Comprehensive study of serine/arginine-rich (SR) gene family in rice: characterization, evolution and expression analysis
- Published
- Accepted
- Received
- Academic Editor
- Sushma Naithani
- Subject Areas
- Agricultural Science, Genetics, Genomics, Plant Science
- Keywords
- Rice (Oryza sativa L.), OsSR genes, Alternative splicing, Expression profile, Abiotic stress
- Copyright
- © 2023 Gao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Comprehensive study of serine/arginine-rich (SR) gene family in rice: characterization, evolution and expression analysis. PeerJ 11:e16193 https://doi.org/10.7717/peerj.16193
Abstract
As important regulators of alternative splicing (AS) events, serine/arginine (SR)-rich proteins play indispensable roles in the growth and development of organisms. Until now, the study of SR genes has been lacking in plants. In the current study, we performed genome-wide analysis on the SR gene family in rice. A total of 24 OsSR genes were phylogenetically classified into seven groups, corresponding to seven subfamilies. The OsSR genes’ structures, distribution of conserved domains, and protein tertiary structure of OsSR were conserved within each subfamily. The synteny analysis revealed that segmental duplication events were critical for the expansion of OsSR gene family. Moreover, interspecific synteny revealed the distribution of orthologous SR gene pairs between rice and Arabidopsis, sorghum, wheat, and maize. Among all OsSR genes, 14 genes exhibited NAGNAG acceptors, and only four OsSR genes had AS events on the NAGNAG acceptors. Furthermore, the distinct tissue-specific expression patterns of OsSR genes showed that these genes may function in different developmental stages in rice. The AS patterns on the same OsSR gene were variable among the root, stem, leaf, and grains at different filling stages, and some isoforms could only be detected in one or a few of tested tissues. Meanwhile, our results showed that the expression of some OsSR genes changed dramatically under ABA, GA, salt, drought, cold or heat treatment, which were related to the wide distribution of corresponding cis-elements in their promoter regions, suggesting their specific roles in stress and hormone response. This research facilitates our understanding of SR gene family in rice and provides clues for further exploration of the function of OsSR genes.
Introduction
Splicing of pre-mRNA is an important post-transcriptional regulatory mechanism in eukaryotes, a pre-mRNA can produce different transcripts by splicing at different splicing sites. It was reported that more than 40% genes in plants undergo AS (Chen et al., 2019a). After transcription, the splicing of pre-mRNA is crucial to the production of mature mRNA, which takes place in the spliceosome. The core of the spliceosome was composed of five small nuclear ribonucleoproteins (U1, U2, U4, U5, and U6 snRNPs) and numerous non-snRNP. SR proteins were important non-snRNP that regulate splicing of pre-mRNA (Long & Caceres, 2009; Wang & Brendel, 2004). The serine/arginine (SR) proteins are well-known splicing factors that play important roles in both the assembly of spliceosomes and the regulation of alternative splicing (AS) (Long & Caceres, 2009).
The serine/arginine (SR) proteins were characterized by the presence of RNA recognition motif (RRM) and the consecutive serine and arginine dipeptides repeats and RNA recognition motif (RRM) (Barta, Kalyna & Reddy, 2010). The consecutive serine and arginine dipeptides repeat functioned as a protein-interaction domain and the RRM provided RNA-binding specificity for SR proteins. Besides, some SR proteins had specific domains which were less well understood, like Zn-knuckles and RGG box (Jin, 2022). The results of plant SR proteins sequence comparison and phylogenetic analysis showed that plant SR proteins were classified into seven different subfamilies, SR, SR45, RSZ, SC, SCL, RS2Z and RS, and the last three of these subfamilies were plant-specific which had unique domains, while the other four subfamilies had orthologs in animals (Reddy & Shad Ali, 2011; Richardson et al., 2011). SR proteins in SCL subfamily had an RRM with a charged extension at the N-terminus. The members of SCL subfamily included dicotyledons, monocotyledons, mosses and green algae. The RS2Z subfamily was found in dicotyledons and monocotyledons, and two Zn-knuckles domains and an extra SP-rich region were present on the proteins in this subfamily. The members of RS subfamily in plants contained two RRMs and the RS domains which were rich in RS dipeptides. The RS subfamily was mainly composed of photosynthetic eukaryotes (Xie et al., 2022).
The SR gene itself undergoes extensive AS. It was shown that 18 SR genes in Arabidopsis thaliana could produce more than 90 transcripts, and the precursor mRNA from the rice SR gene also underwent extensive AS (Reddy & Shad Ali, 2011). Alternative splicing (AS) events that occurred at the NAGNAG acceptor were termed the AS-NAGNAG events, which would cause NAG insertion-deletions in transcripts (Iida, Shionyu & Suso, 2008). In the NAGNAG motif, the first AG is termed the E-acceptor and the second AG was termed I-acceptor (Hiller et al., 2004). AS-NAGNAG events were widespread in mammals and plants, which contributes to the diversity of transcriptome and proteome in different species. It has been reported that AS-NAGNAG acceptors were overrepresented in genes which coded RRM-containing proteins. Genes coding for RNA binding proteins were preferentially equipped with NAGNAG acceptors in human (Akerman & Mandel-Gutfreund, 2006; Iida, Shionyu & Suso, 2008). In Arabidopsis thaliana, NAGNAG acceptors were frequently found in the genome, particularly in the SR genes (Schindler et al., 2008; Shi, Sha & Sun, 2014).
Because of the indispensable role played by SR proteins in both constitutive splicing and alternative splicing of precursor mRNA, it is likely that they will be instrumental in modulating the expression of genes that are essential for plants in different developmental stages. Until now, some studies have revealed that members of the SR gene family played important roles in various biological processes in different species (Ali et al., 2007; Carvalho, Carvalho & Duque, 2010; Park et al., 2020). In Arabidopsis, 19 AtSR genes have been identified (Barta, Kalyna & Reddy, 2010). The function of SR45 has been extensively studied in Arabidopsis thaliana. Studies have shown that SR45 negatively regulated glucose-induced growth by inhibiting abscisic acid (ABA) accumulation and its signaling, thereby inhibiting seedling establishment under adverse conditions (Ali et al., 2007; Carvalho, Carvalho & Duque, 2010). AtSR45a was detected to undergo AS and produced two alternative splicing variants, AtSR45a-1a and AtSR45a-1b. AtSR45a could regulate the response to salt stress by increasing the expression of these two variants and interacting with the cap-binding complex (Li et al., 2021). AtRS40, AtRS41, and AtSCL30 participated in responses to ABA and salt stress in Arabidopsis (Chen et al., 2013; Cruz et al., 2014). Besides, AtSR genes including AtRS40, AtSR34a, AtRSZ22, and AtSR45a etc., were reported to participate in the response to heat stress. Under high temperature, these genes underwent specific AS and produced specific mRNA variants (Filichkin et al., 2010; Ling, Mahfouz & Zhou, 2021; Ling et al., 2018).
In rice, 24 SR genes have been identified (Barta, Kalyna & Reddy, 2010). The OsRSp29, OsRSZp23, and OsSCL26 played roles in stimulating pre-mRNA splicing and promoting the splicing efficiency of downstream genes (Isshiki, Tsumoto & Shimamoto, 2006). The OsSR45 functioned in regulating the response to various stresses, including temperature stress and reactive oxygen species stress at the post transcriptional level by interacting with OsFKBP20-1b which belonged to the immunophilin family in rice (Park et al., 2020). OsSR40, OsSCL57, and OsSCL25 played crucial roles in regulating mineral element absorption and homeostasis in rice by participating in alternative splicing of pre-mRNAs of related genes (Dong et al., 2018).
Despite the fact that SR genes have been identified in rice, further studies on these genes, especially on their biological functions, are still lacking. A comprehensive analysis of SR genes in rice was performed in the present study. The genetic relationship among OsSR genes was analyzed firstly, then we analyzed the structures of the OsSR proteins, the collinear relationships, as well as the promoter sequences, the NAGNAG acceptors, and the expression and alternative splicing patterns in both vegetative and reproductive organs of OsSR genes, and their responses to hormones and abiotic stresses. This study provides a better understanding and establishes the foundation for further functional elucidation of OsSR genes.
Materials and methods
Identification and acquisition of information of OsSR genes
According to the accession number provided in Jin’s article (Jin, 2022), we extracted the sequences of 24 OsSR genes, transcript sequences produced by each gene, and the corresponding encoded protein sequences from the MSU-RGAP (Rice Genome Annotation Project, RGAP) database (http://rice.uga.edu/index.shtml). Meanwhile, the accession numbers of the corresponding genes on the Rice Genome Annotation Project Database (https://rapdb.dna.affrc.go.jp/) were also provided in this study (Kawahara et al., 2013; Sakai et al., 2013). We summarized the alternative splicing information of 24 OsSR genes based on the two databases, MSU-RGAP and RAP DB, which were shown in Table S1. The AS models of OsSR genes except OsRS2Z39 were different in the MSU-RGAP database and RAP DB. We found that the splicing forms of SR genes from the MSU-RGAP database comprised more splicing isoforms compared to the RAP DB, and the alternative splicing information of these genes in the RAP DB was almost included in MSU-RGAP. Moreover, we analyzed the cDNA information in the NBCI datebase with the two databases, which showed identity in MSU-RGAP. Thus, we investigated the alternative splicing pattern as well as the expression of different transcripts produced by the OsSR genes based on the AS models of all 24 OsSR genes on the MUS-RGAP.
Bioinformatics analysis of SR gene family genes
Phylogenetic analysis, conserved motifs, gene structure, tertiary structure prediction
Based on the results of the multiple amino acid sequence alignment done by MEGA 7.0 with ClustalW, MEGA 7.0 was used in this study to perform the phylogenetic analysis with the maximum-likelihood (ML) method and 1,000 bootstrap replicates (Kumar, Stecher & Tamura, 2016). Exon and intron positions in OsSR genes were mapped, and gene structures were deduced using the Gene Structure Display Server (GSDS) (http://gsds.cbi.pku.edu.cn/) (Hu et al., 2015). Conserved motifs of OsSR proteins were analyzed using SMART (Simple Modular Architecture Research Tool) online tool (http://meme-suite.org/tools/meme) (Letunic & Bork, 2018), and then visualized using the TBtools (Chen et al., 2020a). The tertiary structures of OsSR proteins were predicted by SWISS-MODEL (https://swissmodel.expasy.org/) (Waterhouse et al., 2018).
Physicochemical properties and subcellular localizations
The physicochemical properties and subcellular localizations of OsSR proteins were predicted using ExPASy Protparam online (https://web.expasy.org/protparam/) and BUSCA (http://busca.biocomp.unibo.it) (Savojardo et al., 2018), respectively. The NetPhos3.1 service was used to predict the OsSR proteins’ phosphorylation sites (https://services.healthtech.dtu.dk/service.php?NetPhos-3.1) (Blom, Gammeltoft & Brunak, 1999). Meanwhile, the subcellular localization of OsSR proteins was analyzed online (https://croppal.org/) (Hooper et al., 2020).
Syntenic relationships
From the Ensembl plants database, the genomic information of SR genes in rice, Arabidopsis, sorghum, maize, soybean, and wheat was retrieved (http://plants.ensembl.org/index.html). Then, the segment duplication events of OsSR genes and synteny relationships between rice and other species were analyzed by the MCScanX program, and the results were visualized using TBtools (Chen et al., 2020a; Wang et al., 2012).
Cis-acting elements
The key promoter regions (2,000 bp sequences upstream of the translation start codons) of the OsSR genes were retrieved from NCBI (https://www.ncbi.nlm.nih.gov/gene/) and submitted to the PlantCARE online (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) to predict and analyze the regulatory cis-acting elements in promoter regions (Lescot et al., 2002).
Plant materials and growth conditions
Rice (Oryza sativa L. spp. Japonica, var Nipponbare) plants were used in this study. The seeds were sterilized with 20% NaClO solution, soaked with sterile water and incubated at 37 °C for germination. A portion of seeds were transferred to the field with normal water and fertilizer management to continue growing until maturity. Meanwhile, the other portion was transplanted to the 96-well PCR plates with the bottom removed, and grown in the Hoagland solution (CaNO3⋅4H2O 945 mg/L, KNO3 506 mg/L, NH4NO 380 mg/L, KH2PO4 136 mg/L, MgSO4⋅7H2O 493 mg/L, iron salt solution 2.5 mL/L (2.78 g FeSO4⋅7H2O, 500 mL distilled water, 3.73 g EDTA-2Na pH 5.5), microelement five mL/L (KI 0.83 mg/L, H3BO3 6.2 mg/L, MnSO4 22.3 mg/L, ZnSO4 8.6 mg/L, Na2MoO4 0.25 mg/L, CuSO4 0.025 mg/L, CoCl2 0.025 mg/L), pH 6.0) in the greenhouse with a photoperiod of 14/10 h at 28 °C/26 °C (day/night) and relative humidity of 65%. The Hoagland solution was renewed every 2 days.
For tissue-specific expression analysis, the different tissues of rice planted in the field were sampled from vegetative organs and spikelets at different filling stages. For the salt, drought, and phytohormone treatments, the seedlings at the 4-leaf stage were subjected to Hoagland solution with 200 mM NaCl, 20% PEG6000, 100 µM gibberellin (GA) (100 µM) or abscisic acid (ABA), respectively. For cold or heat stress, the seedlings at the 4-leaf stage were moved to the incubator with the temperature of 4 °C and 37 °C, respectively. And the 2nd and 3rd leaves from the seedlings were dissected at different time points under various treatments. The samples without treatments were set with blank control (CK) at the same time. In the CK treatment, the seedlings at the 4-leaf stage were subjected to Hoagland solution and grew in the same incubator as the corresponding treated group.
RNA isolation, RT-PCR and qRT-PCR
Total RNA from different samples was extracted by Total RNA Extractor (Trizol) (Sangon Biotech, Shanghai, China) and reverse transcribed into cDNA using Hifair® III; Reverse Transcriptase (Yeasen, Shanghai, China) according to the instruction book.
The RT-PCR was performed using PrimerSTAR MaxDNA Polymerase (TaKaRa, Shiga, Japan), and the appropriate annealing temperature for PCR according to the properties of primer pairs for different genes. The number of amplification reaction cycles in this study was 30. The RT-PCR experiment for each gene was conducted with 3 biological replicates. The PCR products were determined using electrophoresis on the 1% agarose gels. Specific primers for different OsSR genes and the control gene OsActin used for RT-PCR are listed in Table S7.
For the qRT-PCR experiment, SYBR Green qPCR Master Mix (TOROIVD) was used, and the experiment was conducted on LightCycler 480 II (Roche, South San Francisco, CA, USA). The data was analyzed as previously described using OsActin as the internal standard (Livak & Schmittgen, 2001). The qRT-PCR experiment was carried out using 3 biological replicates, and 3 technical replicates were performed for each biological replicate. The qRT-PCR data was calculated using 2−ΔΔCT method and Student’s t-test. The primer sequences for qRT-PCR are listed in Table S6.
Results
Identification of OsSR genes and their characteristics
Here, we performed the prediction and analysis of the physicochemical properties of the OsSR proteins (Table 1). The results revealed that OsSR proteins ranged in length from 185 amino acids (OsRSZ21a and OsRSZ21) to 502 amino acids (OsSCL57), while the molecular weight varied from 21.02 kDa (OsRSZ21) to 56.83 kDa (OsSCL57). Notably, all of the rice SR proteins were alkaline proteins with the isoelectric point ranging from 8.67 (OsSR40) to 12.37 (OsSR45) (Table 1).
| Subfamily | Revised nomenclature | RAP_Locus | MSU_Locus (Alternative splice form) | Length(bp) | Intron | Exon | Protein length (aa) | Molecular weight (kDa) | Isoelectric point | Instability index | Predicted subcellular location | phosphorylation sites |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCL | OsSCL30a | Os02g0252100 | LOC_Os02g15310.1 | 4,309 | 6 | 7 | 265 | 30.47 | 11.09 | 111.87 | plasma membrane*; nucleus* | 45 |
| OsSCL30 | Os12g0572400 | LOC_Os12g38430.1 | 3,856 | 6 | 7 | 263 | 30.19 | 10.9 | 110.93 | nucleus | 47 | |
| OsSCL57 | Os11g0704700 | LOC_Os11g47830.1 | 7,034 | 13 | 14 | 502 | 56.83 | 10.01 | 100.02 | nucleus | 82 | |
| OsSCL28 | Os03g0363800 | LOC_Os03g24890.1 | 4,646 | 5 | 6 | 243 | 27.78 | 10.83 | 88.03 | nucleus | 35 | |
| OsSCL25 | Os07g0633200 | LOC_Os07g43950.1 | 3,180 | 4 | 5 | 213 | 24.82 | 10.68 | 103.01 | nucleus | 50 | |
| OsSCL26 | Os03g0374575 | LOC_Os03g25770.1 | 3,549 | 4 | 5 | 218 | 25.68 | 11.17 | 114.71 | nucleus | 40 | |
| SC | OsSC32 | Os07g0623300 | LOC_Os07g43050.1 | 4,182 | 7 | 8 | 275 | 32.24 | 11.35 | 118.57 | nucleus | 59 |
| OsSC34 | Os08g0486200 | LOC_Os08g37960.1 | 3,154 | 6 | 7 | 289 | 33.54 | 11.8 | 112.71 | nucleus | 56 | |
| OsSC25 | Os03g0388000 | LOC_Os03g27030.1 | 3,000 | 6 | 7 | 206 | 24.90 | 10.33 | 64.44 | nucleus | 25 | |
| SR45 | OsSR45a | Os05g0105900 | LOC_Os05g01540.1 | 3,826 | 10 | 11 | 426 | 47.75 | 12.19 | 133.86 | nucleus | 86 |
| OsSR45 | Os01g0959000 | LOC_Os01g72890.1 | 5,025 | 11 | 12 | 432 | 48.11 | 12.37 | 160.81 | nucleus, cytoplasm | 77 | |
| RS2Z | OsRS2Z39 | Os05g0162600 | LOC_Os05g07000.1 | 4,237 | 5 | 6 | 338 | 39.02 | 9.83 | 64.03 | nucleus | 45 |
| OsRS2Z37 | Os01g0155600 | LOC_Os01g06290.1 | 3,944 | 5 | 6 | 324 | 36.89 | 11.27 | 96.97 | nucleus* | 57 | |
| OsRS2Z36 | Os05g0120100 | LOC_Os05g02880.1 | 3,489 | 5 | 6 | 323 | 36.22 | 10.83 | 101.82 | nucleus | 61 | |
| OsRS2Z38 | Os03g0285900 | LOC_Os03g17710.1 | 3,333 | 5 | 6 | 335 | 37.52 | 11 | 100.05 | nucleus | 60 | |
| RSZ | OsRSZ21a | Os06g0187900 | LOC_Os06g08840.1 | 3,671 | 4 | 5 | 185 | 21.18 | 11.29 | 103.17 | nucleus | 34 |
| OsRSZ21 | Os02g0789400 | LOC_Os02g54770.1 | 4,678 | 4 | 5 | 185 | 21.02 | 11.24 | 93.82 | nucleus * | 29 | |
| OsRSZ23 | Os02g0610600 | LOC_Os02g39720.2 | 4,269 | 3 | 4 | 203 | 23.20 | 11.33 | 112.07 | plastid*; nucleus* | 35 | |
| RS | OsRS29 | Os04g0118900 | LOC_Os04g02870.1 | 4,490 | 4 | 5 | 245 | 28.78 | 9.94 | 68.28 | nucleus | 31 |
| OsRS33 | Os02g0122800 | LOC_Os02g03040.1 | 3,691 | 4 | 5 | 279 | 32.54 | 9.88 | 60.31 | nucleus* | 30 | |
| SR | OsSR33 | Os07g0673500 | LOC_Os07g47630.1 | 5,111 | 12 | 13 | 296 | 33.14 | 10.64 | 104.32 | nucleus * | 59 |
| OsSR32 | Os03g0344100 | LOC_Os03g22380.1 | 4,789 | 12 | 13 | 286 | 31.94 | 10.54 | 98.23 | nucleus | 55 | |
| OsSR33a | Os05g0364600 | LOC_Os05g30140.1 | 7,169 | 13 | 14 | 294 | 33.42 | 10.92 | 102.83 | nucleus | 64 | |
| OsSR40 | Os01g0316550 | LOC_Os01g21420.1 | 7,570 | 12 | 13 | 292 | 33.50 | 8.67 | 48.14 | nucleus | 29 |
Notes:
(1) The subcellular location was bolded and italicized the corresponding OsSR protein has been experimentally verified.
(2) The subcellular location of the OsSR proteins, determined by mass spectrometric assay, was italicized and marked with *.
According to the BUSCA prediction analysis, OsSR45 was predicted to localize in the chloroplast and nucleus, the remaining 23 OsSR proteins were localized only in nucleus. By the experiments, OsSR45 was observed to co-express and physically interact with OsFKBP20-1b in the nucleus and cytoplasm in vivo (Park et al., 2020). It has been proven experimentally that OsSCL30, which was the SR protein in rice, was visible only in the nucleus (Zhang et al., 2022). The results indicated that different OsSR proteins together with their interaction proteins could function in different intracellular partitions. Besides, the CropPAL data set showed that the subcellular localization of some OsSR proteins had been confirmed by mass spectrometric assay, including OsSCL30a, OsRS2Z37, OsRSZ21, OsRSZ23, OsRS33, and OsSR33 (Table 1).
According to previous research, the mobility of SR proteins was regulated by phosphorylation in Arabidopsis (Tillemans et al., 2006). Notably, we found that all of the OsSR proteins were phosphorylated with numbers of phosphorylation sites ranging considerably from 25 (OsSC25) to 86 (OsSR45a) (Table 1). The difference in physicochemical properties is suggestive of functional differences among the OsSR proteins.
Phylogenetic, motif composition, structure of OsSR proteins and gene structure analysis of OsSR genes
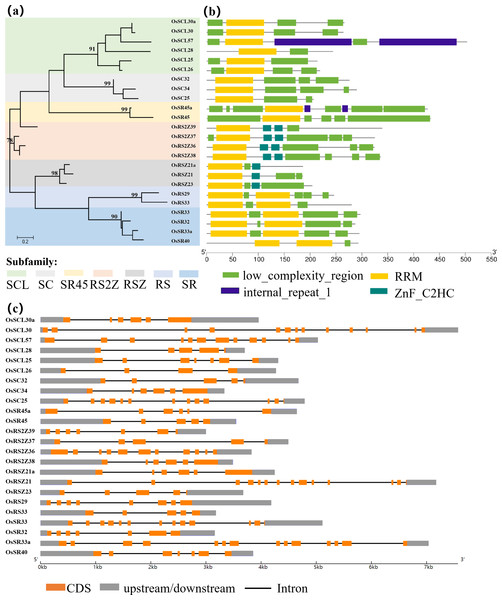
Analysis of the evolutionary relationships among the members of the SR family in rice by a maximum-likelihood phylogenetic tree using amino acid sequences of 24 OsSR proteins was displayed (Fig. 1). A total of 24 OsSR genes could be classified into seven distinct subgroups based on the evolutionary relationships, and the results were consistent with the reported seven subfamilies: SCL, SC, SR45, RS2Z, RSZ, RS, and SR, indicating their conservation within the subfamilies during their evolution. These 7 subgroups contained 6, 3, 2, 4, 3, 2, and four members, respectively. Among them, SR, RSZ, and SC subfamily are common between plants and animals, the remaining subfamilies are plant-specific (Reddy & Shad Ali, 2011), indicating the SR proteins diverged along with the speciation.
Figure 1: Schematic representation of the phylogenetic relationship, gene structures and conserved motifs in OsSR genes.
(A) Maximum-likelihood (ML) phylogenetic tree of OsSR proteins. (B) Distribution of conserved motifs in OsSR proteins. (C) Exon/introns and untranslated regions (UTRs) of OsSR genes. Grey boxes denote UTR (untranslated region); yellow boxes denote exon; black lines denote introns. The length of gene can be estimated using the scale at the bottom.The conserved domains usually have important functions and are closely related to the completion of physiological functions of proteins. Analysis of the conserved domains of OsSR proteins showed that the OsSR proteins within the same subfamily were highly conserved in the types and distribution of conserved domains (Fig. 1B). The members of the SCL and SC subfamilies contained only one RRM domain near the N-terminus, as well as a domain rich in consecutive serine and arginine dipeptide repeats (SR domain) at the C-terminus of the protein (Jin, 2022). Besides, the OsSR proteins in the SCL subfamily also had a domain which could be diverse at the N-terminal. As for two members of the SR45 subfamily, both of them contained one RRM domain and two SR domains which existed in the N- and C-termini of the proteins, respectively. Unlike OsSR proteins in other subfamilies, two and one ZnF_C2HC motif were contained in members of the RS2Z and RSZ subfamily besides RRM and SR domains, respectively. The members in RS subfamily contained two RRMs and the RS domain. Similarly, the four proteins of the SR subfamily also contained two RRM domains, but unlike the RS subfamily, the C-terminal of these proteins was the SR domain.
Furthermore, the prediction of the tertiary structure of these proteins using SWISS-MODEL online server revealed that OsSR proteins were mainly composed of α-helices, β-folds, and random coils (Fig. S1 and Table S2). It was speculated that proteins with different tertiary structures may determine the diversity functions of OsSR genes. We noticed that OsSR protein structures showed differences among different subfamilies, especially among RS subfamily, SR subfamily and other subfamilies (Fig. S1). While in most cases, the OsSR proteins in the same subfamily had similar tertiary structures (Fig. S1).
Gene structural variety might function as a form of evolution for numerous genes (Fedorov, Merican & Gilbert, 2002). The conservation of gene structure is related to the number of introns in eukaryotes (Rogozin et al., 2003). Further exploration of the gene revealed the structural differences and conserved relationships among these OsSR genes. The OsSR genes differed in nucleotide sequence, but they contained the similar number of exons and introns in the same subfamily except for the genes in the SCL subfamily. The number of introns among different subfamilies ranged from 3 to 13 (Fig. 1C). The genes, containing the fewest introns and exons and the most introns and exons, were OsRSZ23 and OsSR33a, belonging to RSZ and the SR subfamily, respectively. The OsSR genes in the SC subfamily usually contained six or seven introns, while the members of the SR45 subfamily contained 10 or 11 introns. All the members of RS2Z and RS subfamily contained five and four introns, respectively. The number of introns of OsSR genes in the RSZ subfamily is four or three. The intron number in SR subfamily was up to 12 or 13.
Segment duplication analysis of OsSR genes
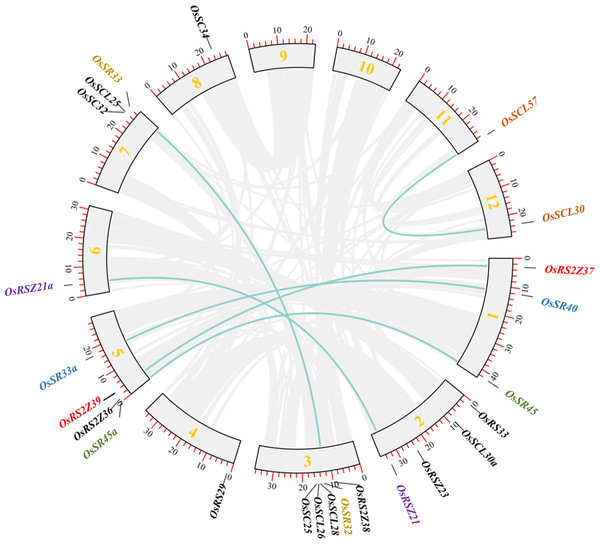
In order to clarify the expansion of rice SR gene family, we analyzed segmental duplication, which is considered as one of the main factors driving the expansion of gene families during evolution in plants (Cannon et al., 2004). As shown in Fig. 2, 12 genes (six pairs), including OsRS2Z37 and OsRS2Z39, OsSR40 and OsSR33a, OsSR45 and OsSR45a, OsRSZ21 and OsRSZ21a, OsSR32 and OsSR33, OsSCL57 and OsSCL30, are implicated in segmental duplication events. Totally, 50% members of OsSR genes showed collinear relationships, indicating that segmental duplication was primarily responsible for the expansions of the SR gene family in rice. Additionally, we found that the Ka/Ks ratios of all OsSR gene duplication pairs were less than 1.0 (Table S3), suggesting that these six duplication pairs underwent purifying selection (Hurst, 2002).
Figure 2: Schematic representations for the chromosomal locations and segment duplications of OsSR genes.
A total of 24 OsSR genes were mapped onto the chromosomes on the basis of their physical location. 1–12 were the chromosome numbers (Chr1- Chr12). The gray lines indicated duplicated blocks. The duplicated OsSR gene pairs were highlighted in green lines.Synteny and orthologous gene pairs of SR genes
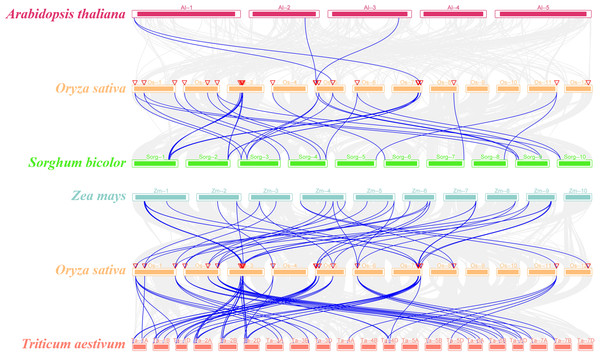
The syntenic relationship of the SR genes between rice and five plant species (Sorghum bicolor, Arabidopsis thaliana, Zea mays, Triticum aestivum, and Glycine max) was examined in this study. There was no orthologous gene between rice and soybean (Fig. S2). While only four pairs of orthologous genes were identified between rice and Arabidopsis (OsSR33a and AtSR34, OsSR33 and AtSR34, OsRS2Z36 and AtRS2Z33, OsRS2Z36 and AtRS2Z32) (Fig. 3). In addition, a total of 20, 40, 64 orthologous SR gene pairs were identified between rice and sorghum, maize, wheat, respectively (Fig. 3 and Table S4).
Figure 3: Synteny analyses of SR genes between rice and four plant species (Arabidopsis thaliana, Sorghum bicolor, Zea mays, and Triticum aestivum).
The gray lines indicated collinear blocks and syntenic SR gene pairs would be highlighted in blue lines.NAGNAG acceptors in OsSR genes
NAGNAG acceptors were termed based on the existence of a NAGNAG acceptor motif, and alternative splicing at NAGNAG acceptors was widespread in the genomes of animals and plants (Akerman & Mandel-Gutfreund, 2006). A scan of OsSR gene products from the information on the MSU-RGAP for signatures associated with NAGNAG acceptors revealed that 14 out of 24 OsSR genes exhibited NAGNAG acceptors. Among these 14 genes, OsSCL26, OsRS2Z36, OsRSZ21a and OsRSZ21 contained 2, 2, 3, 2 NAGNAG acceptors, respectively, and the other 10 genes contained only one (Table 2). Furthermore, we focused on whether alternative splicing occurred on these NAGNAG acceptors. We found that only four OsSR genes, including OsSCL26, OsSC25, OsSR45a and OsSR45, had AS-NAGNAG event on the NAGNAG acceptor, which led to the deletion of a single amino acid at the protein level (Table S1).
| mRNA | |||
|---|---|---|---|
| SR gene | E | I | Motif |
| OsSCL30a | 1 | CAGGAG | |
| OsSCL30 | 1 | CAGGAG | |
| OsSCL26 | 2 | CAGTAG, TAGCAG | |
| OsSC25 | 1 | TAGCAG | |
| OsSR45a | 1 | CAGCAG | |
| OsSR45 | 1 | CAGCAG | |
| OsRS2Z39 | 1 | CAGCAG | |
| OsRS2Z36 | 2 | CAGGAG | |
| OsRS2Z38 | 1 | CAGGAG | |
| OsRSZ21a | 1 | 2 | CAGAAG, GAGCAG, AAGCAG |
| OsRSZ21 | 2 | CAGAAG | |
| OsRSZ23 | 1 | TAGGAG | |
| OsRS33 | 1 | CAGGAG | |
| OsSR33 | 1 | CAGAAG | |
Notes:
Observed NAGNAG motifs and E and I acceptors confirmed by mRNA (from RefSeq) are shown.
The prediction of cis-acting elements on OsSR genes’ promoters
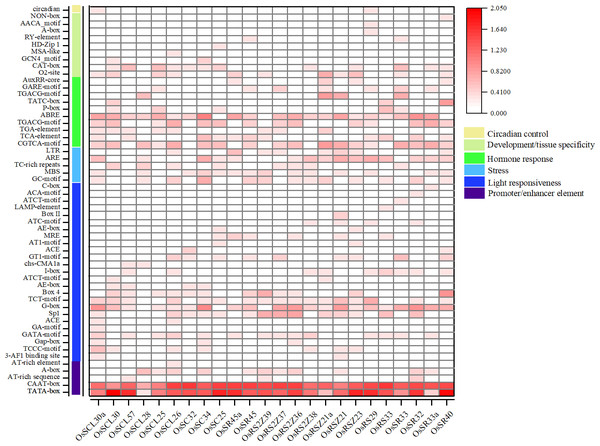
The analysis of the promoter regions of OsSR genes identified six types of cis-elements, including promoter/enhancer elements and elements related to light responsiveness, stress, hormone response, development/tissue specificity or circadian control (Fig. 4 and Table S5). The proportion of cis-acting elements in each of these categories was 9.1%, 45.5%, 9.1%, 18.2%, 16.4%, and 1.8%, respectively.
Figure 4: Cis-acting elements in promoter regions of OsSR genes.
Cis-acting elements were predicted based on 2 kb sequences upstream of coding sequences. The quantity of cis-acting elements was normalized by log 10 (number + 1) and then used for heatmap construction.The cis-acting elements in ‘promoter/enhancer element’ category, which ensure the correct location and start of transcription, were ubiquitously identified in all OsSR genes’ promoters, including CAAT-box, and TATA-box (Fig. 4). The ARE and MBS in ‘stress’ category, which are involved in anaerobic induction and drought responsiveness, respectively, were harbored in most of OsSR genes’ promoters. In ‘hormone response’ category, the cis-acting elements that respond to ABA, auxin, GA, MeJA, and salicylic acid were identified. The ABA, salicylic acid and MeJA responsiveness elements, including the ABA responsive element (ABRE), TCA-motif, TGACG-motif, and CGTCA-motif, were widely presented in the promoters of the OsSR genes. Notably, the ABRE was the most widely distributed hormone-responsive element, which presented in almost all the promoters of the 24 OsSR genes. Among these hormone-responsive elements, GA responsive elements were the most abundant. We found that three out of nine identified hormone-responsive elements were GA response elements (Fig. 4 and Table S5), including P-box, TATC-box, and GARE-motif. As for the ‘development/tissue specificity’ category, GCN4_motif, RY-element, and AACA_motif were identified as seed and endosperm development-related (Fig. 4 and Table S5). In addition, only OsSCL30a and OsRS29 contained circadian related elements in their promoters. Based on these findings, it indicated that OsSR genes may have roles in responding to different hormones and environmental stresses.
Expression patterns and AS of OsSR genes
A series of cis-acting elements related to tissue development, stress response, and hormone response were identified in the promoter region of the OsSR genes. To understand whether the OsSR genes are implicated in the growth and abiotic stress response of rice, we examined the expression profiles and AS patterns of OsSR genes in several tissues or under abiotic conditions (drought, salt, cold, and heat) and hormone treatments (GA and ABA).
Tissue expression profiles of the OsSR genes
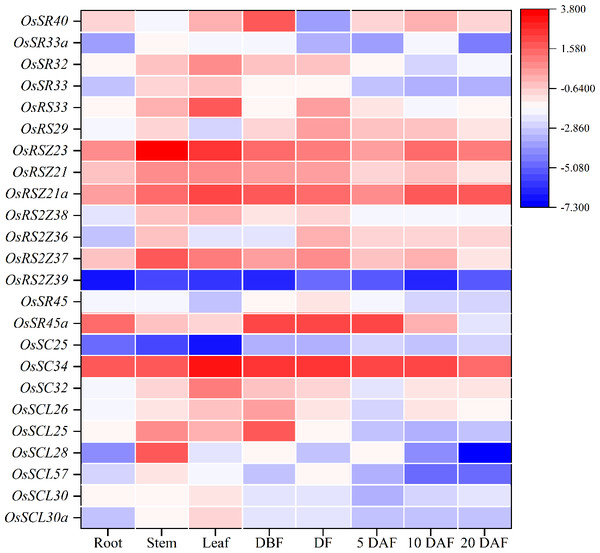
To further characterize the potential biological function, qRT-PCR was used to conduct tissue-specific expression analyses of OsSR genes. Totally, we detected all 24 OsSR genes in eight tissues and organs including root, stem, leaf and spikelets before fertilization, at flowering and 5, 10 and 20 days after fertilization (Table S9). Our results showed that the expression of the OsSR genes was tissue-specific and development phase-dependent (Fig. 5 and Fig. S3). The genes in the SCL subfamily were mainly expressed in stems, leaves and young panicles, and showed a lower expression in grains after 5 days of pollination. Notably, the expression of OsSC32 and OsSC34 in SC subfamily showed leaf preferential expression, whereas the expression of OSC25 was very low in the tested tissues. The OsSR45a in the SR45 subfamily was specifically higher expressed in panicles at DBF, DF, and 5 DAF, while the expression level of OsSR45 was relatively high only in panicles at DF. As for the genes in the RS2Z subfamily, the expression of OsRS2Z39 almost failed to be detected in both vegetative and reproductive organs. Among the remaining three genes in the RS2Z subfamily, OsRS2Z38 was mainly expressed in the stems, leaves, and panicles before flowering, while OsRS2Z36 was highly detected in panicles during the filling period, and OsRS2Z37 was expressed with a relative high level in all tested tissues. The ubiquitous expression of three genes in the RSZ subfamily was observed in 8 tissues with relatively high levels, especially in stems, leaves, and grains after 10 days of fertilization. Furthermore, two RS subfamily genes were highly expressed in panicles at different developmental stages. The high expression in leaves and panicles at DBF was observed for four genes in the SR subfamily.
Figure 5: Expression profiling of the OsSR genes in 8 tissues based on qRT-PCR.
DBF, day before fertilization; DF, day of flowering; DAF, day after fertilization. OsActin was used as control, and each set of data contained three replicates. The comparative Δ CT values of OsSR genes were transformed by log2 to build the heatmap.Alternative splicing of OsSR genes in different tissues
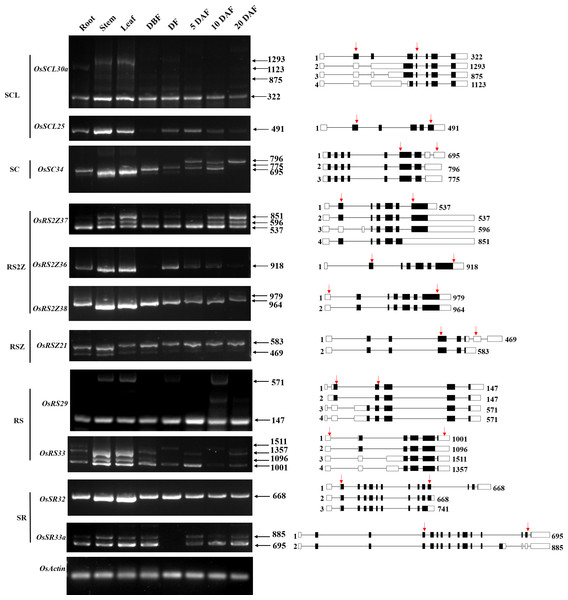
It has been reported that the pre-mRNA of the SR gene which encoded the splicing regulator in different species would undergo extensive AS themselves (Chen et al., 2019b; Isshiki, Tsumoto & Shimamoto, 2006). Until now, the alternative splicing pattern of SR genes and the expression pattern of the corresponding transcripts in different tissues at different developmental stages are still poorly understood in rice. We summarized the alternative splicing of all 24 OsSR genes (Table S1), and the schematic diagrams of alternatively spliced transcripts of the OsSR genes were drawn according to the sequence information provided by the MSU-RGAP database.
To analyze the expression patterns of different transcripts produced by the alternative splicing of OsSR genes, we performed the RT-PCR using primers which were specific to the target genes (Table 3 and Table S7). For 11 selected OsSR genes, RT-PCR analysis was conducted in roots, stems, leaves, and panicles at different developmental stages. The results showed except OsSCL25 and OsRS2Z36, the remaining nine genes exhibited AS actually (Fig. 6).
| SR gene | Size of all predicted transcripts (bp) | Size of amplification product on genome (bp) |
|---|---|---|
| OsSCL30a | (1) 1,269 (322), (2) 2,241 (1,293), (3) 1,822 (875), (4) 2,071 (1,123) | 1,738 |
| OsSCL25 | (1) 1,091 (491) | 1,931 |
| OsSC34 | (1) 1,403 (695), (2) 1,416 (796), (3) 1,392 (775) | 970 |
| OsRS2Z37 | (1) 1,332 (537), (2) 2,351 (537), (3) 2,417 (596), (4) 2,672 (851) | 1,887 |
| OsRS2Z36 | (1) 1,312 (918) | 2,059 |
| OsRS2Z38 | (1) 1,456 (979), (2) 1,442 (964) | 2,855 |
| OsRSZ21 | (1) 1,398 (469), (2) 991 (583) | 957 |
| OsRS29 | (1) 1,234 (147), (2) 1,183 (147), (3) 1,560 (571), (4) 1,579 (571) | 1,206 |
| OsRS33 | (1) 1,349 (1,001), (2) 1422 (1,096), (3) 1,859 (1,511), (4) 1,705 (1,357) | 3,343 |
| OsSR32 | (1) 1,042 (668), (2) 1,003 (668), (3) 1,076 (741) | 2,572 |
| OsSR33a | (1) 1,500 (695), (2) 1,690 (885) | 2,974 |
Notes:
The number in the parenthesis indicates the product size corresponding to the amplified primer used in this experiment.
Figure 6: Expression and AS patterns of OsSR genes.
DBF, day before fertilization; DF, day of flowering; DAF, day after fertilization. The numbers after the black arrows indicate the size of the amplification products. The diagrams on the right are schematic diagrams of alternatively spliced transcripts, red arrows indicate primers, the numbers on the right indicate the expected size of products. For OsRS29, a band with a size of about 300 bp between the target fragments of 147 bp and 571 bp was a non-specific amplification in the spikelets at 10 days after flowering according to the sequencing result.The OsSCL30a belonging to the SCL subfamily produced four transcripts, but the expression of the isoform 1 was dominant compared with other transcripts. The expression of isoform 1 could be observed in various tissues, while isoform 2, isoform 3, and isoform 4 accumulated only in the vegetative tissues including root, stem, and leaf (Fig. 6). The OsSC34 from the SC subfamily produced three transcripts, while the expression of the isoform 1 was much more abundant than the other transcripts, which were mainly accumulated in the leaf and stem (Fig. 6). As for three genes in the RS2Z subfamily, the OsRS2Z36 produced only one transcript (Fig. 6). The OsRS2Z38 produced two transcripts, and the isoform 1 was predominantly accumulated in all tissues. There were four different transcripts that produced by OsRS2Z37, the isoform 1 or 2 was observed in all the tissues while the isoform 3 and isoform 4 were detected in different tissues except the root. Moreover, compared to the other two isoforms, the isoform 1 or 2 of OsRS2Z37 was more abundant in all tissues. The AS pattern of OsRSZ21 belonging to RSZ subfamily in different tissues was analyzed (Fig. 6). Two transcripts of OsRSZ21 were observed, and the isoform 2 expressed more abundantly. The AS expression pattern of two SR genes belonging to RS subfamily in rice was detected in various tissues (Fig. 6). Compared to isoform 3 or 4, the isoform 1 or 2 generated by OsRS29 was more abundant in all tested tissues. For OsRS33, a total of four different transcripts were observed in different tissues, and the isoform 1 was detected in almost all tested tissues. In the SR subfamily, OsSR32 mainly produced isoform 1 or 2 in various tissues (Fig. 6). It was observed that the two variants generated by OsSR33a had equivalent expression levels within the same tissue, such as in the stem, leaf, and spikelet at 5 days after flowering, while for the other tested tissues, isoform 1 produced by OsSR33a was much more abundant compared to isoform 2 within the same tissue.
Expression of OsSR genes in response to abiotic stresses
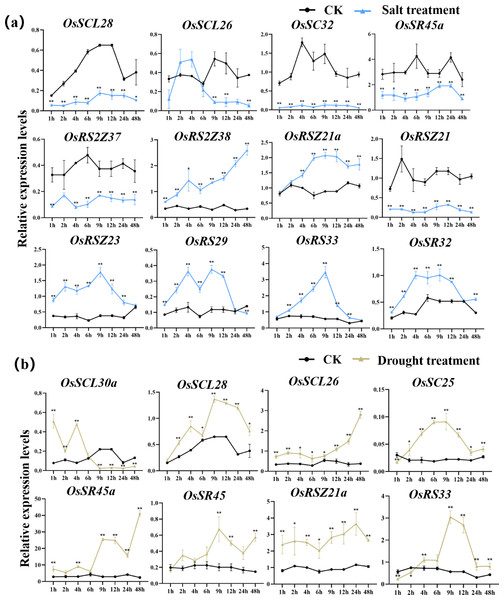
According to the analysis of cis-acting elements in Fig. 4, the response of 24 OsSR genes to different environmental stress were examined (Tables S10–S14). The OsSR genes exhibited different expression patterns in response to the salt stress (Fig. 7A). The OsSCL28, OsSC32, OsSR45a, OsRS2Z37, and OsRSZ21 displayed similar response patterns to salt stress. After being exposed to salt stress for 1 h to 2 days, the expression of these five genes was significantly down-regulated compared to the control. The expression levels of OsRS2Z38, OsRS2Z21a, OsRSZ23, OsRS29, OsRS33, and OsSR32 were significantly induced by salt stress after 1 h of treatment. Among them, the expression levels of OsRS2Z38 and OsRS2Z21a showed a steady increase relative to the control. The response of OsSCL26 to salt stress appeared after 9 h treatment, the expression of OsSCL26 was down-regulated dramatically. The significant and steady induction or inhibition of expression levels were not observed in other OsSR genes, which had the similar expression patterns to the mock treatment (Fig. S4).
Figure 7: Expression of OsSR genes in response to salt (A) and drought (B) stress.
OsActin was used as control. Error bars represent mean ± SE of three biological replicates. ∗P < 0.05 and ∗∗P < 0.01 indicate significant differences compared with CK determined by Student’s t-test.Under drought stress (Fig. 7B), there was the evident increase in the expression level of OsSCL28, OsSCL26, OsSR45a, and OsRSZ21a after treatment for 1 h to 2 days. Different OsSR genes were responsive to drought with various degrees. The expression levels of OsSC25, OsSR45, OsRSZ21, and OsRS33 were considerably up-regulated from 2 h, 9 h, 9 h, and 4 h after treatment, respectively, while OsSCL30a was up-regulated within 4 h of treatment, and the suppressed expression of OsSCL30a was observed after drought treatment for 9 h. The remaining OsSR genes exhibited no obvious patterns in response to drought stress (Fig. S5).
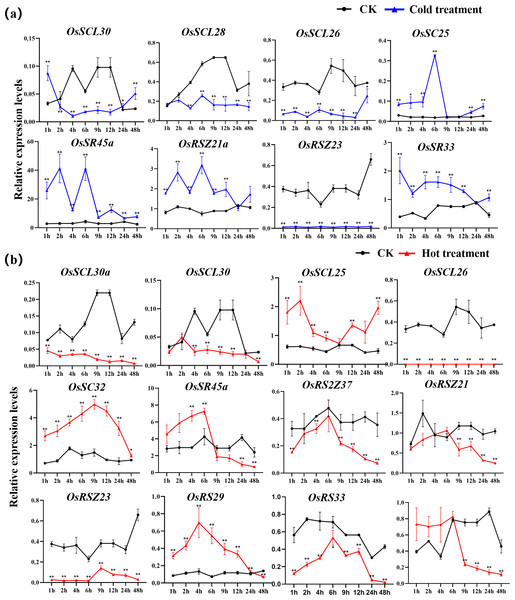
The expression of OsSC25, OsSR45a, OsRSZ21a, and OsSR33 were induced by the cold treatment (Fig. 8A). The induced expression of OsSC25 peaked at 6 h after treatment. The induction of OsSR45a was strong, the expression level of OsSR45a increased by more than 10 times compared to the control within 1 h to 9 h after treatment. Under cold stress, the expression levels of OsSCL30, OsSCL28, OsSCL26, and OsRSZ23 were remarkably decreased. The OsRSZ23 showed an exaggerated response to the cold treatment, its expression was almost completely suppressed under low temperature. Furthermore, the expression levels of other OsSR genes fluctuated, but the changes were slight between treatment and control (Fig. S6).
Figure 8: Expression of OsSR genes in response to cold (A) and hot (B) stress.
OsActin was used as control. Error bars represent mean ± SE of three biological replicates, ∗P < 0.05 and ∗∗P < 0.01 indicate significant differences compared with CK determined by Student’s t-test.The OsSR genes were responsive to high temperature with various patterns and degrees (Figs. 8B and S7). Heat treatment induced the significant down-regulation of OsSCL30a, OsSCL30, OsSCL26, OsRS2Z37, OsRSZ23 and OsRS33 (Fig. 8B). Notably, the expression of OsSCL26 gene was almost completely suppressed under heat stress. The results showed that heat treatment significantly upregulated the expression of OsSCL25 and OsSC32 for 1 h to 2 days (Fig. 8B). After exposure to heat stress within 6 h, the expression of OsSR45a was remarkably induced, and it was observed to be down-regulated after 9 h of treatment. The expression of OsRS29 was up-regulated within 1 day of heat treatment and began to decrease after 1 day. The response of OsRSZ21 and OsSR33 to heat stress appeared after 9 h of treatment, showing a significant down-regulation (Fig. 8B).
Expression of OsSR genes in response to hormones
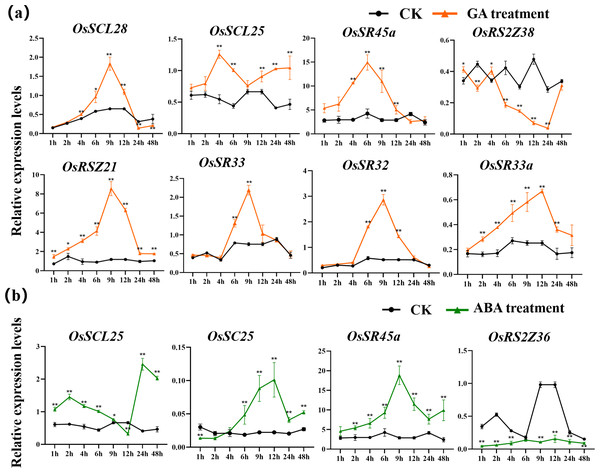
The expression patterns of OsSR genes under different phytohormone treatments were investigated by qRT-PCR (Figs. 9, S8, S9, Tables S10 and S15–S16). We focused on two hormones, ABA and GA, which are essential for plant growth and development. The results showed that OsSCL25, OsRSZ21 and OsSR33a were significantly induced by GA. OsSCL28, OsSR45a, OsSR32 and OsSR33 were induced by GA after treatment for 1 h to 12 h (Fig. 9A), the induced peak values appeared at about 9 h. After being treated with GA for approximately 5 h, there was a considerable decrease in the expression level of OsRS2Z38, then the level gradually increased from 24 h time point and recovered to the similar level compared to the control at 48 h time point (Fig. 9A).
Figure 9: Expression of OsSR genes in response to hormones.
OsActin was used as control. Error bars represent mean ± SE of three biological replicates. ∗P < 0.05 and ∗∗P < 0.01 indicate significant differences compared with CK determined by Student’s t-test.Only a few OsSR genes showed obvious response patterns under exogenous ABA treatment. As compared with the expression under mock treatment, the ABA treatment resulted in a significant increase in that of OsSCL25 and OsSR45a, while the change in expression of OsRS2Z36 was found to be inverse, which was shown to be suppressed by ABA treatment (Fig. 9B).
Discussion
SR proteins, which work as the splicing regulators, play indispensable role in constitutive and alternative splicing of pre-mRNA. The SR gene family has been identified in many plant species, such as Arabidopsis, rice, wheat, maize, cotton and longan (Chen et al., 2019b; Chen et al., 2020b; Jin, 2022; Wei et al., 2022). In this study, we focused on the 24 SR genes in rice, including their classification, gene and protein structure, chromosomal location, evolution, cis-elements, expression profiles, and response to abiotic stresses and hormones.
Domains and physicochemical properties of OsSR proteins
The SR proteins were evolutionarily conserved at the structural level. Previous studies have shown that these conserved domains are essential for the protein to function properly in plants. In Arabidopsis, N- or C-RS domains were necessary for the accurate nuclear localization of atSR30 and atSR45a (Mori et al., 2012). It has been reported that RSZp22, which was the member of the RSZ subfamily in Arabidopsis, displayed speckle-like distribution and localization in the nucleus, and the realization of this accurate localization was inseparable from the presence of RRM and zinc-knuckle in the protein sequence of RSZp22 (Rausin et al., 2010). The analysis of domain and protein structural characteristics in this study laid the foundation for further understanding the function of OsSR. OsSR genes with closer evolutionary relationship were similar in protein domain distribution and protein structure, indicating that OsSR genes belonging to the same subfamily would have similar functions. We found that the OsSR proteins in the same subfamily showed similar motif arrangements and considerable variation among different subfamilies (Fig. 1B). All OsSR proteins were found to have the RRM domain, and the Zn_C2HC domain was contained in the RSZ and RS2Z subfamilies (Fig. 1). In addition, the distribution of domains in OsSR proteins were significantly different among different subfamilies, which may be related to the functional differentiation of OsSR proteins. However, there are few studies on the effect of conservative domains on the proper function of OsSR proteins. Further research could be conducted to edit the RRM, SR or Zn_C2HC domain of OsSR genes by gene editing technology.
The SR proteins are essential nuclear localized proteins that function as splicing factors in splicing of precursor mRNA (Misteli, Cáceres & Spector, 1997). In the current study, subcellular localization of most OsSR proteins was in the nucleus (Table 1), indicating the OsSR proteins could function as splicing factors as those in other species. Intriguingly, SR proteins were also involved in post-splicing activities, which were achieved through continuous shuttling between the nucleus and the cytoplasm (Huang & Steitz, 2001; Michlewski, Sanford & Cáceres, 2008; Swartz et al., 2007). The state of phosphorylation and dephosphorylation was the key factor affecting the dynamic subcellular localization of SR proteins (Jin, 2022; Mori et al., 2012). For example, studies have proved that RSZp22 in Arabidopsis thaliana was a nucleocyto-plasmic shuttling protein, and its shuttling property has been analyzed in details (Rausin et al., 2010; Tillemans et al., 2006). In our study, the OsSR proteins contained different amounts of phosphorylation sites (Table 1), indicating OsSR proteins could function by shuttling between nucleus and the cytoplasm. Further research is needed on dynamic distribution of OsSR proteins and how this process affects post-splicing activities including mRNA export.
The orthologous SR gene pairs between rice and other species provide insights into the evolution and function of the OsSR genes
Synteny refers to the distribution or arrangement of homologous genes within one specie or among different species (McCouch, 2001). Collinearity analysis in this study revealed the distribution of orthologous genes of SR genes between rice and other species, which helped us further understand the origin of the OsSR genes. Compared to a larger number of orthologous SR gene pairs identified between rice and other monocotyledonous plants, no ortholog was found in soybean and only four orthologs of OsSR genes were found in Arabidopsis (Fig. S2, Fig. 3 and Table S4), suggesting that the development of orthologous SR pairs was more probable to occur after the divergence of dicots and monocots. Evidently, multiple TaSR genes were identified as orthologs of single OsSR gene. For instance, TaSR4D, TaSR7A, TaSR6B, TaSR7D and TaSR14D were the orthologs of OsSR33 (Table S4), indicating the expansion of OsSR genes may occur before that of wheat. These orthologous SR genes in different species may have similar functions that are involved in constitutive and alternative pre-mRNA splicing, and post-splicing activities.
The orthologous genes in different species may have originated from a common ancestor. The sequences of orthologous genes are conserved, indicating the conservation of function of these genes (Tang et al., 2008). Understanding the function of these orthologous genes is helpful to reveal and explore the function of OsSR genes. Among the orthologous SR genes identified in other species in this study, there have been some reports on their gene functions. AtSR34 in Arabidopsis, the orthologous gene of rice OsSR33a and OsSR33 has been reported to be related to heat stress response (Ling et al., 2018).
AS-NAGNAG events were not frequent on OsSR genes
NAGNAG splicing produces two distinct isoforms that are distinguished by three nucleotides (NAG, N = A, C, G, T). Due to the fact that SR genes would undergo alternative splicing, one SR gene can generate several transcripts that encode different isoforms, which give the spliceosome greater spatial flexibility, and influence the outcome of splicing (Graveley, 2000). Protein diversity induced by the AS-NAGNAG contributes to this flexibility to some extent. The previous studies have reported that NAGNAG acceptor motifs were frequent in human genes and SR genes in Arabidopsis (Hiller et al., 2004; Schindler et al., 2008). Here, we screened for NAGNAG acceptor tandems in OsSR genes. A total of 19 NAGNAG acceptors were identified in 14 OsSR genes, belonging to seven subfamilies (Table 2). However, AS-NAGNAG events were only observed at the location of the three acceptors. But we only summarized the AS-NAGNAG events of OsSR genes under normal growth conditions. Notably, the different tissues and the change of environmental conditions could affect the alternative splicing rate occurring at the NAGNAG acceptor. For example, the AS-NAGNAG events in Arabidopsis may be mediated by the organ and condition-specific differences of the spliceosome (Schindler et al., 2008). Thus, how these factors affect NAGNAG alternative splicing in OsSR genes remains to be evaluated.
The AS pattern of OsSR genes varied with different tissues
Most SR genes themselves undergo extensive alternative splicing (Reddy & Shad Ali, 2011). This study investigated the alternative splicing pattern as well as the expression of different transcripts produced by the OsSR genes in both vegetative and reproductive tissues (Fig. 6). The alternative splicing patterns of OsSR genes were tissue-specific, which means the expression levels of different transcripts produced by the same OsSR genes varied greatly in different tissues, but most OsSR genes mainly express one transcript in each tissue. Previous studies performed the investigation of all splicing variants of SR genes in Arabidopsis. Notably, the majority of these alternative splicing occurred within the coding region of SR genes, and the AS type on SR genes in Arabidopsis was mainly intron retention (Palusa, Ali & Reddy, 2007). Interestingly, we found in most cases, AS events of OsSR genes occurred in the 3′ or 5′ untranslated regions, which would not cause the corresponding genes to generate new protein coding sequences (Fig. 6 and Table S1). We speculated that such splicing may have an impact on the expression and stability of precursor mRNA (Jin, 2022). Nevertheless, some OsSR genes such as OsSCL30a, OsRS2Z37, OsRS2Z38, etc., could undergo alternative splicing in the coding region and generate transcripts with different CDS, which means they could encode different proteins (Fig. 6 and Table S1). Moreover, these different transcripts produced by the same OsSR gene had tissue expression specificity, and some transcripts could only be detected in specific tissues. For example, isoform 3 and isoform 4 produced by OsRS29 were only detected in stem, leaf and spikelets at 10 days after flowering (Fig. 6), indicating that the proteins encoded by these transcripts were only expressed in specific tissues. Studies have shown that different transcripts of one gene produced by alternative splicing may perform distinct functions. The SR gene SR45 in Arabidopsis could produce two transcripts, and SR45.1 played a role in flower development, while SR45.2 was involved in regulating root growth and development (Zhang & Mount, 2009). Whether there are functional differences between different transcripts produced by the same OsSR gene in rice needs further exploration and research.
OsSR genes may function in plant growth, response to hormones and abiotic stresses
In the current study, we found that the majority of OsSR genes expressed extensively with different levels in stems, leaves, or spikelets. The expression patterns of different OsSR genes were tissue and development stage dependent, indicating their specific functions. Based on the detection results of gene tissue-specific expression (Fig. 5), we speculated that SCL, SC, and RS2Z subfamily genes may be involved in regulating the development of vegetative organs in rice, while the OsSR genes in SR45, RSZ, and RS subfamily were more likely to participate in regulating the formation and filling of grains in rice.
The growth of plants could be profoundly influenced by a variety of environmental conditions. The transcription levels of related genes could be induced, repressed or regulated by various stresses (Palusa, Ali & Reddy, 2007). However, the expression profiles of OsSR genes under various stresses have not been detected till now. Inducing or inhibiting the binding of transcription factors to the corresponding cis-acting sites in the gene promoter region to regulate the expression of downstream genes is an important mechanism to respond to environmental changes (Riechmann et al., 2000). The identification of cis-elements provides clues for determining gene expression patterns under different kinds of stresses. The promoter analysis in this study suggested that OsSR genes played important roles in various stress responses in rice. In the promoter region of OsSR genes, different types of cis-acting elements were discovered, including 10 hormone-responsive and 5 stress-responsive elements (Fig. 4 and Table S5). The expression of genes is influenced by plant growth stage and environment. For the gene whose function is unknown, the distribution of cis-elements in the genes’ promoter region could not directly reflect gene expression, but could provide information for us to further explore the pathway of this gene participating in response. Our results showed the expression of some of OsSR genes were changed after abiotic or hormone treatment (Figs. 7, 8 and 9), indicating that they modulated the response to stresses in rice. These results lay a foundation for further understanding the function of OsSR genes. For example, our experiment showed that under salt treatment, the expression of OsRS33 was significantly upregulated (Fig. 7), which was consistent with the previous study that OsRS33 gene knockout lines were more sensitive to salt stress compared with the wild type (Butt et al., 2022). We found OsSCL30 was obviously and continuously suppressed by the cold treatment, and in fact, OsSCL30 was related to cold tolerance in rice, overexpression of OsSCL30 reduced the tolerance of rice seedlings to low temperature (Zhang et al., 2022).
Besides, we observed that some OsSR genes respond to a variety of stresses simultaneously. A summary of differentially expressed OsSR genes in response to various abiotic stresses was provided in the Table S8. The results indicated that response patterns to abiotic stresses of OsSR genes were time-dependent and varied among different genes. OsSCL30, OsSCL26 and OsRSZ23 responded to both cold and heat stress, suggesting that the expression of these genes was affected by ambient temperature (Fig. 8, Table S8). Moreover, the expression of OsSC25 and OsRSZ21a genes was affected by both drought and cold stress, while the expression of OsSC32 and OsRSZ23 was affected by both salt and temperature stress (Figs. 7 and 8 and Table S8). In addition, OsSCL25 and OsSR45a were found to respond to GA and ABA simultaneously (Fig. 9, Table S8). In Arabidopsis, SR45a responded to ABA and abiotic stresses (Cruz et al., 2014; Ling, Mahfouz & Zhou, 2021). Consistently, we found that OsSR45a, a member in SR45 subfamily, could also respond to multiple stresses simultaneously. The results showed that ABA, GA, salt, drought and temperature stress significantly affected the expression level of OsSR45a (Figs. 7, 8 and 9), indicating that expression pattern of OsSR45a were stress-dependent. Altogether, these results strongly suggest that OsSR genes are critical in response to environmental signals in rice, and the function and mechanism of OsSR genes could be further studied based on the results in this study.
Conclusions
In this study, the comprehensive analysis on OsSR genes gave some insights on their characteristics and functions. It showed that 24 OsSR genes were distributed in seven different subfamilies based on the phylogenetic analysis. Gene structures of OsSR genes, distribution of domains, and protein structure of OsSRs were conserved within each subfamily. There were six segmental duplicated OsSR gene pairs (50%) in the rice genome, indicating segmental duplication played an overwhelming role in the expansion of SR gene family in rice. Most of OsSR genes would undergo AS and the AS patterns varied with different tissues. The majority OsSR genes were expressed in different tissues, while their expression levels varied substantially among different organs, suggesting their extensive functions in vegetative growth or spikelet development in rice. Furthermore, the expression patterns of OsSR genes would change significantly under abiotic stress or hormone treatment, indicating that OsSR genes may participate in the hormone/abiotic stress signaling pathway in rice. The current results will be helpful for better understanding and further study of OsSR genes.
Supplemental Information
Summary of alternative splicing events in rice serine/arginine-rich (SR) genes according to the information from MSU-RGAP and RAP DB
The orthologous gene pairs of SR genes between rice and other four plant spieces
Summary of changes in the expression of the rice serine/arginine-rich (SR) genes in response to abiotic stresses
Predicted protein structure of OsSR proteins
Predicted three-dimensional domains of OsSR proteins. 3D models of the 24 OsSR proteins according to SWISS-MODEL.
Synteny analyses of SR genes between rice (Oryza sativa) and soybean (Glycine max)
Gray lines indicated collinear blocks and syntenic SR gene pairs would be highlighted in blue lines.
Expression profiling of the OsSR genes in 8 tissues based on qRT-PCR
DBF, Day before fertilization; DF, Day of flowering; DAF, Day after fertilization. OsActin was used as control, error bars represent mean ± SE of three biological replicates.
Expression of OsSR genes in response to salt stress
OsActin was used as control. Error bars represent mean ±SE of three biological replicates. ∗P < 0.05 and ∗∗P < 0.01 indicate significant differences com-pared with CK determined by Student’s t-test.
Expression of OsSR genes in response to drought stress
OsActin was used as control. Error bars represent mean ± SE of three biological replicates. ∗P < 0.05 and ∗∗P < 0.01 indicate significant differences com-pared with CK determined by Student’s t-test.
Expression of OsSR genes in response to cold stress
OsActin was used as control. Error bars represent mean ± SE of three biological replicates. ∗P < 0.05 and ∗∗P < 0.01 indicate significant differences com-pared with CK determined by Student’s t-test.
Expression of OsSR genes in response to hot stress
OsActin was used as control. Error bars represent mean ± SE of three biological replicates. ∗P < 0.05 and ∗∗P < 0.01 indicate significant differences com-pared with CK determined by Student’s t-test.
Expression of OsSR genes in response to GA treatment
OsActin was used as control. Error bars represent mean ± SE of three biological replicates. ∗P < 0.05 and ∗∗P < 0.01 indicate significant differences com-pared with CK determined by Student’s t-test.
Expression of OsSR genes in response to ABA treatment
OsActin was used as control. Error bars represent mean ± SE of three biological replicates. ∗P < 0.05 and ∗∗P < 0.01 indicate significant differences com-pared with CK determined by Student’s t-test.