Comprehensive genomic characterisation of the NAC transcription factor family and its response to drought stress in Eucommia ulmoides
- Published
- Accepted
- Received
- Academic Editor
- Imren Kutlu
- Subject Areas
- Agricultural Science, Bioinformatics, Genomics, Molecular Biology, Plant Science
- Keywords
- Eucommia ulmoides, NAC family, Drought-responsive, Gene expression, Phylogenetic analysis
- Copyright
- © 2023 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Comprehensive genomic characterisation of the NAC transcription factor family and its response to drought stress in Eucommia ulmoides. PeerJ 11:e16298 https://doi.org/10.7717/peerj.16298
Abstract
The NAC transcription factor family enhances plant adaptation to environmental challenges by participating in signalling pathways triggered by abiotic stressors and hormonal cues. We identified 69 NAC genes in the Eucommia ulmoides genome and renamed them according to their chromosomal distribution. These EuNAC proteins were clustered into 13 sub-families and distributed on 16 chromosomes and 2 scaffolds. The gene structures suggested that the number of exons varied from two to eight among these EuNACs, with a multitude of them containing three exons. Duplicated events resulted in a large gene family; 12 and four pairs of EuNACs were the result of segmental and tandem duplicates, respectively. The drought-stress response pattern of 12 putative EuNACs was observed under drought treatment, revealing that these EuNACs could play crucial roles in mitigating the effects of drought stress responses and serve as promising candidate genes for genetic engineering aimed at enhancing the drought stress tolerance of E. ulmoides. This study provides insight into the evolution, diversity, and characterisation of NAC genes in E. ulmoides and will be helpful for future characterisation of putative EuNACs associated with water deficit.
Introduction
Eucommia ulmoides Oliver is a highly valued tertiary relict perennial dicotyledonous tree species native to China (Deng et al., 2022). It is widely used in industry because it not only produces wood but is also a valuable raw biomaterial for extracting the active ingredients of Chinese medicine and trans-rubber (Wei et al., 2021; Zhu & Sun, 2018). Compared with cis-rubber, trans-rubber has unique characteristics, such as high hardness, resistance to acid and alkali corrosion, good insulation, and a low thermal expansion/contraction coefficient (Du et al., 2023; Enoki, Doi & Iwata, 2003; Kent & Swinney, 1966; Rose & Steinbuchel, 2005). To improve the viscosity, resilience, elasticity, weather resistance, and tensile strength of trans-rubber, it can be made into cis-rubber by vulcanisation (Yan, 1995). The vulnerability of Hevea brasiliensis to pests and diseases, as well as its narrow habitat, has led to significant challenges for the rubber industry (Tang et al., 2016). E. ulmoides has wide adaptability, few pests and diseases, and its leaves, bark, and pericarp are rich in trans-rubber. Therefore, E. ulmoides is considered an ideal alternative or complementary tree species to H. brasiliensis (Guo et al., 2023; Wuyun et al., 2018). During the growth of E. ulmoides, some environmental factors, such as drought and low-temperature stress, can prevent its full genetic potential, resulting in reduced yield and even plant death (Zuo et al., 2022). The identification and utilisation of resistance genes is the basis for breeding resistant varieties. Transcription factors (TFs), which activate or inhibit their expression by specifically binding to cis-acting elements on the promoters of target genes, play an important role in many biological processes (Oksuz et al., 2023; Yuan et al., 2020). As a plant-specific supergene family, NAC has been demonstrated to play a key role in plant growth and development and in the response to abiotic stress (Du et al., 2022; Hussain et al., 2017). Notably, NAC is very important in plant adaptation to land (Xu et al., 2014). Therefore, NAC family genes have been widely studied in many species. However, the identification and analysis of NAC genes in E. ulmoides have not been emphasised.
The NAC (no apical meristem (NAM), Arabidopsis transcription activator (ATAF1/ATAF2), and cup-shaped cotyledon (CUC2)) family is one of the largest gene families in plants. The N-terminus region has a highly conserved NAM domain, and the C-terminus consists of variable transcriptional regulatory regions, the latter of which have been implicated in specific biological functions (Shao, Wang & Tang, 2015). Increasing evidence suggests that NAC TFs have multiple functions in plant-responses to biotic and abiotic stress. SlNAC1 is involved in the process of fruit softening and fruit pigmentation based on the phytohormone pathway (Ma et al., 2014). NAC13 has important significance in popular responses to salt stress (Zhang et al., 2019). In wheat, overexpression of TaNACL-D1 and TaNAC071-A improves resistance to Fusarium head blight (Perochon et al., 2019) and drought (Mao et al., 2022), respectively; TaNAC30 negatively regulates stripe rust (Wang et al., 2018). Furthermore, TaNAC29 improves salt tolerance by strengthening the antioxidant system (Xu et al., 2015). GhirNAC2 regulates ABA biosynthesis and stomatal closure by regulating GhNCED3a/3c expression, thus playing an active role in cotton drought resistance (Shang et al., 2020). Although NAC TFs are related to various developmental processes and stress responses in plants, the specific functions of most NAC genes remain obscure, especially in E. ulmoides.
The chromosome-level genome of E. ulmoides was recently sequenced (Li et al., 2020); this provides the opportunity to systematically study the NAC gene family and to explore the potential functional involved in E. ulmoides biotic and abiotic responses. In the present study, we performed genome-wide identification and characterisation of NAC proteins based on the genome of E. ulmoides. In addition, we surveyed their expression under drought stress by transcriptome sequencing. This study will lay the foundation for further studies of the molecular mechanisms of NAC TFs in E. ulmoides response to drought stress.
Materials & Methods
Identification of EuNAC proteins from the E. ulmoides genome
The complete genome data of E. ulmoides were obtained from the Gene Warehouse (https://ngdc.cncb.ac.cn/gwh/Assembly/25206/show). The NAC protein sequences of Arabidopsis thaliana were obtained from Arabidopsis Information Resources (TAIR, https://www.arabidopsis.org/index.jsp), and the protein sequences of poplar and Oryza sativa were both derived from the Ensembl Plants website (http://plants.ensembl.org/index.html). The Hidden Markov model (HMM) files of the NAC domain (PF01849) and the NAM domain (PF02365) were obtained from the Pfam database (https://pfam.sanger.ac.uk), which were used for identification analysis. HMMER 3.3.2 (http://hmmer.org/) was then employed to scan the NAC proteins from the E. ulmoides genome with the default parameters. The candidate EuNACs were further validated by the NCBI Conserved Domain Search Service (CD Search) (https://www.ncbi.nlm.nih.gov), SMART (http://smart.embl-heidelberg.de), and Pfam database. Proteins without NAC and NAM domains and duplicates were manually deleted. The molecular weight (MW) and isoelectric point (pI) of each protein were analysed using the ExPASy pI/Mw tool (https://www.expasy.org).
Phylogenetic analysis of EuNAC proteins
The A. thaliana NAC protein sequences were downloaded from the TAIR database (http://www.Arabidopsis.org). Full-length protein sequence multiple alignments were performed using the ClustalW programme (Larkin et al., 2007). MEGA 6.0 software (Hall, 2013) was employed to construct an unrooted phylogenetic tree of E. ulmoides and A. thaliana NAC proteins using the neighbour-joining (NJ) method with 1,000 bootstrap iterations. All EuNAC proteins were classified according to the NAC protein classification criteria in A. thaliana (Ooka et al., 2003).
Conserved motif and gene structure analysis of EuNAC genes
The Gene Structure Display Server (GSDS; http://gsds.cbi.pku.edu.cn/) programme was used to explore the exon/intron structure pattern of the EuNAC genes by comparing their predicted coding sequence with the corresponding full-length gDNA sequence. Multiple Expectation Maximization for Motif Elicitation (MEME) (http://meme-suite.org/) programmes were employed to identify the conserved domains for candidate EuNAC proteins with default parameters. The conserved motifs and exon/intron structure were visualised using Tbtools (Chen et al., 2020).
Genome distribution, selective pressure, and synteny analysis of EuNAC genes
The location of each EuNAC gene on the chromosome was determined based on the E. ulmoides genome annotation file and visualised using TBtools software (Chen et al., 2020). MCScan X software (Wang et al., 2012) with default parameters was employed to analyse duplication events, and the intra-species and inter-species collinearity relationships. Circos software (Krzywinski et al., 2009) and TBtools were used for visualisation. TBtools were also used to calculate the nonsynonymous (Ka) and synonymous (Ks) rates of EuNAC homologous genes. The selection pressure acting on the gene pairs was calculated based on the Ka/Ks ratio, and the dates of each duplication event were further deduced with the formula T = Ks/2 λ, the mean synonymous substitution rate (λ) was assumed to be 6.5 × 10−9 (Liu et al., 2021; Lynch & Conery, 2000).
Promoter region analysis of EuNAC genes
The 2-kb promoter sequences upstream of the EuNAC genes start codon (ATG) were extracted, and the cis-acting elements and their potential related functions were predicted with the PlantCARE online server (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). The 20 cis-acting elements with the highest frequency were visualised using Tbtools (Chen et al., 2020).
Expression analysis of EuNAC genes under drought stress
Two-year-old ‘Qinzhong 1’ grafted potted plants with consistent growth were placed in the Agricultural College of Shihezi University and well managed in the natural environment. Three months later, they were subjected to drought stress treatment. For drought treatment, the soil was saturated with water, and watering was terminated. Leaves were collected at 0, 15, 30, and 45 d and labelled CK, D15, D30, and D45, respectively. Each sample was pooled from six individual plants, and three biological replicates were set for each treatment. Samples were quickly cleaned with distilled water, immediately frozen in liquid nitrogen, and stored at −80 °C for future use.
The above samples were submitted to Beijing Novogen Bioinformatics Technology Co., Ltd. (Beijing, China) for cDNA library construction and transcriptome sequencing. The data were uploaded to the National Center for Biotechnology Information (NCBI) Sequence Read Archive, with accession number PRJNA958614. Gene expression levels were normalised with FPKM (fragment per kilobase per million mapped reads) and visualised with TBtools.
Results
Identification of EuNAC proteins from the E. ulmoides genome
We identified 69 NAC genes in the E. ulmoides genome, named EuNAC1 to EuNAC69, according to their order on the chromosomes (Table S1). The EuNAC proteins varied significantly in length and molecular weight. The length of proteins encoded by EuNAC genes ranged from 86 (EuNAC50) to 617 (EuNAC64) amino acids (Jeong et al., 2008) and the molecular weight varied from 9.81 (EuNAC50) to 70.46 kDa (EuNAC64); the isoelectric points (pIs) ranged from 4.51 (EuNAC59) to 10.01 (EuNAC2). This study also analysed other basic information about 69 NAC genes, including homologous genes in A. thaliana, open reading frame (ORF) length, location coordinates, chromosomal positions, and exon numbers (Table S1).
Phylogenetic analysis and classification of EuNAC proteins
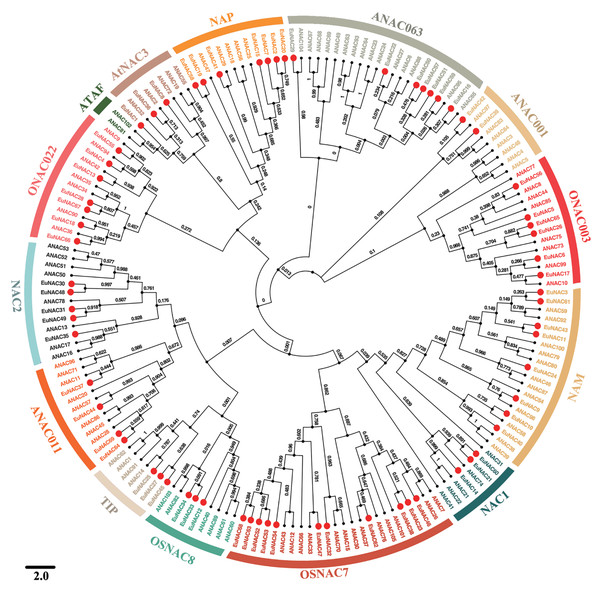
To investigate the evolutionary relationships among EuNAC family genes, MEGA6.0 software was employed to construct a neighbour-joining phylogenetic tree based on full-length protein sequences of NAC in E. ulmoides and A. thaliana (Fig. 1) (Hall, 2013). According to the NAC classification system for A. thaliana, NACs from E. ulmoides were divided into 13 distinct subfamilies, namely NAC2, ONAC022, AtNAC3, NAP, ANAC063, ANAC011, ONAC003, NAM, NAC1, OSNAC7, OSNAC8, TIP, and ANAC011, using a phylogenetic tree; however, no EuNAC members were identified in the ATAF subfamily. Of these 13 subfamilies, OSNAC7 had 11 EuNAC members, which was the most abundant, followed by NAM, which had eight members. ANAC001 was the least frequent with only two members. Phylogenetic analysis revealed that EuNAC proteins had evolved in some diversity, similar to a report in A. thaliana (Ooka et al., 2003).
Figure 1: Phylogenetic relationships among NACs identified in E. ulmoides and Arabidopsis thaliana.
The unrooted phylogenetic tree was constructed by MEGA 6.0 software using the Neighbor-Joining (NJ) method with 1,000 bootstrap iterations. Each subfamily was distinguished by different colors.Conserved motif and gene structure analysis of EuNAC genes
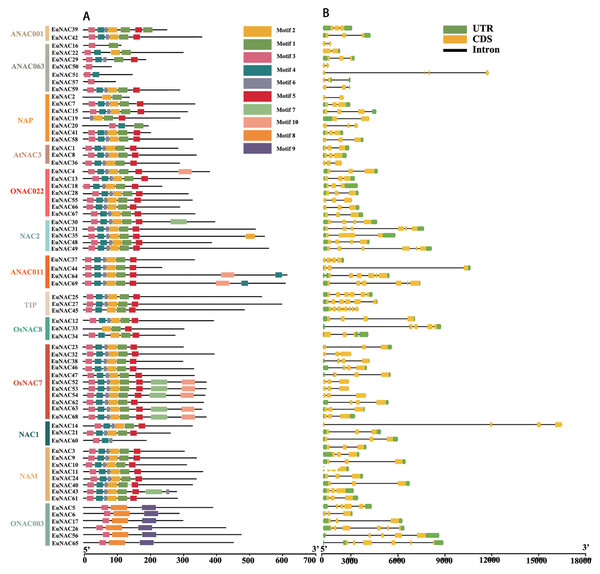
To gain insight into the functional regions of EuNACs, the conserved motifs for each EuNAC protein were analysed using the MEME programme. A total of 10 conserved motifs were identified and named motifs 1–10 (Table S2). These conserved motifs had large variations in length, with a distribution range of 11–50 amino acid residues. As shown in Fig. 2A, motif 3 had the highest frequency in the EuNAC family, and it existed in almost all members except EuNAC2, 19, and 33. In addition, motifs 1, 2, 4, 5, and 6 were very abundant in the EuNAC family, but none of the ONAC003 subfamily members had these motifs. Most of the conserved motifs were distributed in the N-terminus of the NAC proteins, indicating that the N-terminal region plays an important role in NAC gene function. In addition, similar motif compositions existed among different members of the same subfamily, indicating that members of the same subfamily had similar functions.
Figure 2: Motif compositions and DNA structures of NAC gene family in E. ulmoides.
(A) The conserved motif distribution of EuNAC proteins. Different motifs were distinguished by different colored boxes, and black lines represent non-conserved regions. (B) Gene structure of the EuNAC gene. Green boxes represent non-coding regions, yellow boxes represent exons, and black lines represent introns.To investigate the structural features of EuNACs, we analysed the intron/exon distribution patterns of each EuNAC gene. The exon distribution within the EuNAC genes varied from two to eight (Table S1, Fig. 2B). Forty-five (65.2%) genes had three exons, 10 (14.5%) genes had six exons, and EuNAC45 had the largest number of exons, with eight exons (Fig. 2B, Table S1). Forty-nine (71.0%) EuNAC genes possessed less than three exons, indicating a low structural diversity among EuNAC genes.
Genome distribution, selective pressure, and synteny analysis of EuNAC genes
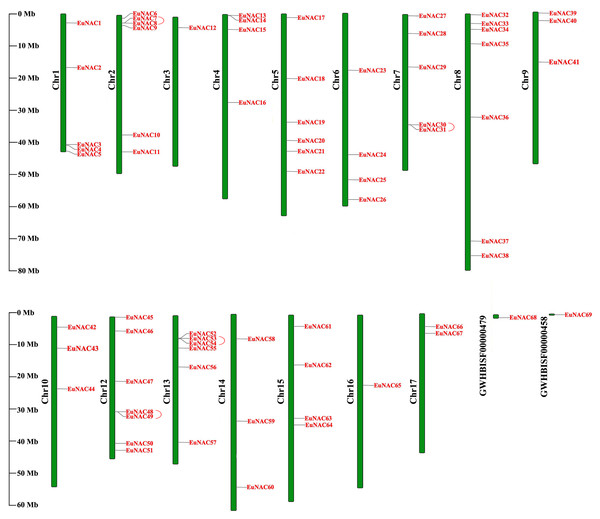
To investigate the distribution of EuNAC genes on the chromosomes of E. ulmoides, TBtools software was employed to map the chromosome locations for all EuNACs identified in this study (Fig. 3). The 69 EuNACs were unevenly scattered on 16 chromosomes and two scaffolds, and the length of each chromosome showed no correlation with the number of genes contained. Chromosomes 8 and 12 had the most EuNACs, both with seven genes. Only one EuNAC was distributed on chromosomes 3 and 16, and no EuNACs were distributed on chromosome 11. Notably, most EuNACs were distributed near the ends of the chromosome.
Figure 3: Distribution of 69 EuNACs on 16 chromosomes and two scaffolds.
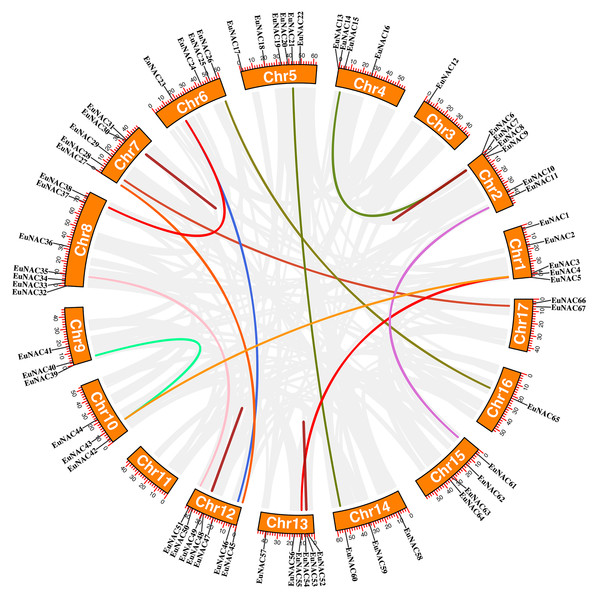
Vertical bars represent the chromosomes of E. ulmoides. The chromosome number is on the left of each chromosome. The scale on the left represents the length of the chromosome.Furthermore, the duplication events of EuNAC gene family members were examined using MCScanX software. A total of 12 pairs of segmental duplications were identified in the EuNAC family, which were distributed across 15 chromosomes, except for chromosomes 3 and 11, and four pairs of tandem replications (EuNAC7/8, EuNAC30/31, EuNAC48/49, and EuNAC53/54) were identified, which were distributed on chromosomes 2, 7, 12, and 13, respectively (Fig. 4, Table S3). The results showed that segmental duplication events might be the crucial driving force in EuNAC gene family expansion. To evaluate the selection pressure of EuNACs, the Ka, Ks, and Ka/Ks for duplicated gene pairs were calculated. In general, a Ka/Ks > 1 indicates positive selection, Ka/Ks = 1 indicates neutral selection, while Ka/Ks < 1 indicates purifying selection (Vahdati & Lotfi, 2013). The Ka/Ks ratios of 16 replicated EuNAC gene pairs were all less than 1, indicating that the evolution of the EuNAC gene family was subjected to purification selection (Table S4).
Figure 4: Schematic representations of the interchromosomal relationships of EuNAC genes.
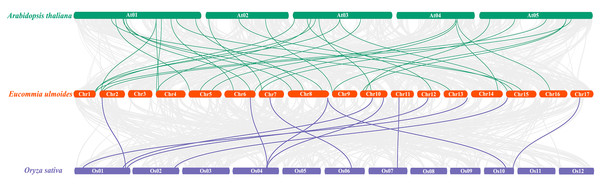
The deep red line represents the EuNAC gene pairs replicated in tandem, while the remaining colored lines represent the EuNAC gene pairs replicated in segments.To further understand the phylogenetic mechanisms of the EuNAC gene family, we constructed a comparative homologous map of NAC genes in E. ulmoides, A. thaliana, and Oryza sativa. In total, 31 EuNACs had a collinear relationship with 27 AtNACs and 10 OsNACs. Thirty-one and eleven pairs of NAC homologous gene pairs were formed between E. ulmoides and A. thaliana and between E. ulmoides and Oryza sativa, respectively (Fig. 5, Table S5). The results indicate that the NAC genes underwent significant evolution and replication after differentiation in monocotyledonous and dicotyledonous plants.
Figure 5: Synteny analysis of NAC genes between E. ulmoides and two representative plant species (Arabidopsis thaliana and Oryza sativa).
Green and purple lines represent syntenic NAC gene pairs of E. ulmoides and A. thaliana and O. sativa, respectively.Promoter region analysis of EuNAC genes
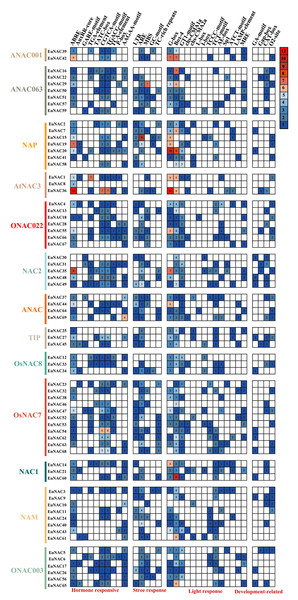
To investigate the potential functions of EuNACs, we employed PlantCARE to predict the cis-acting elements within the 2.0 kb sequence upstream of the initiation codon (ATG) of EuNACs (Table S6). As expected, both TATA and CAAT boxes with good characteristics were found in the results; we also found several other CIS regulators (Table S7 and Fig. S1). They were mined in the promoter region of EuNACs. As shown in Fig. 6, we divided the homeopathic elements into four categories according to their functions. The first category was phytohormone-responsive elements, such as CGTCA-motif, TGACG, TCA-element, and ABRE, wherein ABREs have been associated with ABA responses and TCA-element have been associated with salicylic acid responsiveness. The second category was elements of cis-regulation related to the response to external or environmental pressure. This category includes low-temperature response elements (LTR), which respond to external abiotic stress, abundant cis-regulatory elements (Jeffares, Penkett & Bähler, 2008), which are required for anaerobic induction, and MYB binding site (MBS) elements. Notably, 29 of the 69 EuNAC promoters contained MBS elements that were involved in drought induction as MYB binding sites and could be predicted based on their responses to drought stress treatments. The third category included light-responsive elements, such as G-box, Box-4, and GT1-motif, in which at least one photo-responsive element was detected in almost every promoter region of EuNACs. The last category was cis-regulatory elements related to growth and development. CAT-box and O2-site were mainly detected, which also indicated that most EuNACs may be involved in E. ulmodies meristem expression, zein metabolism regulation, and cell cycle regulation. Finally, based on the above results, EuNACs may be involved in stress response, light, hormones, and growth pathways.
Figure 6: The number of each type of cis-acting element in the promoter region of each EuNAC gene.
Expression analysis of EuNAC genes under drought stress
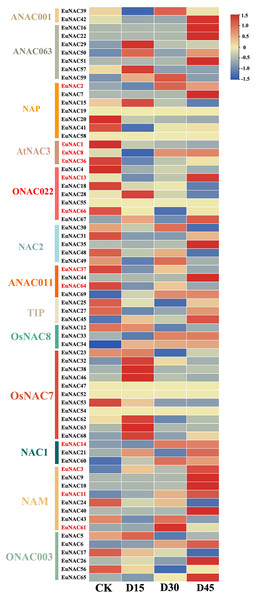
To further investigate the potential function of EuNACs in response to drought stress, comparative transcriptomics of E. ulmoides under drought stress were used to analyse the expression patterns of EuNACs. Of the 69 EuNAC genes, 20 showed high expression levels with FPKM ≥ 20, including EuNAC1, 2, 12, 15, 20, and 25. Forty-three EuNACs showed low expression levels, with FPKM ≤ 20, including EuNAC10, 11, 13, 14, 16, 17, and 18. In addition, EuNAC19, 47, 52, 54, 55, and 58 were not expressed in E. ulmoides leaves (Table S8). Differential gene expression (DEG) analysis showed that 12 EuNAC genes were significantly differentially expressed, of which EuNAC2, 3, 11, and 14 were upregulated after drought stress treatment, and EuNAC1, 36, 37, 64, and 66 were downregulated. The expression levels of EuNAC8 and 13 first decreased and then increased with prolonged treatment time; in contrast, the expression level of EuNAC61 first increased and then decreased (Fig. 7, Table S8). The variable expression patterns of EuNACs may indicate their differential roles in the drought stress response of E. ulmoides. In particular, differentially expressed EuNACs may play a crucial role in E. ulmoides’ response to drought stress.
Figure 7: Expression levels of 69 NAC genes under drought stress in E. ulmoides of leaves.
The expression level was presented based on the transformed data of log2 (FPKM+1) values.Discussion
The NAC transcription factor family is one of the largest gene families in plants. These factors are involved in regulating hormone signalling pathways, biotic and abiotic stress responses, and plant growth and development (Xia et al., 2023; Yuan et al., 2020; Zhang et al., 2019). Several plant genomes have defined the NAC gene family. The evolutionary connection and duplication patterns of the NAC gene family in E. ulmoides can be better understood thanks to the genome of this organism. Previous studies have shown that genes with close evolutionary relationships often share similar functions (Lynch & Conery, 2000). Therefore, by studying the evolutionary relationships across gene families, we can learn more about and perhaps even anticipate how genes operate (Balazadeh et al., 2011; Zhang et al., 2019).
According to our study, the genome of E. ulmoides included 69 NAC genes, which is fewer than that of A. thaliana (117 NAC genes) (Ooka et al., 2003), but similar to K. obovate (79 NAC genes) (Du et al., 2022). Our results indicate that the majority of EuNACs did not experience environmental selection-induced elimination, but rather demonstrated a high level of conservation throughout evolution, underlining the necessity for more research from an evolutionary standpoint. All 69 NAC proteins were divided into 13 subgroups based on their sequence homology and classification relative to A. thaliana (Ooka et al., 2003). NACs in A. thaliana exhibit a high degree of similarity among members of the same class or NAC subgroup. Four EuNACs in the ANAC011 subgroup are orthologous to A. thaliana genes, including AtNAC071 and AtNAC096, which are in charge of tissue reunification, dehydration, and other processes.
Our research indicates that the NAM subgroup has 8 EuNACs that are orthologous to AtNAC054 and AtNAC059 in A. thaliana, which are known to be crucial for organ development, programmed cell death, secondary wall building, and biotic and abiotic stress responses (Kim, Kim & Park, 2007). The seven EuNACs in subgroup NAP are orthologous to AtNAC018, AtNAC025, and AtNAC56 and are essential for leaf senescence (Guo & Gan, 2006). Three genes of the EuNAC gene family’s subgroup TIP are orthologs of the A. thaliana genes AtNAC060 and AtNAC091. These orthologous genes have been demonstrated to be crucial in the stress response and abscisic acid (ABA) signalling (Donze et al., 2014; Jeong et al., 2008; Li et al., 2014). Four EuNACs in the ANAC011 subgroup are orthologous to A. thaliana genes, including AtNAC071 and AtNAC096, which are in charge of tissue reunification, dehydration, and osmotic stress (Asahina et al., 2011; Yang et al., 2020). Five orthologous EuNACs to AtNAC016, which are known to be involved in chlorophyll degradation, are found in the NAC2 subgroup. This indicates that this subgroup of EuNACs may also control how chlorophyll degrades in plants (Sakuraba et al., 2015). Similar to AtNAC003 and AtNAC068, the ANAC001 subgroup also has two EuNACs that are orthologous to them. These genes control salt and osmotic stress tolerance, in addition to DNA damage responses (Xu et al., 2013; Yoshiyama et al., 2014).
Except for individuals from the ONAC003 and ANAC063 subgroups in this investigation, the N-terminus of the EuNAC protein had motifs 1, 2, 3, 4, 5, and 6. ONAC7 comprised motifs 7 and 10, indicating that NAC transcription factors had a highly conserved N-terminus and a very varied C-terminus. The range of EuNAC introns was 2 to 8, which is comparable to the number observed in many plants (Du et al., 2022; Liu et al., 2019). In general, the deletion or insertion of introns can have diverse effects on gene function. Sometimes, intron deletions can lead to the loss of gene function if they result in a frameshift mutation or the removal of critical regulatory sequences. Our analysis supported the findings of Jeffares’ research, which demonstrated that genes susceptible to abrupt changes in stress expression levels have much less intron density (Jeffares, Penkett & Bähler, 2008); EuNACs with fewer introns merit higher consideration if the study objective is to concentrate on genes that react instantly to environmental stress.
As a consequence of our findings, which included the identification of 12 segmental duplications and four tandem duplications in 69 EuNACs, we concluded that segmental duplication served as the primary catalyst for the growth of the EuNAC gene family, in agreement with research on K. obovata (Du et al., 2022). In addition, larger segmental duplications account for most of the A. thaliana genome, and at least four large-scale replication events occurred during the formation of angiosperm diversity (100–200 million years ago) (Vision, Brown & Tanksley, 2000). This might explain why there are more NAC members in A. thaliana, while having a smaller genome than E. ulmoides. Only 10 genes, according to our research, have collinear connections between O. sativa and E. ulmoides. However, we discovered 27 orthologous pairings in A. thaliana, a dicotyledonous plant. These findings demonstrate a closer evolutionary link between dicotyledons and EuNACs than between monocotyledons.
Cis-acting elements are specific DNA sequences located in the promoter region of genes that serve as binding sites for transcription factors (Kaur et al., 2017; Liu, Sun & Wu, 2016). In this study, more than half of the 69 EuNAC promoters included ABRE homeopathic elements, suggesting that these genes may operate via the control of ABA. MBS elements were found in 29 EuNAC s, indicating that these genes may be crucial in response to drought stress.
Research on gene function can benefit from understanding the patterns of gene expression. According to RNA-seq studies, drought stress drastically altered the expression levels of a few EuNAC s in the leaves of E. ulmoides. Significant differential expression was seen in 12 EuNACs. EuNAC1, EuNAC8, and EuNAC36 are identical to ANAC019 (AT1G52890), ANAC055 (AT3G15500), and ANAC072 (AT4G27410), which are members of the AtNAC3 subgroup. Their expression is variably expressed during drought treatment, caused by drought, and stimulated by ABA (Tran et al., 2004). Therefore, we hypothesise that genes EuNAC1, EuNAC8, and EuNAC36 belong to the same subgroup and are drought-responsive genes that control E. ulmoides’ survival ability in drought-stressed environments. Additionally, ANAC054 (At3g15170) and ANAC059 (At3g29035) were identical to EuNAC3, EuNAC11, and EuNAC61 and were grouped into the NAM subgroup, suggesting that they may be crucial in E. ulmoides’ response to drought stress.
Conclusions
In summary, 69 NACs were identified in E. ulmoides in this study. We studied the characteristics of EuNAC genes at the genomic level and analysed expression patterns and responses to drought stress. These TFs can be divided into 13 subgroups according to the NAC classification method of A. thaliana. Chromosomal localisation and homology analysis showed that segmental duplication was the main driving force for EuNAC gene amplification. Our study provides the systematic information, functional framework, and expression patterns under drought stress about EuNAC genes, which will be facilitate the further functional studies of EuNACs in response to drought stress for E. ulmoides.