A nomogram model for predicting lower extremity deep vein thrombosis after gynecologic laparoscopic surgery: a retrospective cohort study
- Published
- Accepted
- Received
- Academic Editor
- Jinhui Liu
- Subject Areas
- Cardiology, Gynecology and Obstetrics, Nursing, Surgery and Surgical Specialties, Women’s Health
- Keywords
- Gynecological surgery, Laparoscopy, Lower extremity deep vein thrombosis, Risk factors, Nomogram model
- Copyright
- © 2023 Zhao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. A nomogram model for predicting lower extremity deep vein thrombosis after gynecologic laparoscopic surgery: a retrospective cohort study. PeerJ 11:e16089 https://doi.org/10.7717/peerj.16089
Abstract
Objective
To investigate the risk factors associated with lower extremity deep vein thrombosis (LEDVT) and to establish a predictive model for patients who undergo gynecologic laparoscopic surgery.
Methods
A review of clinical data was conducted on patients who underwent gynecologic laparoscopic surgery between November 1, 2020, and January 31, 2022. Patients who developed LEDVT after surgery were included as the observation group, while the control group comprised patients who did not experience complications. Multivariate forward stepwise logistic regression models were used to identify independent risk factors associated with LEDVT. A nomogram model was then developed based on these risk factors.
Results
A total of 659 patients underwent gynecologic laparoscopic surgery during the study period, and 52 (7.89%) of these patients developed postoperative LEDVT. Multivariate logistic regression analysis showed that older age (adjusted OR, 1.085; 95% CI [1.034–1.138]; P < 0.05), longer operation duration (adjusted OR, 1.014; 95% CI [1.009–1.020]; P < 0.05), shorter activated partial thromboplastin time (APTT) (adjusted OR, 0.749; 95% CI [0.635–0.884]; P < 0.05), higher D-dimer (adjusted OR, 4.929; 95% CI [2.369–10.255]; P < 0.05), higher Human Epididymis Protein 4 (HE4) (adjusted OR, 1.007; 95% CI [1.001–1.012]; P < 0.05), and history of hypertension (adjusted OR, 3.732; 95% CI [1.405–9.915]; P < 0.05) were all independent risk factors for LEDVT in patients who underwent gynecologic laparoscopic surgery. A nomogram model was then created, which had an area under the curve of 0.927 (95% CI [0.893–0.961]; P < 0.05), a sensitivity of 96.1%, and a specificity of 79.5%.
Conclusions
A nomogram model that incorporates information on age, operation duration, APTT, D-dimer, history of hypertension, and HE4 could effectively predict the risk of LEDVT in patients undergoing gynecologic laparoscopic surgery, potentially helping to prevent the development of this complication.
Introduction
Deep vein thrombosis (DVT) is a common complication that typically occurs within one week after surgery. If venous thrombosis dislodges, it can cause acute pulmonary embolism, which poses a serious threat to the patient’s life (Gutzeit et al., 2020; Hui et al., 2020; Kaya et al., 2021). Recently, with the advancements in surgical technology and equipment, laparoscopic surgery has gained popularity in gynecology due to its advantages such as lesser physical damage, reduced stress response, lower blood loss, and faster recovery (Vedantham, 2020). However, the incidence of lower extremity deep vein thrombosis (LEDVT) after gynecological laparoscopic surgery is 4.0%, which is not lower than that of open surgery due to factors like surgical posture and pneumoperitoneum (Chan & Weitz, 2019; Chong, Bui & Menhaji, 2020; Qu et al., 2015). Gynecological disease is a high risk factor for developing venous thromboembolism (Abu Saadeh et al., 2013). The occurrence of LEDVT can negatively affect the limb motor function of patients and decrease their quality of life, making it crucial to accurately identify high-risk patients for LEDVT (Han et al., 2021; Liu et al., 2006; Moragon-Ledesma et al., 2020).
In current practice, the diagnosis and prediction of LEDVT primarily rely on the Caprini score and Padua score, alongside laboratory examination and ultrasonic imaging examination (Willan, Katz & Keeling, 2019). These scoring systems were initially published in the 1990s and early 21st century and were found to be valuable in predicting the onset of LEDVT in open surgery. However, their predictive efficacy in laparoscopic surgery remains uncertain (Lu et al., 2021; Yang et al., 2019b).
Many previous studies have reported that their model has considerable predictive value for LEDVT (Hu et al., 2022; Tian & Li, 2021; Wu & Cheng, 2020). However, they did not take into account the relevant preoperative biochemical indicators. Some studies have revealed that D-dimer is an independent risk factor for LEDVT (Yago et al., 2020; Zhao, Tian & Zhang, 2021). However, the influence of other biochemical indicators on LEDVT remains unclear. Gynecological patients undergo blood biochemical tests before laparoscopic surgery, which makes it convenient to investigate the relationships between biochemical indicators and LEDVT.
The aim of this study was to identify independent risk factors and develop a predictive model for the occurrence of LEDVT in patients who underwent gynecological laparoscopic surgery.
Methods
Study design and cohort
This retrospective study was approved by the Institutional Review Board of Fujian Provincial Maternity and Children’s Hospital (2022YJ028). The requirement of informed consent was waived due to the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki.
The clinical records and data of patients who underwent gynecologic laparoscopic surgery at our institution between November 1, 2020 and January 31, 2022, were collected and reviewed. This study included patients if they met the following criteria: (1) age ≥ 20 years; (2) underwent elective gynecologic laparoscopic surgery; (3) had complete clinical data; (4) had a normal preoperative color ultrasound examination of lower limb veins or normal D-dimer indicators within three days before surgery; (5) did not use any hormonal drugs before surgery. Patients were excluded if they met any of the following criteria: (1) had a prior diagnosis of LEDVT or pulmonary embolism before admission; (2) were preoperative patients with severe underlying diseases or intolerance to general anesthesia; (3) had liver or kidney dysfunction; (4) were perinatal women.
A total of 659 patients were included in the study. Out of these, 52 patients developed LEDVT after surgery, and were classified as the observation group. The remaining 607 patients who did not develop any complications were chosen as the control group.
Data collection and variables
The clinical records and data of patients were collected by members of the research team. The diagnostic criteria for LEDVT states that the vascular ultrasound must show low echo with a disappearance of blood flow signal in the vascular lumen, and no change should be found after applying pressure with a probe to the blood vessel. The variables in the study include age, height, weight (measured at admission), body mass index (BMI), history of varicose veins, history of hypertension, history of diabetes, operation duration, abdominal air pressure, white blood cell count, neutrophil count, lymphocyte count, platelet count, platelet accumulation, hemoglobin, hematocrit, activated partial thromboplastin time (APTT), thrombin time, international standardized ratio, apolipoprotein A1, apolipoprotein B, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, D-dimer, carbohydrate antigen 125 (CA125), carbohydrate antigen 199 (CA199), Carbohydrate antigen 153 (CA153), carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), squamous cell carcinoma antigen (SCCA), and human epididymis protein 4 (HE4).
Statistical analysis
Data analyses were performed using SPSS version 26.0 (IBM Inc., Chicago, IL, USA). Categorical variables were compared with the chi-squared test. The Student’s t-test was used to compare the continuous variables between the two groups when measurement data met normal distribution and homogeneity of variance. It was described by the median (P25, P75) and the non-parametric rank sum test was used when measurement data did not meet normal distribution and homogeneity of variance. Independent prognostic factors for LEDVT were identified using multivariate full entry logistic regression models. The criterion value for LEDVT prediction was determined through receiver operating characteristic (ROC) curve analysis. The criteria from other studies (age, APTT, D-dimer and history of hypertension) were applied to the entire patient population of this study as to determine a reference predictive model (Han et al., 2021; Liu & Peng, 2022; Liu et al., 2021; Liu et al., 2006). This is followed by Cohen’s kappa coefficient (κ) analysis to compare predictive value between the criteria from other studies and the predictive model suggested by this study (Morrison et al., 2013). The RMS program package of R software (v3.6.2; The R Foundation, Vienna, Austria) was used to build the nomogram predictive model, and the consistency coefficient (C-index) was used to evaluate the predictive value of the predictive model for LEDVT. The bootstrap method was used to sample the data randomly 500 times for internal verification, grab the calibration curve, and evaluate the model’s compliance. A two-sided P-value <0.05 was considered statistically significant.
Results
Participants’ characteristics and univariate analysis
The clinical characteristics of the 659 patients were summarized in Table 1. The univariate analysis revealed no significant difference in height, air abdominal pressure, white blood cell count, neutrophil count, lymphocyte count, platelet count, platelet accumulation, thrombin time, international standardized ratio, low-density lipoprotein cholesterol, apolipoprotein B, triglyceride, CA199, CA153, CEA, AFP, and SCCA between the two groups (P > 0.05). However, compared to the control group, the observation group had significantly higher age, weight, BMI, operation time, history of varicose veins, hypertension, diabetes, D-dimer, CA125, and HE4 (P < 0.05). In contrast, the observation group had a lower rate of hemoglobin, hematocrit, APTT, high-density lipoprotein cholesterol, and apolipoprotein A1 than those in the control group (P < 0.05).
| Characteristics | Non-LEDVT group | LEDVT group | P |
|---|---|---|---|
| Number (%) | 607 (92.1) | 52 (7.9) | |
| Age, mean (SD), years | 39.66 (10.31) | 50.79 (7.55) | <0.001 |
| Weight, mean (SD), kg | 57.16 (8.16) | 60.09 (8.02) | 0.013 |
| Height, mean (SD), meters | 1.59 (0.05) | 1.59 (0.05) | 0.365 |
| BMI, mean (SD), kg/m2 | 22.51 (3.07) | 23.85 (2.99) | 0.003 |
| Operation duration, mean (SD), min | 126.12 (53.32) | 210.19 (98.40) | <0.001 |
| Air abdominal pressure, mean (SD), mmHg | 13.98 (0.478) | 14.06 (0.574) | 0.319 |
| History of varicose vein, n (%) | 0.033 | ||
| Yes | 2 (0.33) | 2 (3.85) | |
| No | 605 (99.67) | 50 (96.15) | |
| Hypertension, n (%) | <0.001 | ||
| Yes | 34 (5.60) | 17 (32.69) | |
| No | 573 (94.4) | 35 (67.31) | |
| Diabetes, n (%) | 0.036 | ||
| Yes | 12 (1.98) | 4 (7.69) | |
| No | 595 (98.02) | 48 (92.31) | |
| White blood cell count, Median (25%, 75%), ×109/L | 5.67 (4.74, 6.63) | 5.64 (4.48, 6.97) | 0.965 |
| Neutrophil count, Median (25%, 75%), ×109/L | 3.06 (2.55, 3.98) | 3.09 (2.42, 4.40) | 0.970 |
| Lymphocyte count, Median (25%, 75%), ×109/L | 1.82 (1.52, 2.13) | 1.77 (1.28, 2.18) | 0.196 |
| Platelet count, Median (25%, 75%), ×109/L | 268 (227, 314) | 276 (231, 339) | 0.503 |
| Platelet accumulation, Median (25%, 75%), % | 0.28 (0.24,0.31) | 0.28 (0.24,0.32) | 0.792 |
| Hemoglobin, Mean (SD), g/L | 124.2(16.724) | 116.73(19.879) | 0.002 |
| Hematocrit, Median (25%, 75%), % | 38.10 (35.70, 40.10) | 36.80 (33.30, 39.35) | 0.013 |
| APTT, Median (25%, 75%), seconds | 27.30 (25.58,28.90) | 24.80 (22.95, 26.35) | <0.001 |
| Thrombin time, Median (25%, 75%), Seconds | 17.00 (16.50,17.40) | 17.20 (16.60, 17.65) | 0.097 |
| International standardized ratio, Median (25%, 75%), % | 0.92 (0.88, 0.96) | 0.93 (0.90, 0.95) | 0.208 |
| D-dimer, Median (25%, 75%), mg/L FEU | 0.20 (0.14, 0.31) | 0.45 (0.21, 1.09) | <0.001 |
| High-density lipoprotein cholesterol, Median (25%, 75%), mmol/L | 1.42 (1.20, 1.61) | 1.28 (1.10, 1.46) | 0.005 |
| Low-density lipoprotein cholesterol, Median (25%, 75%), mmol/L | 3.07 (2.67, 3.58) | 2.88 (2.48, 3.91) | 0.302 |
| Apolipoprotein A1, Median (25%, 75%), g/L | 1.16 (1.04, 1.27) | 1.10 (1.02, 1.21) | 0.033 |
| Apolipoprotein B, Median (25%, 75%), g/L | 0.83 (0.73, 0.96) | 0.85 (0.76, 1.07) | 0.153 |
| Triglyceride, Median (25%, 75%), mmol/L | 1.21 (0.88, 1.77) | 1.17 (0.86, 1.59) | 0.582 |
| CA125, Median (25%, 75%), U/ml | 15.6 (9.78, 24.4) | 25.9 (13.39, 55.45) | <0.001 |
| CA199, Median (25%, 75%), U/ml | 8.01 (4.06, 15.69) | 7.36 (3.53, 29.16) | 0.989 |
| CA153, Median (25%, 75%), U/ml | 8.40 (6.18, 12.25) | 7.5 (5.1, 13.00) | 0.495 |
| CEA, Median (25%, 75%), ng/ml | 1.35 (0.89, 1.80) | 1.60 (1.05, 2.46) | 0.057 |
| AFP, Median (25%, 75%), ng/ml | 2.50 (1.77, 3.51) | 2.19 (1.57, 3.32) | 0.363 |
| SCCA, Median (25%, 75%), ng/ml | 0.80 (0.60, 1.10) | 0.70 (0.58, 1.00) | 0.298 |
| HE4, Median (25%, 75%), pmol/L | 33.16 (24.86, 45.62) | 45.70 (34.80, 66.08) | <0.001 |
Notes:
- APTT
-
activated partial thromboplastin time
- CA125
-
carbohydrate antigen 125
- CA199
-
carbohydrate antigen 199
- CA153
-
carbohydrate antigen 153
- CEA
-
carcinoembryonic antigen
- AFP
-
alpha-fetoprotein
- SCCA
-
squamous cell carcinoma antigen
- HE4
-
epididymis protein 4
Logistic regression analysis
Based on the statistically significant indicators in the univariate analysis above, the factors with significant differences between the two groups were analyzed for collinearity. The results indicated that the variance inflation factor (VIF) was 13.404 for hemoglobin, 13.457 for hematocrit, and less than 10 for the remaining indicators. Consequently, due to the collinearity issue, the two indicators of hemoglobin and hematocrit were excluded from the subsequent multivariate logistic regression analysis.
Multivariate logistic regression analysis revealed that older age (adjusted OR, 1.085, 95% CI [1.034–1.138], P < 0.05), longer operation duration (adjusted OR, 1.014, 95% CI [1.009–1.020], P < 0.05), shorter APTT (adjusted OR, 0.749, 95% CI [0.635–0.884], P < 0.05), higher D-dimer (adjusted OR, 4.929, 95% CI [2.369–10.255], P < 0.05), higher HE4 (adjusted OR, 1.007, 95% CI [1.001–1.012], P < 0.05), and history of hypertension (adjusted OR, 3.732, 95% CI [1.405–9.915], P < 0.05) were independent risk factors for patients with LEDVT (Table 2).
| Characteristic | aOR | 95% Cl | P |
|---|---|---|---|
| Age | 1.085 | 1.034–1.138 | 0.001 |
| Weight | 1.02 | 0.929–1.119 | 0.680 |
| BMI | 0.945 | 0.720–1.240 | 0.683 |
| Operation duration | 1.014 | 1.009–1.020 | <0.001 |
| APTT | 0.749 | 0.635–0.884 | 0.001 |
| D-dimer | 4.929 | 2.369–10.255 | <0.001 |
| High-density lipoprotein cholesterol | 2.202 | 0.216–22.463 | 0.505 |
| Apolipoprotein A1 | 0.095 | 0.001–8.170 | 0.300 |
| CA125 | 1.001 | 0.999–1.003 | 0.431 |
| HE4 | 1.007 | 1.001–1.012 | 0.021 |
| Hypertension | 3.732 | 1.405–9.915 | 0.008 |
| Diabetes | 1.1 | 0.202–5.993 | 0.912 |
| History of varicose vein | 3.823 | 0.191–76.529 | 0.380 |
Notes:
- aOR
-
adjusted odds ratio
- BMI
-
body mass index
- APTT
-
activated partial thromboplastin time
- CA125
-
carbohydrate antigen 125
- HE4
-
epididymis protein 4
All listed covariates in the model were not found to have multicollinearity.
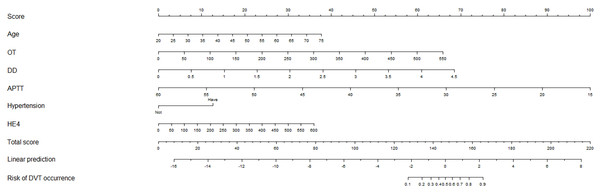
A nomogram model for predicting postoperative LEDVT
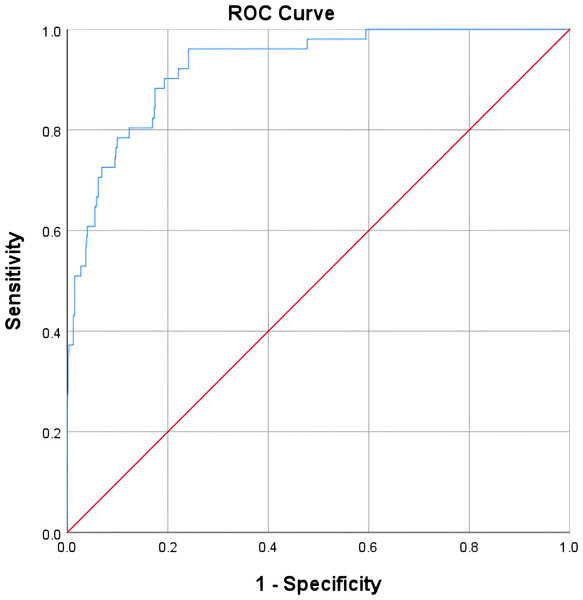
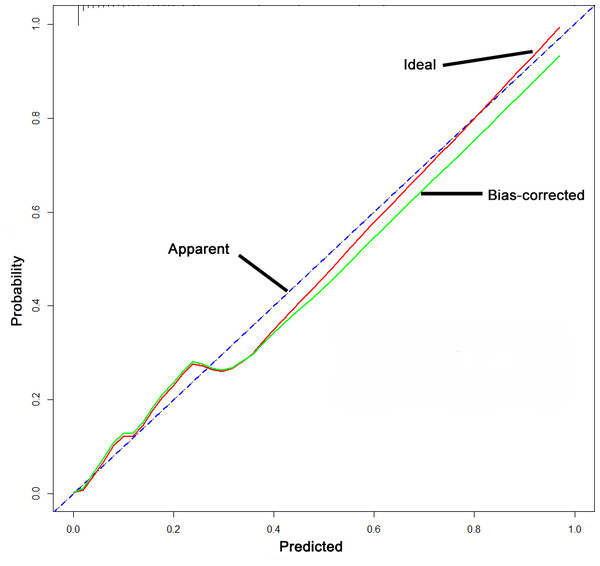
A nomogram predictive model was established for postoperative LEDVT in patients who underwent gynecological laparoscopic surgery according to independent risk factors (Fig. 1). Additionally, a receiver operating characteristic (ROC) curve of the predictive model was established and is shown in Fig. 2. The area under the curve (AUC) was 0.927 (95% CI [0.893–0.961]; P < 0.05), and the value of the Youden index was 0.720, with corresponding sensitivity of 96.1% and specificity of 75.9%. The calibration curve of the nomogram predictive model demonstrated that the C-index was 92.4% (Fig. 3).
Figure 1: Nomogram prediction model of postoperative LEDVT in patients undergoing gynecologic laparoscopic surgery.
Figure 2: Receiver operating characteristic curve for the LEDVT prediction model after gynecological laparoscopy.
Figure 3: Calibration curve of the nomogram predictive model.
Comparison of reference stander and predictive model
The criteria of (age, APTT, D-dimer and history of hypertension) was applied to the entire patient population of this study to determine a reference predictive model (Han et al., 2021; Liu & Peng, 2022; Liu et al., 2021; Liu et al., 2006). Cohen’s kappa coefficient (κ) was 0.375 (P < 0.001) when comparing the prediction of low-risk group between the criteria from other studies and the predictive model suggested by this study were compared.
We also compared the performance of the nomogram model, Caprini score and biochemical indicators (Table 3). The current nomogram model had a better value than Caprini score in sensitivity and specificity. When another model included biochemical indicators (APTT, Thrombin time, D-dimer, CA125 and HE4), it did not have advantage than the current nomogram model.
| Characteristics | AUC, 95% CI | Sensitivity, % | Specificity, % |
|---|---|---|---|
| Model | 0.927 (0.893–0.961) | 96.1 | 79.5 |
| Caprini | 0.684 (0.605–0.763) | 63.5 | 73.3 |
| Biochemical indicators | 0.780 (0.716–0.843) | 78.8 | 64.1 |
Notes:
- AUC
-
area under the receiver operating characteristic curve
- CI
-
confidence interval
Discussion
This research has shown that patients who have undergone gynecological laparoscopic surgery are at risk of LEDVT due to several factors. These risk factors include older age, longer operation duration, shorter activated partial thromboplastin time (APTT), higher D-dimer, higher HE4, and a history of hypertension. To prevent the occurrence of LEDVT in clinics, a nomogram model was established with a sensitivity of 96.1% and a specificity of 75.9%.
Laparoscopic surgery is a commonly employed minimally invasive technique within gynecology. Nonetheless, the incidence of LEDVT remains high due to the utilization of pneumoperitoneum and the patient’s position during surgery (Hu et al., 2022; Wu & Cheng, 2020). A previous study displayed the incidence of LEDVT subsequent to gynecologic laparoscopic surgery to be 7.6–11.55% (Tian & Li, 2021). Consistently, our current study discovered that the incidence of LEDVT following gynecologic laparoscopic surgery was 7.89%. A previous study found various independent risk factors for LEDVT in perinatal women, such as older age, mode of delivery, hypertension, diabetes, history of thrombosis, short activated partial thromboplastin time (APTT), high D-dimer, and fasting blood glucose (Zhao et al., 2018). Additionally, another previous study determined several distinct independent risk factors for LEDVT in patients, such as older age, extended bed rest, high blood viscosity, high D-dimer, and intraoperative blood loss (Evans et al., 2022). These two studies; however, did not examine the value of these risk factors specifically for patients undergoing gynecologic laparoscopic surgery. A previous study established a 16-point evaluation scale for venous thrombosis in gynecological surgery patients. This evaluation scale includes secondary indicators such as age, body mass index (BMI), immobilization preoperatively, specific factors for women, current high-risk disease, past and family medical history, treatment and medication history, surgery and anesthesia duration and mode, special conditions during surgery, posture during surgery, D-dimer, fibrinogen (FIB), APTT, and platelet count (Yang et al., 2019a). However, this study failed to disclose the risk factors specifically for laparoscopic surgery patients and could not predict the risk of LEDVT.
In the present study, we determined that prolonged duration of surgery is a significant risk factor for LEDVT independent of other factors. This prolonged surgical time can result in extended periods of laying down for patients, leading to the relaxation of lower limb muscles along with blood stasis in veins, thereby increasing the chance of LEDVT. Researchers have identified an elevated D-dimer level as a reliable indication of an abnormal blood coagulation system. Furthermore, previous studies have indicated that increased D-dimer levels hold immense importance in identifying LEDVT among peripheral diseases (Komatsu et al., 2020; Li et al., 2019a).
D-dimer, a primary index of coagulation function, holds great significance in the diagnosis, curative effect, and prognosis of thrombotic diseases. The elevation of D-dimer levels indicates active thrombosis or fibrinolytic activity in the blood vessels of patients. This study revealed that an increase in D-dimer is another independent risk factor for LEDVT, which is consistent with several previous studies (Jiang et al., 2021; Li et al., 2019b). However, a previous study showed that D-dimer is a risk factor for venous thromboembolism but not an independent risk factor due to the inclusion of fibrinogen degradation products, which interact with D-dimer (Shen et al., 2020). APTT, a vital indicator for screening the endogenous coagulation system, can reflect the status of coagulation factors in the body. In a hypercoagulable state or thrombosis, a decrease in APTT value indicates that the coagulation and fibrinolysis systems are unbalanced, leading to a hypercoagulable state and a higher risk of LEDVT (Zhang et al., 2019). Hypertension is another risk factor for LEDVT. Most patients with hypertension exhibit dysfunction of the renin-angiotensin-aldosterone system, leading to an increase in water and sodium retention, interstitial fluid, and decreased plasma content in blood vessels, which raises blood viscosity (Barber & Clarke-Pearson, 2016). In hypertension, vascular endothelial cell function is disordered, producing more oxygen-free substances, inactivating more vasodilators, causing vascular inflammation, activating the blood coagulation system, and promoting the onset of LEDVT (Laws et al., 2018). HE4, a member of the whey acidic protein domain-putative extracellular protease inhibitor protein family, serves as a biomarker for malignancy risk evaluation in various neoplastic diseases, especially ovarian cancer (Chhikara et al., 2012; Scaletta et al., 2017). HE4 produced by epithelial or fibroblast-derived cells can exacerbate tissue fibrosis and result in tissue damage (LeBleu et al., 2013; Zhang et al., 2020). An activity could represent an important factor in promoting thrombosis.
A nomogram model was developed in this research to predict the risk of LEDVT in gynecological patients. The model demonstrated a significant predictive capability, with a sensitivity of 96.1% and a specificity of 75.9%. It has been observed that the predictive performance of the model is higher than that of previous studies.
This study had several limitations. Firstly, due to the retrospective nature of this study, selection bias was inherent. Secondly, the model’s external validation was not performed despite the high predictive value for LEDVT, which restricted its application. Thirdly, the application of the model is restricted to patients undergoing gynecological laparoscopic surgery due to a limited number of patients.
Conclusion
The present study, for the first time to our knowledge, identified the independent risk factors for LEDVT in gynecological laparoscopic surgery patients as old age, prolonged surgical duration, shortened APTT, elevated D-dimer levels, a history of hypertension, and high HE4. Our nomogram model utilizing these factors accurately predicts the likelihood of LEDVT and may be applied to prevent its occurrence.