Antifungal effects of eugenol on Candida albicans adherence to denture polymers
- Published
- Accepted
- Received
- Academic Editor
- Héctor Mora-Montes
- Subject Areas
- Biochemistry, Microbiology, Mycology, Dentistry
- Keywords
- Candida albicans, Denture base polymers, eugenol, Denture cleansers, Fungal adherence
- Copyright
- © 2023 Zanul Abidin et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Antifungal effects of eugenol on Candida albicans adherence to denture polymers. PeerJ 11:e15750 https://doi.org/10.7717/peerj.15750
Abstract
Background
The study’s objective is to assess the adherence of C. albicans in different types of denture polymers and the effectiveness of eugenol and commercialized denture cleansers in the removal of C. albicans. Three types of denture base polymers (Lucitone® 199 (High-Impact PMMA), Impact® (conventional PMMA) and Eclipse® (UDMA)) and two hard denture reline materials (Kooliner® and Tokuyama® Rebase II Fast) were used in this study.
Methods
Three hundred samples were prepared (6 × 2 mm disc shape) and divided into five groups of denture polymers (n = 60) and further subjected into five treatment groups (Polident®, Steradent, distilled water, eugenol 5-minutes, and eugenol 10-min). Three samples were extracted from each treatment group for baseline data (n = 12). Baseline data were used to calculate the initial number of C. albicans adherence. A 0.5 ml immersion solution from each specimen was cultured on YPD agar and incubated for 48 h at 37 °C. Visible colonies were counted using a colony counter machine (ROCKER Galaxy 230).
Results
The result showed that the denture base polymer significantly affected the initial adherence (p = 0.007). The removal of C. albicans was also considerably affected by the denture base polymers and denture cleansers (p < 0.05). Lucitone®, Tokuyama®, and Kooliner® denture base polymers immersed for 3 min in eugenol showed the best results of removal.
Discussion
This study’s overall results showed that all denture polymers used as denture bases had an effect on C. albicans initial adherence and removal from the denture base, and eugenol is comparable to commercialised denture cleansers in reducing the number of attached C. albicans on denture base polymers.
Introduction
According to Zain et al. (1997) denture stomatitis is among the most common oral mucosa lesions diagnosed in Malaysia, with a high prevalence of approximately 33.5%, while Al-Maweri et al. (2013) reported a lower prevalence of denture stomatitis in patients attending Hospital Universiti Sains Malaysia. These different may be due to the latter study used a convenience sample and the former study samples were randomly sampled from national population.
Denture stomatitis is a lesion usually seen on the maxilla and mandibular denture-bearing area, and as the name suggested, it is related to wearing dentures. However, it is more common on the hard palate of the maxillary denture compared to the mandibular region due to more area of contact between the maxillary denture and oral tissues. The causes of this lesion are mainly due to poor oral hygiene of denture wearers, continuous or prolonged wearing of the denture, particularly overnight, wearing poorly constructed dentures, pharmacological reaction (allergic reaction), and C. albicans infection in combination with all the factors mentioned above.
C. albicans is the most common species found as commensal members in the microflora of a healthy human. According to Scully & Felix (2005), C. albicans is the most common species isolated from denture stomatitis cases. The presence of saliva and its flow help in the self-cleansing effect on the oral mucosa. However, the presence of pellicle promotes the adherence of Candida spp. to the denture base polymer. In patients with xerostomia, artificial saliva is used to lubricate the oral environment, to keep the mouth moist, and to improve self-cleansing of the oral cavity. A study by Hahnel et al. (2010) reported that saliva substitute films influence the initial adherence of C. albicans and the artificial saliva with lysozyme and lactoferrin may be effective in preventing C. albicans from adhering to oral surfaces. Hence, artificial saliva with these substitutes could serve as a first step in a therapy plan targeting the oral microbial including C. albicans.
A study by De Freitas Fernandes et al. (2011), reported that polymethyl methacrylate resin surface of denture polymer may promote on microbial colonization. Many studies had shown that surface roughness of denture base material or denture liner does have an impact on microbial adherence (Meirowitz et al., 2021; Rapone et al., 2022; Di Fiore et al., 2022). A study by Verran & Maryan (1997) has found less adherence of Candida spp on rough surface of denture base than the smoother surface. However, Moura et al. (2006) had reported that surface roughness and polymerization method have no impact on Candida adherence, but the presence of saliva does have an impact on Candida adherence.
Many over-the-counter denture cleansers have been developed recently with more added functions such as effervescent effect (mechanical plaque removal by bubbling effect) or fluoride additive (prevention of dental caries especially in partial dentate patients) and rapid action to clean the denture. Ramage et al. (2019) compared two denture cleansing methods, using a denture cleanser alone and brushing with a dentifrice, and found that using a denture cleanser daily is more effective than brushing with dentifrice to inhibit the growth of C. albicans on denture surface.
In the last century, researchers found that eugenol (4-allyl-2-methoxy phenol), the main compound of clove (Syzygium aromaticum), has antibacterial and antifungal properties (Kim, Marshall & Wei, 1995; Ouattara et al., 1997). eugenol is a polyphenol component that can be found in a variety of plant sources, including clove oil which has medicinal and nutritional benefits (Ohkubo & Shibata, 1997; Rana, Rana & Rajak, 2011). It is also can be obtained in other sources such as clove or African Basil plant (Ocimum gratissium), holy basil plants (Ocimum tenuiflorum, Ocimum sanctum), Golden Shower flowering plant (Cassia fistula), lanoline bush plant (Zieria smitii), fairy fans wildflower plant (Clarkia breweri), Billygoat weed plant (Ageratum Conyzoides) and also in whole tobacco plant (Reddy et al., 2007).
The mechanism by which eugenol triggers mortality in Candida spp are uncertain and not fully understood. The chemical compound in eugenol generated significant morphological alterations in Candida spp cells and cytoplasmic component leakage, indicating an effect on the cell membrane (Nakamura et al., 2004; Chami et al., 2005). Several authors have demonstrated that fungicidal concentrations of eugenol against C. albicans cause a significant decrease in cell ergosterol content which may interfere with H+-ATPase activity (Pinto et al., 2009; Ahmad et al., 2010a; Ahmad et al., 2010b; Khan, Ahmad & Cameotra, 2013). It is possible that the primary purpose of eugenol to destroy the permeability of cell membranes, leading them to degrade or dissolve. Additionally, previous studies have demonstrated that eugenol can substantially increase the permeability of phospholipid bilayers in cell membranes (Woranuch & Yoksan, 2013; Wang et al., 2010). Braga et al. (2007) also found that eugenol could alter the C. albicans cell envelope, thus reducing the ability of C. albicans to colonise host tissue; the cell envelope referred to combination layer of the plasma membrane, the periplasmic space of the cells, the cell walls of also the fibrous layer on the outer region of the C. albicans wall. The eugenol resulted in a significant alteration in the morphology of the envelope of C. albicans. According to that study, the mechanism is still unclear but may be due to the present of lipophilic and carvacrol compounds founds in eugenol that change the fluidity and permeability of cell membranes by penetrating between the fatty acyl chains that make up the lipid bilayers.
Because of its diverse range of biological and functional properties, eugenol may be a topic of considerable interest. Therefore, the present study aims to investigate the effectiveness of eugenol and commercialised denture cleansers in reducing the initial adherence of C. albicans to denture polymers.
Materials & Methods
Microbiological preparation
C. albicans (ATCC 14053) (The American Type Culture Collection (ATCC), USA) was used in adherence assay in this study. The strain was rehydrated in sterile distilled water and inoculated onto Yeast Peptone Dextrose (YPD) agar media (BD Difco, Franklin Lakes, NJ, USA). This was followed by incubation at 37 °C for 24 h, and then the colonies were subcultured on fresh YPD agar slants and stored at 4 °C. Stocks for long-term storage were also prepared in 20% glycerol and kept at −70 °C.
Denture based polymers
Three types of denture base polymers (Lucitone® 199 (High-Impact heat-polymerised PMMA), Impact® (conventional heat-polymerized PMMA) and Eclipse (UDMA-based light-cured resin)) (Table 1) and two types of hard denture reline polymers (Kooliner® and Tokuyama® Rebase II Fast) (Table 2) were used in this study. Sixty-five denture base samples, discs of size 6.0 mm diameter × 2.0 mm height, were fabricated from each denture base (n = 325). Samples were fabricated according to the manufacturer’s instructions and polished for further use in the experiment. For the polishing procedure, the discs were trimmed with grind and polishing machine (Buehler Beta, Lake Bluff, IL, USA) using 240 grit silicon carbide (3 min for both sides at 60 rpm) followed with 1000 grit silicon carbide (5 min for both sides at 300 rpm) until reaches to a final thickness of 1 ± 0.2 mm. Digital electronic caliper (Mitotuyo Digimatic Vernier Caliper, Mitotuyo Corp, Kawasaki, Japan) was used to control thickness for each specimen. Grinding and polishing procedure were performed by the same operator with constant motion and light minimal pressure.
| Material type | Product Name | Main composition | Manufacturer | Batch number |
|---|---|---|---|---|
| High-Impact heat-polymerizing Polymethyl methacrylate | Lucitone® 199 | Powder: Polymethylmethacrylate Liquid: Methylmethacrylate, ethylene glycol dimethacrylate | Dentsply, International Inc. York, PA, USA. | Powder: Lot 120220 Liquid: Lot 111026 |
| Conventional heat-polymerizing Polymethyl methacrylate (PMMA) | Impact® Acrylic Denture Base | Powder: Polymethylmethacrylate Liquid: MMA, ethylene glycol dimethacrylate | Dental export of London, London, England. | Powder: Lot 11NOV147 Liquid: Lot 22745 |
| Light Activated Urethane Dymethacrylate (UDMA) | Eclipse® Base Plate | Single paste component Urethane dimethacrylate, Stearyl Acrylate | Dentsply, International Inc. York, USA. | Lot 030909 |
| Polymer | Product name | Main composition | Manufacturer | Batch number |
|---|---|---|---|---|
| Hard denture reline | Kooliner® | Powder: Polyethyl methacrylate, Benzoyl peroxide, Silica, Crystalline-Quartz Liquid: Isobutyl methacrylate, 2,4-Dihydroxy benzophenone | GC Dental Product | Lot 1201079 |
| Hard denture reline | Tokuyama® Rebase II | Powder—Polyethyl methacrylate, Benzoyl peroxide Liquid-2-(Acetoacetoxy) ethyl methacrylate and 1,9-nonanediol Dimethacrylate Tokuso Resin Hardener II—sodium sulfite and sodium bicarbonate | Tokuyama® Dental America Inc. | Lot 139E62 |
Sample groups
Next, denture base samples were randomly divided into five groups (n = 13 per group). All samples were soaked in chlorhexidine 0.12% for one hour, and were washed thoroughly with distilled water, and subjected to sonication methods for 10 min and were repeated ten times consecutively (Table 3). One specimen from each denture base group was taken aside and not subjected to any adherence assay and used as a negative control. The remaining twelve samples from each denture base group were subjected to the adherence assay for 3 h at 37 °C with C. albicans suspension prepared at an optical density (OD550nm) of 0.144, which is equivalent to 106 cells/ml. Before that, the samples were soaked in artificial saliva for 30 min (Table 3). Three samples were extracted from each treatment group for baseline data (n = 15) and labelled as E1. Baseline data were used to calculate the initial number of C. albicans adherence. The denture base polymers were then soaked in five types of denture cleansers Polident®, Steradent, sterile distilled water, eugenol 3 min, and eugenol 10 min and labelled as E2 (Table 4). 0.5ml of the immersion solution from each specimen of E1 and E2 groups were then cultured on YPD agar and incubated for 48 h at 37 °C.
| Solution | Product Name | Main composition | Manufacturer | Batch number |
|---|---|---|---|---|
| Artificial saliva | Kin Hydrat Spray | Xylitol, Potassium Chloride, Sodium Chloride, Calcium Chloride, Potassium Thiocyanate, Sodium Saccharin | LABORATORIOS KIN S.A, Barcelona, Spain | Lot 12C01 |
| YPD Broth | Difco YPD Broth | Yeast extract, Peptone, Dextrose | Difco Laboratories | Lot 2100348 |
| Chlorhexidine 0.12% | Oradex antibacterial mouthwash | Chlorhexidine Gluconate | Fortune Laboratories Sdn. Bhd | Lot 1212057B |
| Product name | Main Composition | Manufacturer | Batch Number |
|---|---|---|---|
| Polident® | Citric Acid, Sodium Carbonate, Potassium Peroxymonosulfate, Sodium Perborate Monohydrate | Glaxo Smith Kline | Lot FD999101 |
| Steradent | Potassium Peroxymonosulfate Sulfate, Sodium Carbonate, Citric Acid, Sodium Carbonate Peroxyhydrate, Sodium Sulfate, Sulfamic Acid, Malic Acid. | Reckitt Benckiser | Lot 0308943 |
| eugenol | 2-methoxy-4-(2-propenyl)phenol* 4-Allyl-2-methoxyphenol | Fluka Analytical | Lot BCBD3793 |
| Sterile Distilled Water (dH2O) | Hydrogen, Oxygen |
Adherence analysis
Visible colonies were counted using a colony counter machine (ROCKER Galaxy 230) and represented as colony-forming units (CFU).
Data analysis
Data collected were entered and analysed in SPSS version 22. The assumption for normality was not met; therefore, non-parametric analysis was used. The Kruskal-Wallis test was used to analyse the initial adherence of C. albicans to the denture polymers. To analyse eugenol’s effectiveness and commercialised denture cleanser on the removal of C. albicans, the Mann–Whitney U test was used with Bonferroni correction for multiple comparisons. A statistically significant level was set to a p value of 0.05.
Results
Initial adherence of C. albicans on different types of denture polymers
The amount of initial adherence of C. albicans on different types of denture polymers are shown in Table 5.
| Denture polymer | Initial Adherence (CFU/ml) | Kruskal-Wallis | ||
|---|---|---|---|---|
| Median ± IQR (102) | X2 | Df | p-value | |
| Lucitone® | 24 ± 4a,b,e | 47.70 | 4 | 0 .000 |
| Impact® | 20 ± 3a,c | |||
| Eclipse® | 22 ± 9e | |||
| Kooliner® | 21 ± 3b,d | |||
| Tokuyama® | 24 ± 8c,d | |||
Notes:
Significant at p < 0.05.
a,b shows significant different differences between groups (post hoc analysis using Mann–Whitney U with Bonferroni correction).
A Kruskal-Wallis H test showed that there was a statistically significant difference in the initial adherence of C. albicans among different denture polymers, X2(4) = 47.70, p < 0.001. The initial adherence of C. albicans to denture polymers ranges from 20 ± 3 CFU/ml (Impact) to 24 ± 8 CFU/ml (Tokuyama) with a mean rank initial adherence of 161.60 for Lucitone 199®, 131.00 for Tokuyama, 104.00 for Eclipse, 89.60 for Kooliner and 78.80 for Impact.
Post hoc analysis showed that the initial adherence of C. albicans on Impact® (20 ± 3 CFU/ml) and Kooliner® (21 ± 3 CFU/ml) were significantly lower than Lucitone 199® (p = 0.007). However, no statistically significant difference was observed in the initial adherence of C. albicans on the Lucitone 199®, Eclipse and Tokuyama® denture polymers (p > 0.05).
Effectiveness of eugenol and commercialised denture cleansers in removing C. albicans from denture base polymers
The effectiveness of eugenol and denture cleansers in removing Candida was measured by the amount of C. albicans removed from the denture base polymers in CFU/ml. The amount of C. albicans removed from the denture polymers varies among the groups and ranges from 2,740 ± 30 CFU/ml to −53 ± 685 CFU/ml upon immersion in the denture cleansers, as shown in Table 6. The negative value shown in distilled water would suggest the growth of Candida. The removal of Candida is significantly affected by the denture base and denture cleanser (p < 0.001).
| Denture base | Denture Cleanser | Colonies removed (CFU/ml) | Kruskal-Wallis | ||
|---|---|---|---|---|---|
| Median ± IQR | X2 | df | p-value | ||
| Impact® | Polident® | 2140 ± 55a,b | 40.05 | 4 | <0.001 |
| Steradent | 1863 ± 30b,c | ||||
| Eug-3 | 1983 ± 10d | ||||
| Eug-10 | 2010 ± 5c,e | ||||
| dH2O | 67 ± 325a,d,e | ||||
| Lucitone® | Polident® | 2267 ± 15f,g | 42.67 | 4 | <0.001 |
| Steradent | 2140 ± 20h,i | ||||
| Eug-3 | 2650 ± 10g,h,j | ||||
| Eug-10 | 2523 ± 0i,k | ||||
| dH2O | 260 ± 365f,j,k | ||||
| Eclipse | Polident® | 2740 ± 30l,m,n | 42.56 | 4 | <0.0001 |
| Steradent | 1700 ± 50n,o | ||||
| Eug-3 | 2163 ± 10p,q | ||||
| Eug-10 | 1590 ± 5m,q | ||||
| dH2O | 67 ± 730l,o,p | ||||
| Tokuyama® | Polident® | 1837 ± 75r | 40.30 | 4 | <0.001 |
| Steradent | 1803 ± 140s | ||||
| Eug-3 | 2733 ± 10r,s,u | ||||
| Eug-10 | 2600 ± 10t | ||||
| dH2O | 410 ± 425t,u | ||||
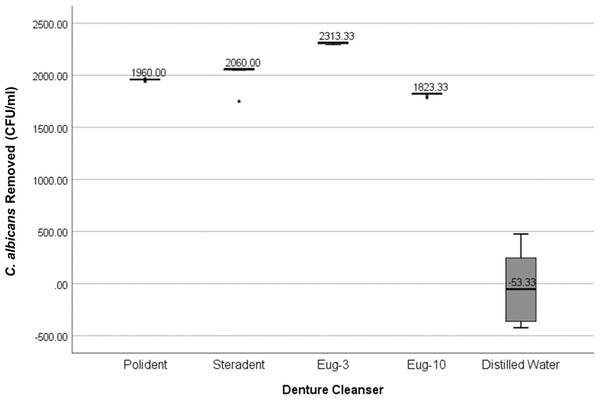
| Kooliner® | Polident® | 1960 ± 5v | 40.12 | 4 | <0.001 |
| Steradent | 2060 ± 10w | ||||
| Eug-3 | 2313 ± 10x,y | ||||
| Eug-10 | 1823 ± 10y | ||||
| dH2O | −53 ± 685v,w,x | ||||
Notes:
Eug-3: 3 min immersion in eugenol.
Eug-10: 10 min immersion in eugenol.
Significant at p < 0.05. Superscript letters show significant different between groups (post hoc analysis using Mann–Whitney U with Bonferroni correction).
Impact® denture base polymer
For Impact® denture base, immersion in all denture cleanser solutions significantly removed C. albicans colonies by at least 28 folds compared to distilled water (67 ± 325 CFU/ml) (p < 0.05). There is no significant difference between three- and ten-minute immersion in eugenol with pairwise comparison (p > 0.05) and both did not differ significantly with other cleansers except between ten minutes immersion in eugenol (2010 ± 5 CFU/ml) and Steradent (1863 ± 30 CFU/ml).
Lucitone 199® denture base polymer
Immersion of Lucitone 199® denture base in three- and ten-minute immersion in eugenol significantly removed more C. albicans colonies (2650 ± 10 CFU/ml and 2523 ± 0 CFU/ml respectively) compared to other denture cleansers and they did not differ significantly. However, immersion in eugenol for three minutes significantly removed C. albicans compared to Polident® and Steradent, and immersion in ten minutes eugenol only differ significantly from Steradent denture cleanser.
Eclipse denture base polymer
Three minutes of immersion in eugenol removed more C. albicans (2,163 ± 10 CFU/ml) compared to ten minutes of immersion (1,590 ± 5 CFU/ml) and the difference was significant (p < 0.05). Immersion in ten minutes of eugenol also differs significantly with Polident® (2,740 ± 30 CFU/ml).
Tokuyama denture base relining material
No significant difference was found between three- and ten-minute immersion in eugenol (2,733 ± 10 CFU/ml and 2,600 ± 10 CFU/ml respectively). However, three minutes of immersion in eugenol differ significantly from other cleansers (Steradent (1,803 ± 140 CFU/ml) and Polident (1,837 ± 75 CFU/ml)) (p < 0.05). The colonies of C. albicans removed from the Tokuyama® denture base after immersion in distilled water (44 ± 685 CFU/ml) are four folds less compared to after immersion in other denture cleansers (p < 0.05).
Kooliner denture base relining material
For Kooliner® denture base, the highest number of C. albicans colonies removed was after three minutes of immersion in eugenol (2,313 ± 10 CFU/ml), which was significant compared to the ten minutes of immersion in eugenol (1,823 ± 10 CFU/ml), and both differ significantly. Both three- and ten-minute immersion did not differ significantly from other denture commercial denture cleansers.
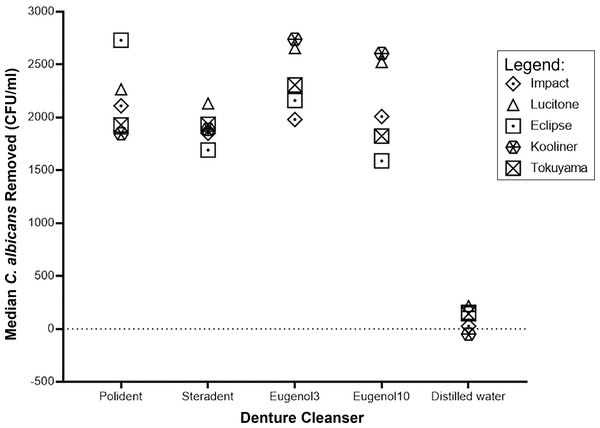
The effect of denture cleansers varies with different types of denture base polymers. In general, immersed in distilled water removed the least number of C. albicans whilst immersed in eugenol solution for three minutes yields the greatest number of C. albicans removed from all the denture bases (Figs. 1 and 2).
Figure 1: Graph of C. albicans removed (CFU/ml) by each denture cleanser.
The amount of C. albicans removed after immersion in denture cleansers from all types of denture base materials.Figure 2: Graph of median C. albicans removed (CFU/ml) by denture cleanser.
The median of C. albicans removed after immersion in denture cleansers varies in denture bases.Discussion
Microbial adherence to denture polymers surfaces can be measured by using several different quantitative methods such as colony-forming unit (CFU) assay, scanning electron microscopy (SEM), fluorescence microscopy, crystal violet assay or optical density (OD) (Cheng et al., 2019). The colony-forming unit (CFU) assay used in this study provided a simple and reproducible method commonly used for measuring the initial adherence between Candida and denture polymers (Sieuwerts et al., 2008).
The adherence of Candida to denture base can be affected by various factors, such as the experiment environment (in-vivo or in-vitro) and the physical and mechanical properties of the denture materials to name a few (Gacon, Loster & Wieczorek, 2019; Sampaio-Maia et al., 2012; Radford et al., 1998). In the present study, the experiment was conducted in-vitro with more precise control of experimental conditions, but the initial adherence may depend mainly on the denture polymers itself. Yildirim et al. (2005) reported that in the adherence of Candida to solid surfaces, both hydrophobic and electrostatic interactions are involved depending on the physiochemical properties of the surfaces of both solids and Candida.
Three different denture base materials used in this study were among the commonly used materials for the fabrication of denture base. The light-activated urethane dimethacrylate (UDMA) (Eclipse®) was used with a different curing mode. A survey by Hashem et al. (2014) reported that UDMA denture base polymer has better mechanical and physical properties in terms of surface hardness and flexural strength than PMMA. This material is a timesaving since it eliminates the investing, de-waxing, and packing in flask stages as in the conventional method. Besides that, it has a shorter curing time compared to PMMA.
The initial adherence of C. albicans on different types of denture polymers
Our study showed that denture base polymer type significantly affected the initial adherence of C. albicans; the initial C. albicans adherence to denture polymers ranged from 20 ± 3 CFU/ml (Impact) to 24 ± 8 CFU/ml (Tokuyama®). In contrast to our findings, Nevzatoğlu et al. (2007) found that the C. albicans adherence was higher for cold-polymerized resins than heat-polymerized ones, regardless of the surface finishes. These discrepancies in the results may be partly attributed to the differences in the physicochemical properties of the denture polymers and Candida species used. The chemical composition of the denture polymers surface may also influence C. albicans adhesion much more than the average surface roughness (Yildirim et al., 2005). This result contradicts the previous study by Devarhubli, Subbarao & Patil (2012), who reported a lower adhesion of Candida in Lucitone 199® heat-cured denture base material.
The lowest value of adhesion between the two reline materials was found with Kooliner® (21 ± 3 CFU/ml), while Tokuyama® Rebase II Fast recorded a value of 24 ± 8 CFU/ml. The result coincides with the previous study for the initial adherence study, although artificial saliva was used in the present study to replace the human saliva sample. Hahnel et al. (2010) and Silva et al. (2012) reported that artificial saliva did promote adherence of C. albicans to denture base material. Artificial saliva was used because whole human saliva might cause a particular problem due to its complex biochemical and physical-chemical properties; its inherent variability and instability would also be posed as a challenge that will affect the study (Schipper, Silletti & Vingerhoeds, 2007). Therefore, human saliva could be inappropriate for a standardised in vitro study; rather artificial saliva may be essential for a well-justified and controlled experiment (Pytko-Polonczyk et al., 2017).
The effectiveness of eugenol and commercialised denture cleansers in removing C. albicans from denture base polymers
Four different denture cleanser solutions, namely Polident®, Steradent, eugenol (3-minutes and 10-minutes immersion time) and distilled water (control group), were used to determine the effectiveness in removing C. albicans after initial adherence to denture polymers. The concentration of 2% eugenol was chosen in the study as it was reported that 2% clove oil could inhibit the growth of some mycotoxigenic molds and yeasts (Matan et al., 2006; Mandras et al., 2016; Marchese et al., 2017).
Distilled water was used in this study as a control group and as predicted immersion in the distilled water showed lowest removal value of C. albicans compared to the study group for all denture base polymers. This exhibited that distilled water alone has no capacity to eliminate the C. albicans from the denture polymer surfaces.
The data described from this study indicates that all four cleansers effectively removed the C. albicans greater than 70% from the denture polymers surfaces regardless the immersion time. This result was agreeable with the previous study by De Freitas Fernandes et al. (2011), who reported that a denture cleanser could reduce Candida spp. cells count on denture polymers. Ghazal et al. (2019) also stated that denture cleanser could effectively detach the adhered C. albicans from denture base resin. The active ingredients in Polident® and Steradent® might help to eliminate some of the Candida colonies (Han, Liu & Cai, 2020). Polident® contains sodium perborate, a hydrogen peroxide source. When these tablets are dissolved in water, sodium perborate forms an alkaline peroxide solution. This solution releases oxygen, which loosens debris or adherence of microorganisms by mechanical means.
Eugenol was significantly more effective than other denture cleansers in removing C. albicans from Lucitone® and Tokuyama® denture base polymers, even after 10 min immersion. This was supported in the previous study by Fontenelle et al. (2011), who reported that eugenol was significantly active against Candida spp. Being an antifungal agent, eugenol could inhibit the growth of C. albicans (Silva et al., 2017) and showed good potential to inhibit spoilage fungi, yeast, and bacteria (El-Saber Batiha et al., 2020). eugenol was also found to modify the cell membrane’s morphology of C. albicans, thus reducing its colonization on host tissues (Latifah-Munirah, Himratul-Aznita & Mohd Zain, 2015; Rajkowska, Nowicka-Krawczyk & Kunicka-Styczyńska, 2019). Although, the longer immersion time may lead to deleterious effects on the denture base polymers or not, could not be concluded without further study. This study demonstrated a significant greater C. albicans removal value following a 3-minute immersion compared to 10-minute immersion in eugenol (p < 0.001), suggesting 3 min immersion time is acceptable for eugenol. Lucitone®, Tokuyama® and Kooliner® denture base polymers immersed for 3 min in eugenol showed the best results of removal.
Conclusions
This study demonstrated that C. albicans could adhere to all denture polymers even with artificial saliva. Different types of denture polymer polymerising systems allowed the different number of C. albicans in the initial adherence. All denture cleansers in the present study were suitable for all denture polymers in reducing the number of attached C. albicans. It is safe to say that eugenol was as effective as commercialised denture cleansers in reducing the number of attached C. albicans on denture polymers. This is because eugenol is a potent antifungal, especially to C. albicans besides, as an effective pain reliever. However, there are a few limitations with utilizing eugenol as the base compound for the potential denture cleanser, such as allergic reaction, and an unpleasant taste of spiciness, to name a few. Further studies could be recommended to investigate the addition of some materials to neutralize the effect of the eugenol. Lower percentage and less immersion time could also be investigated to overcome these limitations. This study design did not simulate the clinical situation ideally, as artificial saliva was used to replace human saliva to form the pellicle for attachment of C. albicans. Further randomized clinical trials are recommended to assess the effect of eugenol as denture cleanser inside the oral environment.
Furthermore, the surfaces of the denture polymer used in this study have been polished to a smooth finishing, standardising the surfaces. In contrast, in clinical situations, the surface of the intaglio surface was rougher, which might promote the adherence of C. albicans. Therefore, it is necessary to conduct further studies to simulate the actual clinical situation.