, a total of 361 cis-elements in the promoter regions of DoGRFs and DcGRFs were categorized into three groups: plant development-related (9%), stress-responsive (39%), and hormone-related (52%).
Within the plant growth and development category (33/361), we identified five cis-elements involved in endosperm expression (GCN4-motif), cell cycle regulation (MSA-like), meristem expression (CAT-box), circadian control (circadian), and zein metabolism regulation (O2-site), with CAT-box accounting for the largest proportion.
In the stress responsiveness category (141/361), we identified cis-elements responsive to light (ACE, G-box, GT1-motif, and Sp1), low-temperature (LTR), defense and stress (TC-rich repeats), and anaerobic induction (ARE). Additionally, more than half of the cis-elements (187/361) were related to phytohormones, responding to various phytohormones such as ABA, auxin, GA, MeJA, and salicylic acid. Notably, MeJA-responsive and light-responsive cis-elements were the most abundant in both D. officinale and D. chrysotoxum.
These results suggest that MeJA-induced or suppressed GRF genes, along with those responding to various abiotic stresses, may play a role in photosynthesis (Fig. 5).
Figure 5: Information of cis-acting elements in GRF genes of D. officinale and D. chrysotoxum.
(A) The gradient orange colors and numbers in the grid indicate the number of different cis-elements. (B) The different colors histogram indicates the number of cis-elements in each category. (C) The ratio of different cis-acting elements in D. officinale and D. chrysotoxum is shown as pie charts.Expression patterns of GRFs in different tissues and under MeJA treatments
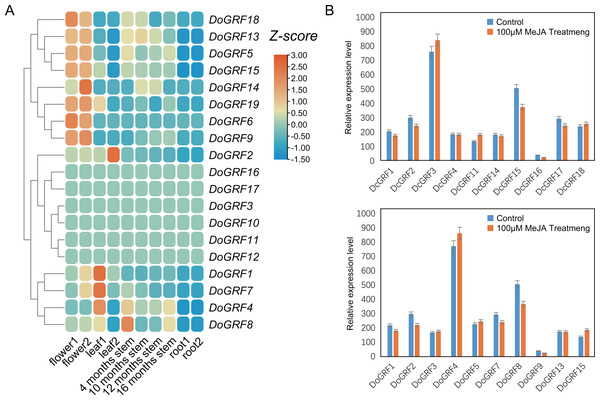
To investigate the potential biological functions of GRFs in D. officinale, we analyzed the tissue-specific expression of DoGRFs using transcriptome data and created a heat map (Fig. 6A) based on FPKM values from roots, leaves, flowers, and stems at four different growth stages of D. officinale. The heat map revealed that more than half of the DoGRFs were expressed in stems, flowers, and leaves, but not in roots of D. officinale. Different expression patterns were observed in the four stages of stem development, with most genes showing the highest expression at 4 months. Previous studies have associated GRFs with stem elongation (Van der Knaap, Kim & Kende, 2000). Additionally, cis-acting element analysis showed that the CAT-box, related to stem and root meristem expression, accounted for the highest proportion of growth and development-related elements. Considering the presence of gibberellin-related elements and CAT-box, it can be speculated that DoGRF8 may play a significant role in stem elongation in D. officinale.
Figure 6: Expression analysis of GRF s in different tissues and MeJA treat.
(A) Expression profiles of GRF genes of D. officinale in different tissues including root, stem, leaf and flower. Z-score transformed FPKM values. (B) Relative expression levels of DoGRF s and DcGRF s under MeJA treatments.Furthermore, cis-element analysis revealed a significant number of MeJA response elements within DoGRFs and DcGRFs. To explore the potential biological functions of GRFs under MeJA treatment, we selected 10 DoGRFs and 10 DcGRFs based on the expression results mentioned above and determined their expression levels using qRT-PCR (Fig. 6B). Among them, four genes were up-regulated, ten were down-regulated, and the remaining GRFs showed no significant changes in expression levels. These results suggest that MeJA treatment may affect the proper functioning of GRF genes in Dendrobiums.
3D structure prediction of GRF proteins of D. officinale and D. chrysotoxum
To explore the effect of protein structure on function, 19 DoGRFs and 18 DcGRFs were submitted to the SWISS-MODEL website for protein 3D structure prediction. Ultimately, 24 high-quality models with more than 30% consistency were generated (Table S1). The QMEAN DisCo Global value and GMQE value provided by the SWISS-MODEL website serve as quality evaluation standards. The QMEAN DisCo Global values of DoGRFs ranged from 0.76 to 0.87, and the GMQE values ranged from 0.72 to 0.88. The QMEAN DisCo Global values of DcGRFs ranged from 0.80 to 0.87, and the GMQE values ranged from 0.61 to 0.88. Overall, the models exhibited good quality. Detailed data can be found in the attached table.
All 24 constructed models were Hom-Dimer Oligo-State, indicating a relatively conserved function. In both D. officinale and D. chrysotoxum, the GRF gene family exhibited two different protein structures due to variations in rotation angles. Similar protein structures are likely to have similar functions, while different protein structures may contribute to the functional diversity of GRFs in Dendrobiums.
Protein-protein interaction networks of DoGRFs and DcGRFs
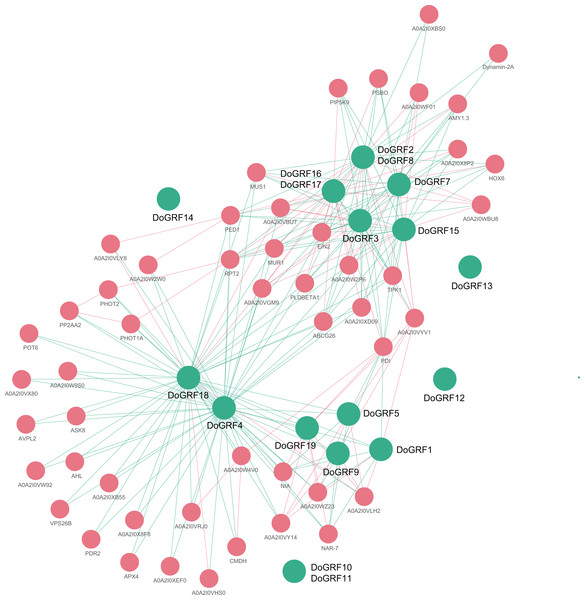
In order to gain a better understanding of the potential biological functions and regulatory networks of GRF genes, we predicted and constructed interaction networks between GRF proteins and related proteins in D. officinale and D. chrysotoxum, respectively. Our findings revealed complete consistency in the interactions between related proteins of both species, identifying a total of 65 related proteins and 233 connections. Among them, DoGRF18 protein interacted with 43 proteins, while DoGRF18 protein interacted with 38 proteins (including GRF proteins and related proteins), suggesting their involvement in multiple biological processes. On the other hand, five GRF proteins did not show any connections to related proteins. Additionally, based on homology and co-expression analysis, DoGRF12, DoGRF16, DoGRF17, and PBSO (oxygen-evolving enhancer protein 1, chloroplastic) exhibited the closest interaction relationship, with CMDH (Malate dehydrogenase) also being among the related proteins. PBSO and CMDH are known to play essential roles in photosynthesis. Hence, our results indicate that plant growth and development, encompassing multiple processes, may represent the most significant function of DoGRFs and DcGRFs (refer to Fig. 7 for details).
Figure 7: Protein-protein interaction (PPI) networks of GRF proteins in D. officinale.
Green and pink circles represent GRF and related proteins, respectively.Discussion
The evolution of GRFs is conserved within Dendrobium genus
The GRF family, a group of small transcription factors, plays crucial roles in various plant biological processes, including phytohormone responses, regulation of growth and development, and stress responses (Vercruyssen et al., 2015). For instance, Hewezi et al. (2012) focused on the study of GRFs in A. thaliana and found that highly expressed AtGRF1 and AtGRF3 in roots had a balanced expression that affected root growth. Gibberellin treatment, as a plant hormone, has been shown to increase the expression of several GRFs in rice and B. rapa. Additionally, AtGRF7 mutants exhibit greater tolerance to drought and salinity stress compared to wild-type and AtGRF7 overexpressor lines (Liu et al., 1998; Kim et al., 2012). While the genome-wide identification of GRFs has been reported in various plant species, such as nine genes in A. thaliana, 13 genes in O. sativa, and 17 genes in Z. mays (Kim, Choi & Kende, 2003; Choi, Kim & Kende, 2004; Zhang et al., 2008), studies on the evolution and function of GRFs in Dendrobiumspecies are still lacking despite the availability of high-quality D. officinale and D. chrysotoxum genome sequences.
The GRF family has been documented to undergo significant expansions/contractions among different plant lineages. For example, there are a total of nine and nine GRF genes in Vitis vinifera and A. thaliana, respectively (Hu et al., 2023; Kim, Choi & Kende, 2003), while 17 genes are found in B. rapa (Wang et al., 2014). On the contrary, Z. mays and Gossypium raimondii have 17 and 19 GRF genes, respectively (Zhang et al., 2008; Cao et al., 2020), whereas Sorghum bicolor has eight genes (Shi et al., 2022). Comparative analysis reveals significant expansion/contraction events among these species. In our study, we identified 19 and 18 GRFs in D. officinale and D. chrysotoxum, respectively. Although the gene numbers of GRFs vary between Dendrobium orchids and A. thaliana, P. equestris, and C. ensifolium, the evolution of the GRF gene family remains conserved within the genus of Dendrobium. For example, (i) the GRF genes among Dendrobium species have formed eight pairs of orthologous genes, which account for 43% of the total GRF genes. Importantly, we identified a pair of positively selected genes (DoGRF10 and DoGRF11), suggesting that DoGRFs have undergone positive selection pressure. These findings directly demonstrate the conservation of GRF evolution among Dendrobiumspecies. (ii) Collinearity analysis suggests that the GRF genes have experienced both expansion and contraction events in other plant lineages, but the most abundant homologous genes are found between D. officinale and D. chrysotoxum. (iii) A total of 17 gene duplications, with 8 and 9 repeats, were identified in D. officinale and D. chrysotoxum, respectively, indicating that gene duplication has been a driving force for GRF gene evolution, leading to a conserved gene family among Dendrobium orchids.
The GRF gene family are important for plant development, stress response and hormone response among Dendrobium species
The GRF genes are members of an important plant-specific family that have been studied for their crucial role in central developmental processes in plants, including stem and leaf development, seed formation, flowering, and root development. For example, AtGRF4 of A. thaliana has been reported to have various functions, such as cell proliferation in leaves, the shoot meristemless/stm mutant phenotype, and embryonic development of cotyledons (Kim & Lee, 2006; Gonzalez, Beemster & Inzé, 2009). Pajoro et al. (2014) revealed the role of miR396a in flower formation in A. thaliana, where it regulates GRF transcript levels and determines sepal-petal identity. Additionally, a regulatory network involving miR396 and its targets, including bHLH74 and GRFs, plays a central role in normal root growth and development (Debernardi et al., 2012; Bao et al., 2014). Recent research has highlighted the significant effects of GRF genes in photosynthesis, phytohormone signaling, and growth under adverse environmental conditions. For instance, (1) AtGRF5 stimulates chloroplast division, leading to an increase in the number of chloroplasts per cell in 35S:GRF5 leaves and a consequent increase in chlorophyll levels, thereby maintaining a higher rate of photosynthesis (Vercruyssen et al., 2015). (2) Van der Knaap, Kim & Kende (2000) first reported that the GRF member OsGRF1 regulates GA3-induced stem elongation and transcriptional activity (Kim & Kende, 2004). (3) Further functional classification of the putative downstream targets of AtGRF1 and AtGRF3 has revealed that most of them are involved in defense responses and disease resistance processes (Liu et al., 2014).
Consistently, our results confirm that GRF genes have diverse biological functions related to plant development, stress response, and hormone signaling. For example, (i) GRFs play an important role in plant development. In our study, we detected 33 cis-elements involved in plant development, accounting for 9.14% of all predicted cis-elements. For instance, the expression of DoGRF8 was closely related to stem development in D. officinale. Similar results were observed for DoGRF1, DoGRF2, DoGRF7, and DoGRF14, which were related to the development of flowers and leaves. (ii) As epiphytes growing at high altitudes above 800m, Dendrobiumspecies have developed mechanisms to accumulate anti-stress substances, enhancing their ability to respond to harsh environments. In our study, we identified 141 cis-elements involved in stress response, accounting for 39.06% of all detected cis-elements. Moreover, based on our analysis of MeJA treatment, we found that DoGRF4 and DoGRF15, which have been documented in D. officinale, were up-regulated, indicating their enhanced function in stress response in harsh habitats. (iii) We identified a total of 46 and 39 cis-elements involved in light responsiveness in D. officinale (23.12%) and D. chrysotoxum (24.07%), respectively, which may be related to the special photosynthetic pathway of Dendrobiums.
The biological function of GRF gene family were closely related to the protein structure, gene evolution or duplication events and protein interaction
As reported by Wang et al. (2022), different gene families exhibit different functions, and even the same gene family may have various functions. Consequently, in this study, we found that the GRF genes contain diverse biological functions. Our comparative analysis suggests that the biological function of the GRF gene family is closely linked to protein structure, gene evolution or duplication events, and protein interactions.
Firstly, we detected a total of 24 distinct 3D structures of GRFs, indicating diverse biological functions among Dendrobiumspecies. Secondly, gene evolution and duplication events also affect the biological function of GRF genes. For example, (i) DoGRF13 and DcGRF3 show homologous relationships with A. thaliana, O. sativa, V. planifolia, and each other; (ii) DoGRF7, DoGRF2, DoGRF8 and DcGRF17, DcGRF2, DcGRF15 show homologous relationships with O. sativa, V. planifolia, and each other; (iii) Collinearity analysis detected 3 pairs of GRFs with close relationships among Dendrobiumspecies (DcGRF17-DoGRF7, DcGRF2-DoGRF2, DcGRF15-DoGRF8). These results indicate that GRFs have a conserved evolutionary history within the Dendrobium genus. However, GRFs also show a diversified evolutionary history among orchid species and other plant lineages. For example, (i) Vpl04Ag09642 of V. planifolia has homologous pairs with two DoGRFs (DoGRF8 and DoGRF2); (ii) AT1G78300 of A. thaliana has homologous pairs with two DcGRFs (DcGRF11 and DcGRF7). Considering the conserved evolutionary history within the Dendrobium genus but diversified evolutionary history among different plant lineages, we suggest that gene evolution and duplication events affect the biological function of GRF genes.
Thirdly, interactions between different GRF proteins also affect their biological functions. We detected a total of 233 interactions between 15 GRF proteins and 50 related proteins. Among them, three DoGRFs (DoGRF12, DoGRF16, and DoGRF17) have the closest interaction relationship with PBSO (oxygen-evolving enhancer protein 1, chloroplastic). CMDH (Malate dehydrogenase) is also present in related proteins, indicating a possible correlation between GRFs and photosynthesis in Dendrobium s. Therefore, we suggest that the biological function of the GRF gene family is closely related to protein structure, gene evolution or duplication events, and protein interactions.
Conclusions
In the current investigation, we identified and verified a total of 19 DoGRFs and 18 DcGRFs in the genomes of D. officinale and D. chrysotoxum, respectively. The DoGRFs and DcGRFs are distributed randomly across various chromosomes and classified into six subfamilies. We conducted a comprehensive analysis of gene structure, molecular evolution, interaction networks, and expression profiles to gain insights into the evolution of GRF genes in studied Dendrobiumspecies. Our findings provide important information on the evolution of GRF genes in Dendrobiumspecies.
Supplemental Information
The detailed information of the predicted three-dimensional (3D) proteins
Primer sequences for qRT-PCR
The raw data showed that four genes were up-regulated under Methyl-Jasmonic Acid/Methyl Jasmonate (MeJA) treatment, which means DoGRFs and DcGRFs play a crucial role in stress response.
Ka, Ks and Ka/Ks values for duplication gene pairs in C. ensifolium, P. equestris and A. thaliana
Synonymous (Ks) and non-synonymous (Ka) substitution rates of duplicate gene pairs (Ka/Ks ratios).