Side-effects of hyperthermic intraperitoneal chemotherapy in patients with gastrointestinal cancers
- Published
- Accepted
- Received
- Academic Editor
- Jian Zhang
- Subject Areas
- Drugs and Devices, Gastroenterology and Hepatology, Oncology
- Keywords
- Hyperthermic intraperitoneal chemotherapy, Gastrointestinal cancers, Side effects, Gastric cancer, Colorectal cancer
- Copyright
- © 2023 Hu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Side-effects of hyperthermic intraperitoneal chemotherapy in patients with gastrointestinal cancers. PeerJ 11:e15277 https://doi.org/10.7717/peerj.15277
Abstract
Background
Hyperthermic intraperitoneal chemotherapy (HIPEC) produces unwanted side-effects that are mainly caused by chemotherapeutic drugs in the treatment of gastrointestinal (GI) cancers, and these effects have not been systematically summarized. The aim of this article was to provide a comprehensive overview of the side-effects of HIPEC for GI cancers and propose practical strategies for adverse event management.
Methodology
PubMed, Web of Science, and the Cochrane Library were systematically searched for side-effects of HIPEC in GI cancers prior to October 20, 2022. A total of 79 articles were included in this review.
Results
Adverse events, such as enterocutaneous digestive fistulas, GI tract perforation, neutropenia, postoperative bleeding, ventricular tachycardia, hyperglycemia, hypocalcemia, renal impairment, encapsulating peritoneal sclerosis, scrotal ulceration, and sarcopenia were described, and their clinical management was discussed. These side-effects involve the digestive, hematopoietic, circulatory, metabolic, and urinary systems. Effective methods for adverse event management included an expert multidisciplinary team, replacing chemotherapy drugs, using Chinese medicine, and careful preoperative assessments.
Conclusion
The side-effects of HIPEC are frequent and can be minimized by several effective methods. This study proposes practical strategies for adverse event management of HIPEC to assist physicians in choosing the optimal treatment method.
Introduction
Cancer is a major public health problem and the leading cause of death worldwide, with approximately 19.3 million new cases and 10.0 million deaths globally in 2020 (Sung et al., 2021). Gastrointestinal (GI) cancers account for 26% of the global cancer incidence and 35% of all cancer-related deaths (Arnold et al., 2020). The pathophysiology and pathogenesis of GI cancer is complex and multifactorial. Microbiota dysbiosis, unchecked inflammasome activities perpetuating chronic inflammation, the cyclooxygenase (COX)–2/prostaglandin (PGE)2 pathway, and excess adiposity play important roles in the molecular and pathophysiological basis of GI cancers (LaCourse, Johnston & Bullman, 2021; Man, 2018; Murphy, Jenab & Gunter, 2018; Sender, Fuchs & Milo, 2016). With the development of medical technology and a deeper understanding of the pathogenesis of GI cancers, current therapeutic modalities for the treatment of GI cancers include surgery, chemotherapy, radiotherapy, and immunotherapy. Hyperthermic intraperitoneal chemotherapy (HIPEC), an emerging therapeutic modality, is currently used as an essential component of treatment, to improve the disease-free and overall survival of patients with primary and metastatic GI cancers (González-Moreno, 2006; Klempner & Ryan, 2021; Loggie & Thomas, 2015; Verwaal et al., 2008).
In 1980, Spratt et al. (1980) first performed HIPEC-based treatment in a patient with pseudomyxoma peritonei; thereafter, the use of HIPEC was explored in patients with GI cancers (Hirose et al., 1999; Kaibara et al., 1989). In 1994, Hamazoe, Maeta & Kaibara (1994) used mitomycin C (MMC) at 10 mg/mL to prevent peritoneal recurrence of gastric cancers. As soon as the abdomen was closed after gastric resection, patients were administered this treatment while under general anesthesia on the operating table. There is a 53–66% probability that a patient with metastatic gastric cancer will develop peritoneal metastases (PM) (Dong et al., 2019). HIPEC along with cytoreductive surgery (CRS) is the only therapeutic modality that has resulted in long-term survival in specific groups of patients. As a palliative treatment in advanced PM with intractable ascites, HIPEC has been shown to control ascites and reduce the need for frequent paracentesis (Goéré et al., 2013; Seshadri & Glehen, 2016). HIPEC plus CRS achieved great survival benefits in patients with peritoneal cancer (PC) of colorectal origin (12.6–22.3 months). However, this treatment also produced several side-effects which greatly hinder the application of HIPEC in the treatment of GI cancer (Mancebo-González et al., 2012; Stiles et al., 2020; Verwaal et al., 2003).
The side-effects of HIPEC (mainly caused by chemotherapeutic drugs) in patients with GI cancers are poorly defined. This review describes the current knowledge regarding the mechanism of action, safety, and side-effects of HIPEC in the treatment of GI cancers and explores the current knowledge gaps. With the aim of improving preoperative planning, preventing morbidity, and enhancing surveillance, we provide physicians with the latest information to assist them in choosing the optimal method of combined or primary treatment and predict which patients are at risk of experiencing side-effects.
Survey methodology
We systematically searched for relevant studies in PubMed (1,050), Web of Science (1,628), and the Cochrane Library (203) prior to October 20, 2022. The Medical Subject Headings or key words used were the following: (“hyperthermic intraperitoneal chemotherapy,” or “intraperitoneal thermo-chemotherapy,” or “HIPEC,”) AND (“gastrointestinal cancers,” or “GI cancers,” or “gastrointestinal tumors” or “GI tumors,” or “Gastric Cancer,” or “Colon Cancer,” or “Rectal Cancer,” or “Colorectal Cancer,” or “Appendiceal Cancer,” or “Peritoneal Cancer”). The exclusion criteria included the following: duplicate literature, and literature not specified in these key words, comprising other types of cancer patients, other cancer indices, other study outcomes, and studies lacking original or complete data. Table 1 shows the 16 studies selected which cover the typical side-effects of GI cancers.
| Reference | Country | Type of study | No. of patients and primary site |
Chemotherapeutic agents | Intraabdominal temperature and duration time |
Side effects | Morbidity (Percentage, %) |
|---|---|---|---|---|---|---|---|
| Lee et al. (2022) | Korea | Retro | 124 colorectal cancer patients | MMC (35 mg/m2) | 90 min at 41–43 °C | Neutropenia | 62.9 |
| Lambert et al. (2009) | USA | Retro | 117 patients appendiceal cancer patients | MMC (29.1 mg/m2) | 90 min at 40 °C | Neutropenia | 39 |
| Hakeam et al. (2018) | Saudi Arabia | Retro | melphalan: 46 CIS+MMC: 35 |
Melphalan (60 mg/m2) OR CIS (60 mg/m2) + MMC (30 mg/m2) | 60 min | Leukopenia and thrombocytopenia | melphalan: 25.7/60 CIS + MMC: 17.3/68.8 |
| Kemmel et al. (2015) | France | RCT | 45 PC of colorectal cancer patients | MMC (32.5 mg/m2) | 90 min at 42.5 °C | Neutropenia | 40 |
| van Vugt et al. (2015) | The Netherlands | Retro | 206 peritoneal carcinomatosis of colorectal cancer. | MMC (35 mg/m2) | 90 min at 41–42 °C | Sarcopenic | 43.7 |
| Mor et al. (2022) | Israel | Retro | 191 GI cancer patients | NA | NA | Gastrointestinal anastomotic leaks | 17.8 |
| Elias et al. (2001) | France | Retro | 64 Colorectal adenocarcinomas | MMC (20 mg/m2) and CDDP (200 mg/m2) |
60 min at 41–44 °C | Perforation | 7.8 |
| Valle et al. (2016) | Australia | Retro | 778 peritoneal surface malignancy patients | NA | NA | Enterocutaneous fistula | 5.8 |
| Sugarbaker et al. (2006) | USA | Retro | 356 appendiceal mucinous malignancy patients | MMC; 5-Fu (600 mg/m2) |
90 min at 41.5 °C | Hematological; gastrointestinal | 28; 26 |
| Levine et al. (2018) | USA | RCT | 121 Appendiceal cancer patients | MMC (40 mg) oxaliplatin (200 mg/m2). |
120 min at 40 °C | Hematologic toxicity | NA |
| Ye et al. (2018) | China | Retro and cohort study | 99 peritoneal carcinomatosis patients | CP (60 mg/m2) and 5-Fu (700–800 mg/m2) | 60–90 min at 41–45 °C | Acute kidney injury | 90.9 |
| Kapoor et al. (2019) | USA | Retro | 23 gastric or gastroe- sophageal adenocarcinoma patients |
CP (106.6 ± 10.9 mg/%BSA) and MMC (16 ± 1.6 mg/%BSA) | 60 min at 39–42 °C | Hypocalcemia, hypophosphatemia, and hypomagnesemia | 94%, 84% and 9.7% |
| Mangan et al. (2019) | UK | Case report | A 65-year-old man with colonic tumour | Oxaliplatin, 5-Fu and MMC | NA | Encapsulating peritoneal sclerosis | NA |
| Stewart et al. (2018) | USA | Retro | 85 appendiceal or colorectal peritoneal cancer patients | MMC and oxaliplatin | 57 min | Hyperglycemia | 86 |
| DiSano et al. (2019) | USA | Retro | 115 adenocarcinomas of gastro- intestinal origins |
MMC OR MMC + CP |
NA | Hyperglycemia | MMC: 39 MMC + CP: 86 |
| Smibert et al. (2020) | Australia | Retro | 100 colorectal cancer and pseudomyxoma peritonei patients | NA | NA | Infectious complication | 43 |
Note:
MMC, Mitomycin C; CP, cisplatin; 5-Fu, 5-fluorouracil; CIS, cisplatin plus; CDDP, Cisplatinum; RCT, randomized trial; Retro, retrospective study.
Rationale for hyperthermic intraperitoneal chemotherapy
The rationale of HIPEC is based on the concept of peritoneal dialysis; when chemotherapy is retained in the peritoneal cavity by the peritoneal-plasma barrier, small nodules of cancer are exposed on the abdominal and pelvic surfaces (Dedrick et al., 1978; Flessner, 2005). Normal tissue cells can withstand 47 °C for 1 h under high-temperature conditions, while malignant tumor cells can only withstand 43 °C for 1 h (Garofalo et al., 2006). Large-volume perfusate-containing chemotherapeutic drugs are heated to a certain temperature and continuously circulate, then remain for a certain period of time in the abdominal cavity of the patient, which can effectively kill and remove the residual cancer cells and minute lesions in the body cavity (Kusamura et al., 2008; Ye et al., 2020). Various methods for delivering HIPEC have been proposed, all of which are variations of two modalities: the open and closed techniques. The open technique ensures optimal distribution of heat and cytotoxic solutions, with the disadvantages of heat loss and leakage of cytotoxic drugs. The closed technique prevents heat loss and drug spillage and increases drug penetration but does not ensure homogeneous distribution of the perfusion fluid (Lotti et al., 2016).
The theoretical basis of HIPEC for GI cancers is as follows: first, tissue penetration of the intraperitoneal chemotherapy is facilitated by moderate hyperthermia (41–42 °C) (Sugarbaker, Van der Speeten & Stuart, 2010). When the temperature at a tumor site is >42 °C, cell killing phenomena are evident, such as destruction of the cell membrane, denaturation of proteins, and irreversible damage of tumor cells, while normal cells remain intact (Sticca & Dach, 2003; Zhang et al., 2016). Second, thermal effects can activate heat shock proteins to induce antitumor effects in the autoimmune system and enhance anticancer immune responses via exposure to heat shock protein 90 (Zunino et al., 2016). The peritoneal cavity is continuously perfused with a heated chemotherapy solution to provide a high intraperitoneal drug concentration (van Ruth et al., 2004). Higher HIPEC flow rates improve peritoneal heating efficacy and lead to more rapid heating of the peritoneum and greater peritoneal/outflow temperature gradients. Shear forces generated by fluid flow during treatment can lead directly to tumor cell death (Furman et al., 2014). Finally, the synergistic effect of hyperthermia and chemotherapy inhibits proliferation and induces cell death via the apoptotic pathway (Cesna et al., 2018; Tang et al., 2006).
Side-effects of HIPEC in GI cancers
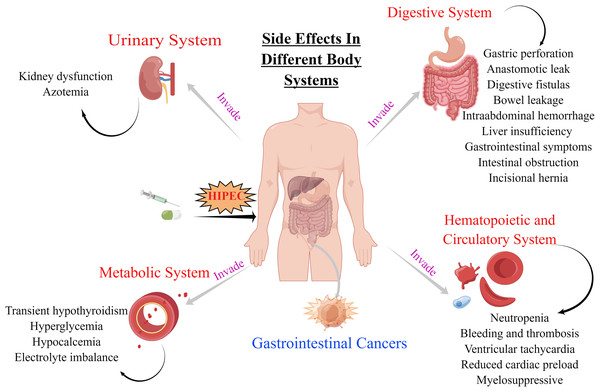
The decision to undergo HIPEC involves careful consideration of both the potential benefits and the possible risks of therapy including side-effects that occur if HIPEC therapy is not well controlled. These side-effects involve the digestive, hematopoietic, circulatory, metabolic, and urinary systems (Table 1 and Fig. 1). The side-effects of HIPEC vary among individuals as well as the specific agents used in the adjuvant regimen and the dose and duration of treatment.
Figure 1: Side effects of HIPEC for GI cancers in digestive, hematopoietic and circulatory, urinary and metabolic systems.
The original figure was created using figdraw (www.figdraw.com).Substantial short- and long-term side-effects are associated with chemotherapy. Short-term side-effects include the toxic effects of chemotherapy, whereas long-term side-effects include later complications of treatment arising after the conclusion of adjuvant chemotherapy. Complications significantly affect the survival rate of patients after CRS/HIPEC. One study that summarized and analyzed the perioperative complications of 225 consecutive patients who underwent CRS/HIPEC showed that the incidence rates of low- and high-grade complications were 38.7% and 15.6%, respectively (Tan et al., 2020). Moreover, the survival outcomes of patients without postoperative complications were significantly better than those of patients with severe complications. In addition, intraoperative blood loss was associated with greater odds of developing postoperative complications (Tan et al., 2020). Oemrawsingh et al. (2019) found that age was also a significant risk factor, reporting a 26.7% occurrence rate of serious adverse events (grade >3) among older adults and a 10.4% rate among younger adults.
Side-effects on the digestive system
Side-effects affecting the digestive system include gastric perforation, anastomotic leak, digestive fistulas, bowel leakage, intra-abdominal hemorrhage, liver insufficiency, gastrointestinal symptoms, and intestinal obstruction.
The effect of certain chemotherapeutic agents on wound healing and intestinal anastomosis leakage is difficult to assess in cancer patients because of the short-term survival and combination with adjuvant treatment. Previous studies identified a higher PC index, more packed cells transfused, pelvic peritonectomy, more anastomoses, and colonic resections as factors associated with GI leaks (Mor et al., 2022). Among the 185 patients included in that study, 16 (8.6%) developed enterocutaneous digestive fistulas, and a median of 18 days (range 9–56) was observed for spontaneous fistula closure in 14 (87.5%) patients (Halkia et al., 2015). Mor et al. (2022) reported GI leaks in 17.8% (34/191) of patients, and conservative management of GI leaks was used in most cases, whereas reoperation was required in 44.1% of the cases. Valle et al. (2016) reported an enterocutaneous fistula rate of 5.8% diagnosed after 13 days and a 5.7% mortality rate. Patients who had a CC2 score (nodules between 2.5 mm and 2.5 cm) cytoreduction, who had an abdominal vacuum-assisted closure device, or who smoked had a higher risk of developing a fistula.
In a study by Zappa, Savady & Sugarbaker (2010), an incidence rate of 6% was reported for GI tract perforation after CRS and HIPEC. This may result from vascular compromise, delay in wound healing from chemotherapy, seromuscular tears related to traction on the stomach wall, and point pressure on the greater curvature from a long-term indwelling nasogastric tube. It may be possible to prevent this complication by reperitonealizing the greater curvature if seromuscular tears occur (Zappa, Savady & Sugarbaker, 2010). In HIPEC treatment, higher temperatures increased the possibility of damage to the normal tissues (Di Miceli et al., 2012). A prospective cohort study found that CRS-HIPEC can be associated with significantly worse bowel-related quality of life (QOL) and social function due to anxiety, embarrassment, and altered body image after the creation of a stoma (Bayat et al., 2020).
Side-effects on the hematopoietic and circulatory systems
Inhibition of hematopoiesis and neutropenia are common side-effects of HIPEC, whereas thrombosis, ventricular tachycardia, and decreased cardiac preload are relatively rare. Although there are multiple reasons for the delay or dose reduction in chemotherapy, bone marrow suppression remains a major cause (Denduluri et al., 2015; Soff et al., 2019). Lee et al. (2022) found that MMC-induced mild and severe neutropenia occurred in 24.2% and 38.7% of the patients, respectively, and severe neutropenia developed significantly earlier than mild neutropenia and lasted significantly longer. Schnake, Sugarbaker & Yoo (1999) found that patients who presented with obesity and anemia had an increased risk of developing profound postoperative neutropenia, which can result in high mortality and morbidity rates. Therefore, reduced chemotherapy doses are necessary in certain patients to prevent the development of this condition (Schnake, Sugarbaker & Yoo, 1999). Lambert et al. (2009) found that the incidence of neutropenia in patients with appendiceal cancer after MMC-HIPEC was 39%. Additionally, female sex and MMC dose per body surface area were independent risk factors for neutropenia (Lambert et al., 2009).
Kemmel et al. (2015) suggested that MMC pharmacokinetics may be a predictor of severe neutropenia in HIPEC, as plasma MMC concentrations increased 30 min (T30) and 45 min (T45) after HIPEC commencement, and neutropenia and its severity increased. Levine et al. (2018) found that both mitomycin and oxaliplatin were associated with minor hematologic toxicity; however, mitomycin resulted in a slightly lower QOL and higher hematologic toxicity than oxaliplatin in HIPEC. Oxaliplatin may be preferred in patients with leukopenia and mitomycin in patients with thrombocytopenia (Levine et al., 2018). A meta-analysis that included 3,268 patients found postoperative bleeding incidence rates within 30 days ranged from 1.7% to 8.3%, and venous thromboembolism incidence rates within 90 days ranged from 0.2% to 13.6% after CRS + HIPEC (Lundbech et al., 2022). Thix et al. (2009) reported a case of ventricular tachycardia during HIPEC with cisplatin (CP) in a patient with moderate cardiac insufficiency, which may have been caused by high plasma CP levels with concomitant low magnesium levels.
Side-effects on the metabolic system
Metabolic system side-effects include persistent hypothyroidism, hyperglycemia, hypocalcemia, and electrolyte imbalance. DiSano et al. (2019) found that the rates of hyperglycemia in patients undergoing CRS and HIPEC are high, which likely represent a stress response, but do not appear to adversely affect long-term outcomes or hospital stays. Tharmalingam et al. (2020) reported that a patient with severe symptomatic hypocalcemia after HIPEC likely suffered from a profound inflammatory reaction with transient hypoparathyroidism, which led to symptoms of significant neuromuscular excitability. In the study by Stewart et al. (2018), most patients (86%) suffered from intraoperative hyperglycemia, with values up to 651 mg/dL. Insulin was required in 66% of the patients, and 91% of the patients experienced peak hyperglycemia within an hour of perfusion, which resolved by postoperative day 1 in 91% of the patients. Hyperglycemia may is caused by using a carrier solution containing dextrose; therefore, the use of carrier solutions containing dextrose needs to be carefully considered and further investigated (Stewart et al., 2018).
Side-effects on the urinary system
Renal impairment is also a common problem after HIPEC. However, whether it is caused by chemotherapeutic agent toxicity or patient kidney function changes remains controversial (Ceresoli, Coccolini & Ansaloni, 2016). A meta-analysis suggested that HIPEC is associated with a high risk of respiratory failure and renal dysfunction (Desiderio et al., 2017). In their study, Ye et al. (2018) found that CP application during HIPEC increased nephrotoxicity; when comparing the CP HIPEC group with the non-CP HIPEC group, urea nitrogen and creatinine levels were significantly higher in the CP HIPEC group. For patients at high risk of acute kidney injury during HIPEC treatment, strict monitoring of renal function, active diuretic therapy, and prophylactic drugs should be applied (Ye et al., 2018).
Other side-effects
A number of other side-effects have been observed during or after HIPEC. Only a few cases of encapsulating peritoneal sclerosis (ERS) secondary to HIPEC have been reported. ERS is a rare surgical complication and a serious and potentially fatal complication of continuous ambulatory peritoneal dialysis that can occur after intraperitoneal treatment (Aihara et al., 2003; Mangan et al., 2019; Takebayashi et al., 2014). HIPEC and dimethyl sulfoxide treatment can result in scrotal ulceration with the presence of intractable and constant scrotal pain along with erythema and induration progressing to eschar (Bartlett et al., 2019). In a study with 206 patients, 90 (43.7%) were classified as sarcopenic. Sarcopenia was associated with a significant increase in reoperations, and skeletal muscle mass depletion was associated with an increased rate of postoperative complications in patients undergoing CRS-HIPEC for colorectal PC (van Vugt et al., 2015). A previous case report described a rare pulmonary complication secondary to intraperitoneal administration of MMC. Moreover, this should be considered as it was the cause of serious pulmonary toxicity. However, there was no fluid collection or other evidence of an anastomotic leak in the abdominal computed tomography scan. Therefore, an abdominal source of pulmonary toxicity was unlikely (Abel, Kokosis & Blazer, 2017). Smibert et al. (2020) reported that infections after HIPEC were noted in the surgical site, respiratory tract, and urinary tract. These included Clostridium difficile infection, and postoperative sepsis. In most cases, infection onset was within 7 days postoperatively, and the median length of hospitalization was 19 days (Smibert et al., 2020).
Strategies for toxicity workup and management
There are several ways to address the challenges posed by the side-effects presented in this article. Recent evidence strongly suggests that an expert multidisciplinary team, including experienced surgeons and medical oncologists, should be established for HIPEC to better control hyperthermia and drug selection on an individual patient basis. One important consideration is improving the efficiency of HIPEC. A drug that enters the circulation may have little secondary therapeutic effects, but its systemic effect should be low enough to minimize its side-effects (De Smet et al., 2013; Rezaeian, Sedaghatkish & Soltani, 2019). Photothermal inorganic nanoparticles responsive to near-infrared light provide new opportunities for simultaneous and targeted delivery of heat and chemotherapeutics to tumor sites in pursuit of synergistic effects to enhance efficacy (Zhang, Wang & Chen, 2013).
Choosing a comparatively new and promising class of anticancer agents, such as ripretinib, for HIPEC not only improves median progression-free survival and acceptable safety profiles, but also reduces the associated adverse reactions (Blay et al., 2020). Neutropenia can be effectively treated with filgrastim, which is the original recombinant human granulocyte colony-stimulating factor widely used for preventing neutropenia-related infections and mobilizing hematopoietic stem cells (Dale et al., 2018). Assessment of platelet and leukocyte counts prior to CRS/HIPEC may help predict the development of thrombocytopenia and leukopenia (Hakeam et al., 2018). Bouhadjari et al. (2016) found that amifostine may reduce severe renal impairment when cisplatin (CP) is used in HIPEC. Aihara et al. (2003) suggested that bowel obstruction does not improve with conservative treatment, and PC recurrence has been excluded through thorough examination. Moreover, an early laparotomy can resolve symptoms of bowel obstruction and restore QOL (Aihara et al., 2003). In addition, Somashekhar et al. (2020) suggested that minimizing perioperative temperatures to <36.0 °C may decrease perioperative surgical site infections in these patients after CRS and HIPEC (Eng et al., 2018). A study from India found that CP was a safer drug when used alone, followed by MMC, and adriamycin combined with CP had higher morbidity and worse side-effects (Somashekhar et al., 2020).
Traditional Chinese medicine (TCM) focuses on overall treatment of the individual, and the focus of Chinese herbal medicine is to reduce the side-effects of treatment. The various herbs used in the TCM formula are thought to have synergistic effects or reduce side-effects, that is, the characteristic of “Jun Chen Zuo Shi” of TCM formulas (Gao et al., 2021). TCM, such as Ginseng, Huang-Qi, BanZhiLian, TJ-48, Huachansu injection, and Shenqi Fuzheng injection, play an important role in reducing side-effects after surgery or chemotherapy by inhibiting cancer cell proliferation, regulating immunity, and suppressing angiogenesis (Law et al., 2012; Li et al., 2008; Qi et al., 2015). TCM may serve as a dietary herbal supplement in the treatment of GI cancers and may decrease the side-effects of chemotherapeutic agents used in HIPEC.
Regarding the management of rarely reported side-effects, such as ERS, the patient who underwent laparotomy, total enterolysis, and peritonectomy, had a satisfactory recovery, started a normal diet within 7 days, and was discharged from the hospital within 14 days after a postoperative stay without complications (Mangan et al., 2019). In the case of pulmonary toxicity (Abel, Kokosis & Blazer, 2017), the treatment with empiric antibiotics and diuretics was not effective enough, and phenylephrine and intermittent bilateral positive airway pressure were also administered for blood pressure and respiratory support. Early identification and timely use of topical mitigating agents, such as dimethyl sulfoxide (DMSO), may prevent progression to scrotal necrosis and requires surgical debridement. More effective strategies may be geared toward prevention with thorough washout following HIPEC. Preprocedural radiologic imaging or intraoperative visualization of the patent processus vaginalis, internal inguinal canal plugs, and patient education with anticipatory guidance are suggested in the event that a reaction occurs (Abdul Aziz, Wang & Teo, 2015; Bartlett et al., 2019).
There is a potential risk that the above interventions may cause other issues. Nonetheless, these measures are very effective for toxicity inspection and management in HIPEC, and the benefits of these interventions outweigh the risks of further issues arising.
Conclusions
HIPEC is currently used as an essential component of treatment to improve the disease-free and overall survival of patients with primary and metastatic GI cancers. High complication rates are a misperception from early CRS/HIPEC experiences and should no longer deter the referral of patients to experienced centers or impede clinical trial development. However, the treatment has led to unwanted side-effects in the digestive, hematopoietic, circulatory, metabolic, and urinary systems. These side-effects vary depending on the specific agents used in the adjuvant regimen as well as on the dose and the duration of treatment. In addition, there is considerable variability in the side-effect profile across individuals.
While HIPEC has proven to be effective in optimizing the efficacies of GI cancer treatments, traditional chemotherapy is subject to side-effects, and heat delivery is often challenging. Future studies will require tailored patient selection, timing, and optimal HIPEC regimens to improve the effectiveness of this specialized treatment for patients with GI cancer. Careful preoperative assessment of patients is paramount to ensure favorable patient outcomes following this complex procedure. Our study may also provide a rationale for concurrent treatment with drugs that protect against or compensate for the side-effects of chemotherapy. The decision to undergo HIPEC involves careful consideration of the potential benefits and risks of therapy. In future research, additional experimental and molecular epidemiological studies should explore ways to reduce the side-effects of HIPEC in patients with GI cancer.