Plant-exuded chemical signals induce surface attachment of the bacterial pathogen Pseudomonas syringae
- Published
- Accepted
- Received
- Academic Editor
- Jesús Campos-García
- Subject Areas
- Biochemistry, Microbiology, Plant Science
- Keywords
- Type III secretion, Pseudomonas syringae, Inter-Kingdom signaling, Biofilm, Arabidopsis
- Copyright
- © 2023 O’Malley et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Plant-exuded chemical signals induce surface attachment of the bacterial pathogen Pseudomonas syringae. PeerJ 11:e14862 https://doi.org/10.7717/peerj.14862
Abstract
Many plant pathogenic bacteria suppress host defenses by secreting small molecule toxins or immune-suppressing proteins into host cells, processes that likely require close physical contact between pathogen and host. Yet, in most cases, little is known about whether phytopathogenic bacteria physically attach to host surfaces during infection. Here we report that Pseudomonas syringae pv. tomato strain DC3000, a Gram-negative bacterial pathogen of tomato and Arabidopsis, attaches to polystyrene and glass surfaces in response to chemical signals exuded from Arabidopsis seedlings and tomato leaves. We characterized the molecular nature of these attachment-inducing signals and discovered that multiple hydrophilic metabolites found in plant exudates, including citric acid, glutamic acid, and aspartic acid, are potent inducers of surface attachment. These same compounds were previously identified as inducers of P. syringae genes encoding a type III secretion system (T3SS), indicating that both attachment and T3SS deployment are induced by the same plant signals. To test if surface attachment and T3SS are regulated by the same signaling pathways, we assessed the attachment phenotypes of several previously characterized DC3000 mutants, and found that the T3SS master regulator HrpL was partially required for maximal levels of surface attachment, whereas the response regulator GacA, a negative regulator of T3SS, negatively regulated DC3000 surface attachment. Together, our data indicate that T3SS deployment and surface attachment by P. syringae may be co-regulated by the same host signals during infection, possibly to ensure close contact necessary to facilitate delivery of T3SS effectors into host cells.
Introduction
Pseudomonas syringae are Gram-negative bacteria capable of infecting a diverse range of plant species. Under environmental conditions that favor disease, P. syringae invade through wounds or natural openings in the leaf surface to colonize the intercellular space, or apoplast (Hirano & Upper, 2000). Once inside the apoplast, P. syringae can proliferate to high levels and ultimately cause visible symptoms including chlorosis and the formation of water-soaked necrotic lesions. P. syringae infection can drastically reduce the photosynthetic capacity and productivity of diseased plants (Lamichhane, Messéan & Morris, 2015), thereby limiting overall agricultural yield and product quality (Xin, Kvitko & He, 2018).
P. syringae overcomes host immune responses by deploying a type III secretion system (T3SS), a syringe-like apparatus that delivers proteins termed effectors directly into host cells. Within the host, effector proteins target and suppress immune defense signaling pathways, allowing for pathogen growth in the apoplast. The expression of T3SS-encoding genes is under direct control of HrpL, an alternative sigma factor that directly binds to a hrp box motif within the promoters of T3SS-associated genes to activate their expression (Xiao & Hutcheson, 1994; O’Malley & Anderson, 2021). Although T3SS-associated genes are a primary component of the HrpRS-HrpL regulon, additional T3SS-independent genes have altered expression levels in mutants lacking hrpL, suggesting a broader and more complex role for this pathway in regulating gene expression (Buell et al., 2003; Ferreira et al., 2006; Lam et al., 2014).
Genes that encode T3SS components and effectors are not constitutively expressed and must be induced during infection (Rahme, Mindrinos & Panopoulos, 1992; Salmeron & Staskawicz, 1993). Regulation of T3SS-encoding genes is complex, with distinct inputs from different classes of plant-derived metabolites, as well as from general environmental conditions such as pH. Synthetic media that mimic apoplast conditions, namely an acidic pH, low nitrogen-to-carbon ratio of available nutrients, and the presence of a simple sugar such as fructose, can stimulate expression of T3SS-associated genes (Huynh, Dahlbeck & Staskawicz, 1989; Rahme, Mindrinos & Panopoulos, 1992; Salmeron & Staskawicz, 1993; O’Malley & Anderson, 2021). Recently, specific amino acids and organic acids exuded from Arabidopsis seedlings were identified as inducers of T3SS gene expression (Anderson et al., 2014), and genes required for T3SS induction by two of these metabolites, aspartic acid and glutamic acid, were identified (Yan et al., 2020). Whether these same plant-derived metabolites can also induce T3SS-independent responses in P. syringae remains unknown.
Upon entry into the leaf apoplast, P. syringae shed their flagella and transition from motile to sessile (Chinchilla et al., 2006; Buscaill et al., 2019; Bao et al., 2020). Electron micrograph imaging of P. syringae within the apoplast revealed an apparent close association of bacteria with plant cell surfaces, though the status of these non-motile bacteria, and how they may attach, is not fully understood (Bestwick, Bennett & Mansfield, 1995; Misas-Villamil, Kolodziejek & van der Hoorn, 2011; Rufián et al., 2018). This motility transition may be mediated in part by GacA, a global regulator of virulence known to promote flagellar motility in P. syringae and other bacteria (O’Malley et al., 2020, López-Pliego et al., 2021). However, the status of these non-motile bacteria within the plant host, and the mechanisms by which they may attach, are not fully understood. The syringe-like ultrastructure of the T3SS itself implies that the T3SS may span the plant host cell wall to deliver effectors. The T3SS consists of a ring-like structure that spans both bacterial inner and outer membranes, and an extracellular ~2 µm-long filament termed the pilus likely traverses the plant cell wall, a barrier ranging in thickness from 100 nm to 10 µm (O’Neill & York, 2003; Cornelis, 2006). Based on similarities between T3SS pilus length and plant cell wall thickness, P. syringae likely require close physical contact with the plant cell wall to successfully deliver effectors into host cells, yet mechanisms that may facilitate close physical contact are unknown. Under normal plant growth conditions, the leaf apoplast is an air-filled space with only a thin layer of water associated with the surrounding cell wall (Sattelmacher, 2001). As a consequence, close association between P. syringae and plant cells may be driven in part by bacterial colonization of this air-water interface. However, P. syringae may also actively alter its physiology to physically attach to plant cells to facilitate effector delivery and access cell nutrients.
In this work we report that P. syringae attaches to physical surfaces in response to specific metabolites exuded by plant tissues. Surface-inducing activity was present in exudates prepared from Arabidopsis seedlings and tomato leaf tissue. To identify the bioactive compounds, we tested a panel of metabolites present in Arabidopsis exudates and found that several metabolites previously found to induce the T3SS (Anderson et al., 2014) also stimulate surface attachment, suggesting co-regulation of these distinct processes in P. syringae by the same host signals. Although the molecular nature of this surface attachment is not known, surface attachment was influenced by known genetic regulators of the T3SS. The T3SS regulator HrpL was required for maximal attachment during this response. Conversely, GacA, a negative regulator of T3SS-encoding genes, suppressed surface attachment. Together, our data introduce a model in which T3SS and surface attachment are co-induced by plant signals encountered by P. syringae during infection.
Materials and Methods
Preparation of media, metabolite stocks and crystal violet staining solution
P. syringae were routinely cultured on King’s B (KB) medium (King, Ward & Raney, 1954) solidified with 1.5% agar. For all surface attachment assays, a modified hrp-inducing minimal medium (MM) (10 mM K2HPO4/KH2PO4 (pH 6.0), 7.5 mM (NH4)2SO4, 3.3 mM MgCl2, 1.7 mM NaCl) was prepared and autoclaved prior to use (Huynh, Dahlbeck & Staskawicz, 1989; Anderson et al., 2014). For biofilm assays in glass culture tubes, MMR minimal medium (7 mM Na-glutamate, 55 mM mannitol, 1.31 mM K2HPO4, 2.2 mM KH2PO4, 0.61 mM MgSO4, 0.34 mMCaCl2, 0.022 mM FeCl3, 0.85 mM NaCl) was prepared as described (Farias, Olmedilla & Gallegos, 2019). Autoclaved MM and MMR media were stored at room temperature. Sugar and metabolite stocks were prepared at concentrations of 1 M and 20 mM, respectively, in deionized water, 0.2 µM filter sterilized, and stored at 4 °C. A crystal violet staining solution was prepared by dissolving crystal violet dye in water to a concentration of 0.05% (w/v). The stain solution was then vacuum filtered through a 0.45 µM membrane to remove any undissolved dye and stored at room temperature.
Bacterial strains and growth conditions
DC3000 hrpL− and DC3000 AC811 strains were previously reported (Zwiesler-Vollick et al., 2002; Chatterjee et al., 2003). A complete list of all strains used in this study is provided in Table S1. P. syringae were maintained in 25% glycerol stocks at −80 °C. Bacteria were streaked onto KB agar plates supplemented with antibiotics rifampicin (50 µg/mL), spectinomycin (150 µg/mL), and kanamycin (30 µg/mL) as necessary and grown at room temperature for 2 days prior to use.
Preparation of plant exudates
Arabidopsis thaliana wild-type, mkp1, and mkp1 mpk6 plants, all ecotype Wassilewskija, were previously described (Anderson et al., 2014). Arabidopsis seeds were germinated and grown for approximately twelve days on agar plates containing 0.5× Murashige & Skoog (MS) medium (Murashige & Skoog, 1962). To prepare exudates, seedlings were removed from agar and immersed in water in a 15 mL conical vial at a density of approximately four seedlings per mL. After overnight incubation, the seedlings were removed and the remaining liquid (exudate) was sterilized using a 0.2 µm syringe filter. To prepare tomato leaf exudates, 0.8 cm2 leaf disks were punched from fully-expanded 5-week-old Solanum lycopersicum (tomato) cv. Rio Grande leaflets. The isolated leaf disks were floated on water for 24 h at a density of 3 disks/mL. The resulting exudate was sterilized using a 0.2 µm syringe filter. All exudates were either used immediately or stored at −20 °C.
P. syringae surface attachment assays
P. syringae were scraped from the surface of KB agar plates and suspended in 1 mL of water. To remove residual KB medium, bacteria were pelleted by centrifugation at 21,000×g for 1 min, then resuspended in 1 mL of water. This process was repeated twice for a total of three washes. Washed bacteria were adjusted to an optical density at λ = 600 nm (OD600) of 1.0 (~1 × 109 bacterial colony forming units/mL), then 50 µL inoculated into the well of 24-well polystyrene assay plates containing 450 µL of MM supplemented with 50 mM of sugar (fructose, sucrose, galactose, mannitol, glucose) and/or 200 µM of citric acid, aspartic acid, 4-hydroxybenzoic acid, glutamic acid, glycine, threonine, valine as indicated. For testing of plant exudates, 20 µL of OD600 = 1.0 bacteria were inoculated into 100 µL of exudate or water mixed with 100 µL of MM supplemented with or without 100 mM of sugar (fructose, sucrose, galactose, mannitol, glucose) in 96-well polystyrene assay plates. For fructose dose response assays in the presence or absence of seedling exudate, the concentration of fructose in MM was increased up to 250 mM as indicated. After inoculation of bacteria, assay plates were sealed with 3M micropore tape and incubated at room temperature under constant light for 16 h without shaking. To stain surface attached cells, a pipette was used to gently aspirate the supernatant from each well. The assay plate wells were then washed with 1 mL of water prior to staining with 0.5 mL of 0.5% (w/v) crystal violet stain for 15 min at room temperature. After removing the staining solution, stained wells were washed three times with water. Stained wells were then destained by addition of 0.5 mL of 95% ethanol and a Tecan Spark 10M microplate reader was used to measure the Absorbance at λ = 562 nm (Abs562) of the destaining solution. To rule out that the differences in attachment are due to growth differences between the compared strains, planktonic and attached bacteria were enumerated by serial dilution plating. To collect bacteria for dilution plating, the liquid was removed from an assay well and reserved as the planktonic cell fraction. Attached cells were resuspended by addition of 0.5 mL of MM to each well and scraping the well surface with a pipette tip. The resuspended cell fractions were vortexed for ~10 s to disaggregate cells. Planktonic and attached cell fractions were then serial diluted and spotted onto KB agar plates supplemented with rifampicin. After incubating the agar plates for 1–2 days at room temperature a light microscope was used to count colony forming units (cfus) on plate surfaces.
For attachment assays in glass culture tubes, washed bacteria were inoculated into KB medium, MM or MMR to a final OD600 = 1.0 (~1 × 109) in a total volume of 2.0 mL in sterile borosilicate glass culture tubes. Culture tubes were incubated at room temperature without shaking. After 72 h, the liquid in each culture tube was removed by pipetting, and the interior of each tube gently washed with 3 mL of water, then stained with 2 mL 0.05% CV staining solution. After 15 min, the CV staining solution was removed and tubes were washed three times with water. Tubes were air-dried overnight, then photographed.
Results
Plant exudates induce attachment of P. syringae to physical surfaces
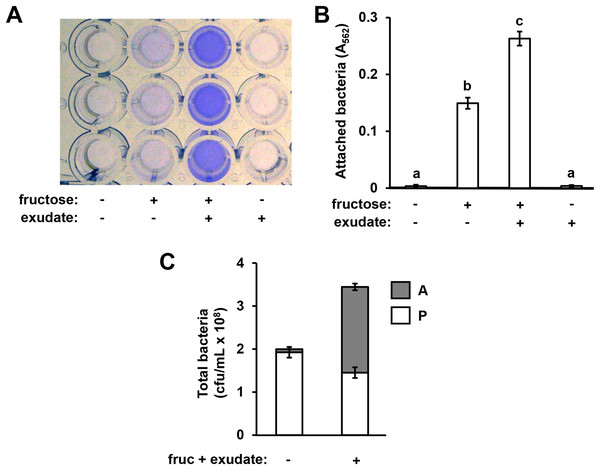
In previous work we reported that exudates prepared from Arabidopsis seedlings, when supplemented with a simple sugar such as fructose, can induce the expression of T3SS-associated genes in P. syringae pv. tomato DC3000 (Anderson et al., 2014). During these experiments we routinely cultured DC3000 within the wells of polystyrene assay plates. After culturing DC3000 with seedling exudates, we observed that the inner surface of assay plate wells had become cloudy in appearance, suggesting that bacteria, and/or metabolic products exuded by bacteria, were forming a residue in response to the exudate treatment. To assess if this residue was bacteria physically attaching to the assay plates, we cultured DC3000 in a minimal medium (MM), or MM supplemented with Arabidopsis seedling exudate and/or fructose. After 16 h, we decanted the liquid from assay plate wells and stained the wells with crystal violet, a non-specific stain of bacterial cells routinely used to detect biofilms (O’Toole & Kolter, 1998). Significantly higher levels of crystal violet (CV) staining occurred in wells that had contained DC3000 cultured in both fructose and Arabidopsis exudate relative to wells that had contained DC3000 cultured in fructose only (Figs. 1A and 1B). No CV staining was detected in wells that contained DC3000 in MM lacking fructose and exudate (Figs. 1A and 1B). Higher levels of CV staining were detected in wells where DC3000 had been incubated in fructose alone compared to the medium-only control wells, albeit to levels lower than observed with both fructose and exudate (Figs. 1A and 1B). By contrast, no CV staining occurred in wells that contained DC3000 and plant exudates in the absence of fructose. We also incubated DC3000 with exudate prepared from tomato leaf tissue and observed similar patterns of CV staining (Fig. S1). To determine if CV staining is due to surface attachment of viable bacteria, we scraped the attached material from the surface of assays wells and plated serial dilutions of these samples, as well as serial dilutions of the supernatant containing planktonic material, on King’s B (KB) agar. Surface-attached colony-forming units increased fifteen-fold in wells containing fructose and exudate relative wells containing MM alone, confirming that CV staining is indeed a proxy for levels of surface-attached viable bacteria (Fig. 1C). Total bacterial levels (planktonic plus attached) increased less than two-fold, indicating that increased attachment under these conditions is not due to large changes in overall bacterial growth (Fig. 1C). Together, these observations suggest that signals within both Arabidopsis and tomato exudates induce surface attachment of DC3000 in a fructose-dependent manner.
Figure 1: Arabidopsis seedling exudates induce P. syringae surface attachment.
P. syringae DC3000 were cultured in minimal medium (MM) alone, or in MM supplemented with 50 mM fructose and/or Arabidopsis seedling exudate. Cultures were incubated in wells of a 24-well polystyrene microtiter plate without shaking. After 16 h, microtiter plate wells were washed with water to remove unattached bacteria. The assay wells were then either stained with crystal violet (CV), or attached cell fractions were collected to enumerate bacterial levels by serial dilution plating on KB agar. (A) Photograph of plate wells after CV staining and desorption of stain from walls by addition of 95% ethanol. (B) Graphed are means of Absorbance at λ = 562 nm (A562) measurements of wells after CV staining. Lowercase letters are statistical groupings based on multiple pairwise t-tests, p < 0.05. Error bars are standard error; n = 6. (C) Graphed are means of bacterial colony forming units (cfu/mL) from attached (A) and planktonic (P) cell fractions. Error bars are standard error; n = 4. Results are representative of at least three independent experiments.A variety of simple sugars, as well as the sugar alcohol mannitol, can induce T3SS gene expression in DC3000 (Salmeron & Staskawicz, 1993; Rahme, Mindrinos & Panopoulos, 1992). We therefore tested a panel of sugars for surface attachment-inducing activity. Based on CV staining, we detected increased levels of DC3000 attachment in response to all sugars tested, albeit to different levels (Fig. S2). Fructose, galactose, or mannitol combined with exudate elicited the highest levels of attachment, whereas relatively little attachment was observed in response to sucrose or glucose combined with exudate (Fig. S2A). In the absence of seedling exudate, lower but significant levels of surface attachment were observed in MM containing fructose, galactose, or mannitol, whereas MM containing glucose or sucrose did not significantly induce attachment (Fig. S2B). A slight yet significant decrease in attachment was observed in MM containing sucrose relative to MM alone (Fig. S2B). We conclude that all sugars tested, alone or in combination with exudate, can induce attachment and have varying levels of bioactivity.
A diverse mixture of soluble metabolites, including plant-derived sugars, are present in plant exudates (Anderson et al., 2014). Because various sugars were able to elicit surface attachment, we reasoned that attachment may be induced by plant-derived sugars present in exudates. To assess this possibility, we incubated DC3000 with or without Arabidopsis exudate in the presence of increasing amounts of fructose. Regardless of the concentration of fructose added (up to 250 mM), the presence of exudate significantly increased the amount of attached bacteria (Fig. S2C). These data suggest that attachment-inducing signals in exudates are not sugars, but distinct signals that function synergistically with exogenously provided sugar to induce attachment.
Exudates from Arabidopsis mkp1 stimulate lower levels of P. syringae surface attachment
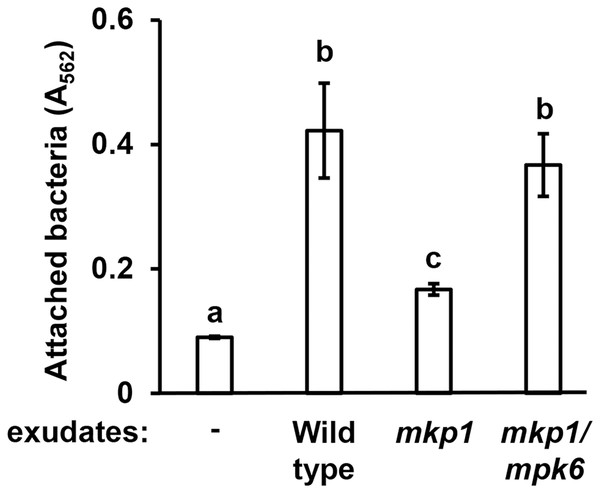
Arabidopsis MITOGEN-ACTIVATED PROTEIN KINASE PHOSPHATASE 1 (MKP1) is a negative regulator of immune-activated MAPK signaling pathways and resistance to bacterial disease (Jiang et al., 2017; Anderson et al., 2011). Arabidopsis mkp1 mutants are more resistant to P. syringae infection, and this enhanced resistance requires a functional MAP kinase 6 (MPK6) gene. The molecular basis for this enhanced resistance phenotype of Arabidopsis seedlings is decreased exudation of T3SS-inducing signals (Anderson et al., 2014). In this regard, mkp1 seedlings exude lower levels of T3SS-inducing metabolites, whereas exudation of these metabolites from mkp1 mpk6 double mutant is restored to wild type levels (Anderson et al., 2014). To assess if the abundance of surface attachment-inducing signals may be genetically regulated in a similar fashion, we evaluated mkp1 and mkp1 mpk6 exudates for their capacity to induce surface attachment. Compared to exudate from wild type plants, exudate from mkp1 plants stimulated significantly lower levels of surface attachment by DC3000. Conversely, the level of attachment induced by mkp1 mpk6 exudate was not significantly different from wild type levels (Fig. 2). These data show that, similar to T3SS-inducing signals, MKP1 negatively regulates attachment-inducing signals in exudates in an MPK6-dependent manner.
Figure 2: Exudates from Arabidopsis mkp1 seedlings elicit lower levels of P. syringae surface attachment.
DC3000 were cultured for 16 h in MM containing 50 mM fructose and supplemented with or without exudate from wild type (Ws, ecotype Wassilewskija), mkp1, or mkp1/mpk6 Arabidopsis seedlings as indicated. Graphed are means of Absorbance (A562) measurements of crystal violet-stained wells. Small case letters are statistical groupings based on multiple pairwise t-tests, p < 0.05. Error bars are standard error; n = 3. Data are representative of three independent experiments.To further characterize the nature of attachment-inducing signal(s), we used chloroform to fractionate exudates from wild type and mkp1 plants into aqueous and organic phases, then measured levels of surface attachment of DC3000 in response to metabolites extracted into each phase. Surface attachment was induced only by the aqueous fraction of WT and mkp1 exudates (Fig. S3). These data are consistent with our previous finding that the T3SS-inducing activity of seedling exudate is present only in the aqueous phase after chloroform extraction (Anderson et al., 2014), suggesting that T3SS and surface attachment may be induced by the same hydrophilic signal(s).
P. syringae surface attachment is induced by known T3SS-inducing metabolites
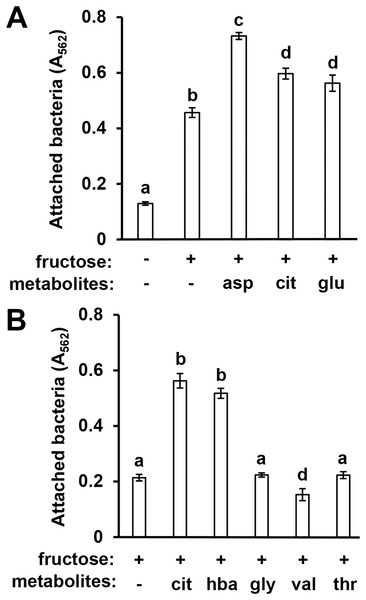
In previous work we identified specific T3SS-inducing metabolites present in Arabidopsis seedling exudates and decreased in abundance in mkp1 exudates (Anderson et al., 2014). To assess whether surface attachment is induced by these same signals, we tested three T3SS-inducing metabolites—aspartic acid, glutamic acid, and citric acid—for their ability to induce DC3000 attachment. All three metabolites individually, in combination with fructose, induced significantly higher levels of surface attachment compared to fructose only control wells (Fig. 3A). We also observed that 4-hydroxybenzoic acid, a T3SS-inducing metabolite associated with secondary metabolic pathways, induced attachment, indicating that compounds from diverse metabolic pathways possess attachment-inducing activity (Fig. 3B). Several amino acids present in Arabidopsis exudates that do not induce T3SS genes, namely glycine, valine and threonine (Anderson et al., 2014), did not induce attachment (Fig. 3B). These data show a close correlation between T3SS- and attachment-inducing activities of plant-derived metabolites.
Figure 3: Known T3SS-inducing metabolites induce P. syringae surface attachment.
(A) DC3000 were cultured for 16 h in minimal medium (MM) with or without 50 mM fructose, or MM with 50 mM fructose and 200 µM aspartic acid (asp), citric acid (cit), or glutamic acid (glu). Graphed are means of Absorbance (A562) measurements after crystal violet (CV) staining of wells. Statistical groups were determined by multiple pairwise t-tests, p < 0.05. Error bars are standard error; n = 4. (B) DC3000 were cultured for 16 h in MM with 50 mM fructose and 200 µM cit, 4-hydroxybenzoic acid (hba), glycine (gly), valine (val), or threonine (thr). Graphed are means of A562 measurements of CV-stained wells. Small case letters are statistical groupings based on multiple pairwise t-tests, p < 0.05. Error bars are standard error; n = 4. Data are representative of at least three independent experiments.P. syringae surface attachment is influenced by T3SS master regulator HrpL and global regulator GacS-GacA
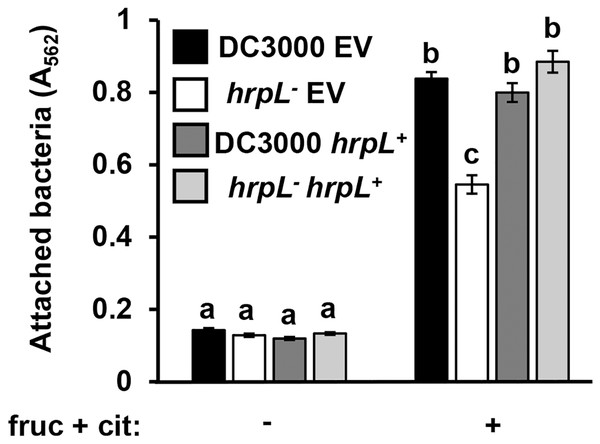
Because surface attachment and type III secretion are induced by the same metabolites, we next tested whether these responses are dependent on the same signaling pathways in P. syringae. We first tested attachment of a DC3000 hrpL− insertion mutant and its wild type parental strain carrying either a complementing plasmid (pVSP61::hrpL) or an empty vector control (Zwiesler-Vollick et al., 2002; Sreedharan et al., 2006). Surface attachment of DC3000 hrpL− in response to fructose and citric acid was partially attenuated, exhibiting a ~40% reduction in attachment relative to wild type DC3000 (Fig. 4). Introduction of pVSP61::hrpL into DC3000 hrpL− restored metabolite-induced attachment to wild type levels (Fig. 4). These results indicated that the observed surface attachment is dependent on both hrpL-dependent and -independent processes in DC3000.
Figure 4: P. syringae surface attachment is partially dependent on T3SS master regulator HrpL.
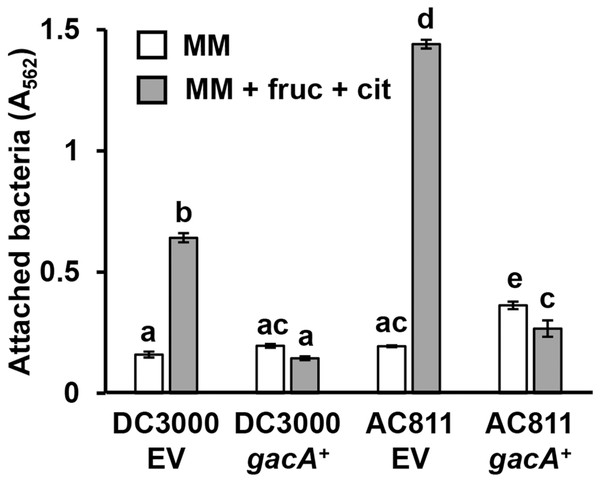
DC3000 and DC3000 hrpL−, harboring either a pVSP61::hrpL complementing plasmid (hrpL+) or pVSP61 empty vector (EV), were incubated in minimal medium (MM) supplemented with 50 mM fructose and 200 µM citric acid for 16 h. Graphed are means of Absorbance (A562) measurements of crystal violet-stained microtiter plate wells. Lowercase letters denote statistical groups based on ANOVA with multiple pairwise t-test comparisons and Tukey’s post-hoc HSD test, p < 0.05. Error bars are standard error; n = 3. Data are representative of three independent experiments.GacS-GacA is a two-component system that regulates the expression of various virulence traits in pseudomonads (Heeb & Haas, 2001), including biofilm formation in some species (Parkins, Ceri & Storey, 2001; Li et al., 2015). To investigate whether GacS-GacA also regulates surface attachment in DC3000, we measured attachment of AC811, a Tn5 insertion mutant of gacA (O’Malley et al., 2019; Chatterjee et al., 2003), in response to fructose and plant metabolites. We observed significantly higher levels of surface attachment of the AC811 mutant in response to fructose and citric acid relative to its wild type DC3000 parental strain (Fig. 5), indicating that surface attachment may be negatively regulated by GacA. Accordingly, levels of surface attachment were suppressed to sub-wild type levels in a complemented strain of AC811 expressing gacA under its native promoter (AC811 gacA+). The repression of surface attachment below wild type levels in both DC3000 gacA+ and AC811 gacA+ is likely due to gacA overexpression in these complemented strains, as previously described (O’Malley et al., 2020). We conclude from these results that GacA functions as a negative regulator of surface attachment by DC3000 in response to fructose and citric acid.
Figure 5: P. syringae surface attachment is repressed by the global response regulator GacA.
DC3000 and DC3000 gacA::Tn5 (strain AC811) carrying either pBBR1-MCS1::gacA (gacA+) or pBBR1-MCS1 empty vector (EV) were incubated in minimal medium (MM) supplemented with 50 mM fructose and 200 µM citric acid for 16 h. Graphed are means of Absorbance (A562) measurements of crystal violet-stained microtiter plate wells. Lowercase letters denote statistical groups based on ANOVA with multiple pairwise t-test comparisons and Tukey’s post-hoc HSD test, p < 0.05. Error bars are standard error; n = 3. Data are representative of three independent experiments.T3SS-inducing metabolites enhance P. syringae attachment at the air-liquid interface in static cultures
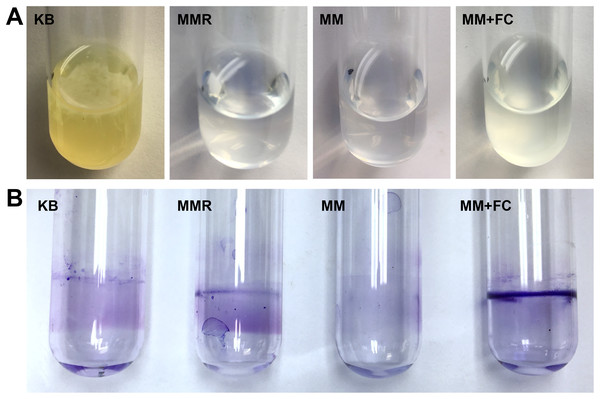
We also assessed whether T3SS-inducing metabolites induce surface biofilm formation at the air-liquid interface, a phenomenon previously described in DC3000 (Farias, Olmedilla & Gallegos, 2019). To investigate, we cultured DC3000 under static conditions in borosilicate glass culture tubes containing rich KB medium, or containing MM with or without fructose and citric acid. We also cultured DC3000 in MMR, a defined minimal medium previously used to assess P. syringae biofilm dynamics (Farias, Olmedilla & Gallegos, 2019; Robertsen et al., 1981). MMR differs from MM by addition of mannitol and sodium glutamate, and contains higher levels of iron and calcium (Farias, Olmedilla & Gallegos, 2019). To assess bacterial attachment, we visually observed culture tubes for 72 h, followed by CV staining of culture tube surfaces. A pellicle, or floating biofilm formed at the air–liquid interface, was visible on the surface of DC3000 in KB as early as 48 h post-inoculation, whereas no pellicles were ever visible in tubes containing DC3000 in MMR or in MM with or without fructose and citric acid (Fig. 6A). These results were distinct from those reported previously, where pellicle formation was observed in MMR, suggesting potential differences in experimental conditions. Upon removal of media and CV staining of culture tubes, we observed only diffuse CV staining on the walls of tubes that contained DC3000 in MM (Fig. 6B). A slight yet more defined ring of CV staining at the air-liquid interface was apparent on the walls of tubes that contained DC3000 in KB or MMR (Fig. 6B). In contrast, a dense ring of CV staining at air-liquid interface was observed in tubes that contained DC3000 in MM supplemented with fructose and citric acid (Fig. 6B).
Figure 6: T3SS-inducing metabolites enhance surface attachment by P. syringae at the air-liquid interface in glass culture tubes.
P. syringae DC3000 were incubated in borosilicate glass tubes in King’s B (KB) rich medium, MMR medium, or minimal medium (MM) with or without 50 mM fructose and 200 µM citric acid. Tubes were maintained at room temperature without shaking for 72 h before aspirating the cultures and staining the culture tube surfaces with crystal violet (CV). Pictured are culture tubes after CV staining, with visible rings of bacterial attachment at the level of the air-water interface. Results are representative of three independent experiments.Discussion
In this work, we discovered that specific plant-derived metabolites induce P. syringae to attach to surfaces. We established that surface attachment is a genetically-encoded response in DC3000, with T3SS master regulator HrpL necessary for maximal attachment, and the global response regulator GacA functioning to suppress attachment. These results support a model in which surface attachment is a process co-activated by T3SS-inducing plant metabolites, possibly as a means to ensure close contact with host cells necessary for T3SS deployment during infection.
While our results demonstrate that P. syringae is capable of physical attachment to surfaces, the mechanism(s) of adhesion remains unknown. Among bacterial pathogens, a variety of extracellular structures mediate attachment to surfaces, and attachment processes can be reversible or irreversible. The gall-forming plant pathogen Agrobacterium tumefaciens forms reversible attachments to host surfaces through pili, which are multiple distinct types of hair-like extracellular appendages (Wang, Haitjema & Fuqua, 2014). Similarly, pili mediate initial contact of the opportunistic animal and plant pathogen Pseudomonas aeruginosa with host epithelial cell surfaces (Bucior, Pielage & Engel, 2012). Structural components of flagella also contribute to reversible surface association by P. aeruginosa (Merritt et al., 2007; Schniederberend et al., 2019). Following initial reversible surface contact, bacterial cells often undergo phenotypic differentiation and become irreversibly attached (Sauer et al., 2002; Hinsa et al., 2003). These cells become anchored to surfaces through attachment factors such as extracellular polysaccharides (EPS) and/or cell-surface proteins such as adhesins or carbohydrate-binding lectins, providing strong surface adhesion. Numerous potential attachment factors are genetically encoded in P. syringae, including various pili and extracellular adhesins (Buell et al., 2003), though their roles in virulence remain unknown. P. syringae has been observed to form biofilms, multicellular communities in which bacteria are embedded in a self-produced matrix comprised of EPS or other polymeric substances. When grown in rich media, P. syringae forms biofilms consisting of EPS such as alginate and levan (Laue et al., 2006), as well as pellicles containing both alginate and cellulose (Farias, Olmedilla & Gallegos, 2019). Less is known about biofilm formation during infection, though the production of various EPS is required for epiphytic fitness and/or full virulence of select P. syringae strains on host plants, suggesting a role for biofilm formation in host infection (Yu et al., 1999; Arrebola et al., 2015; Helmann, Deutschbauer & Lindow, 2019; Heredia-Ponce et al., 2020). Further analysis of the genetic basis of P. syringae attachment observed in this study will be necessary to determine the physical factor(s) that promote the surface association.
Our analysis of the AC811 gacA::Tn5 strain indicates that surface attachment by P. syringae may be negatively regulated by the GacS-GacA two component system. Recently we reported that GacA also functions as a negative regulator of T3SS and a positive regulator of flagellar motility (O’Malley et al., 2019; O’Malley et al., 2020). Based on these previous findings, we hypothesized that GacS-GacA signaling is activated on the leaf surface, where it functions to suppress production of the T3SS and promote flagellar motility for bacteria to gain access to the leaf interior through stomata or other openings (O’Malley et al., 2020). Suppression of the T3SS by GacA is also hypothesized to optimize fitness of epiphytic bacteria, given that the T3SS is critical for P. syringae survival within the apoplast but is largely dispensable on the leaf surface (Helmann, Deutschbauer & Lindow, 2019). Based on the data presented here, we propose that P. syringae surface attachment may be similarly repressed on the leaf surface by activated GacS-GacA, and potentially de-repressed within the apoplast due to inactivated GacS-GacA. Analysis of the activation of GacS-GacA within the host plant environment will be necessary to fully evaluate this model.
Upon apoplast colonization, P. syringae shed their flagella and undergo a motile-to-sessile transition (Chinchilla et al., 2006; Yu et al., 2013; Helmann, Deutschbauer & Lindow, 2019; Bao et al., 2020; Buscaill et al., 2019). As such, it is plausible that P. syringae surface attachment may occur coincident with with this loss in motility, potentially as a means to facilitate T3SS deployment. Our observations that P. syringae attachment is induced by metabolic signals that are highly abundant in the leaf apoplast, such as glutamic acid and aspartic acid, lends further support to this hypothesis (Rico & Preston, 2008; Kumar et al., 2017). The extended length of the P. syringae pilus is hypothesized to facilitate delivery of T3SS effectors across the plant cell wall, which can be up to 10 nm in thickness (O’Neill & York, 2003). Although the P. syringae T3SS pilus can extend several µM in length, significantly longer than the ~50–600 nm T3SS filament produced by animal pathogenic γ-proteobacteria (Cornelis, 2006), close association with the plant cell wall is likely required for the T3SS pilus to contact the host plasma membrane and deliver effectors. Physiochemical effects of bacterial association with plant cell walls, such as local changes in osmolarity and pH in association with surfaces vs. bulk liquid, may additionally influence effector delivery and pathogen dynamics during infection (Hong & Brown, 2010; Kimkes & Heinemann, 2020).
Multiple T3SS-inducing signals, including aspartic acid and citric acid (Anderson et al., 2014), as well as a simple sugars, increased surface attachment of P. syringae. Conversely, glycine, valine, and threonine, compounds that were previously identified as non-inducers of T3SS (Anderson et al., 2014), failed to elicit surface attachment. This apparent close correlation between induction of T3SS and surface attachment, as well as partial requirement of T3SS regulator HrpL for surface attachment, suggests that both responses may be partially regulated by the same signaling pathways in P. syringae. In various pathogens, nonmotile or surface-associated cells exhibit increased T3SS expression and virulence, suggesting that co-regulation of attachment and T3SS may be a common virulence strategy (Soscia et al., 2007; Tamayo, Patimalla & Camilli, 2010). The T3SS translocon is required for initial adhesion and bacterial aggregate formation on host cell surfaces by various pathogens, including P. aeruginosa and the biofilm-forming plant pathogen Xanthomonas citri (Hernandes et al., 2013; Santos et al., 2019; Tran et al., 2014; Zimaro et al., 2014). These studies indicate that the T3SS translocon itself may function as a surface adhesin, though the underlying mechanism(s) have not been established. Beyond the potential role of the T3SS itself in mediating surface interaction, various T3SS-independent genes are co-regulated by HrpL and may impact attachment (Lam et al., 2014). How T3SS deployment and surface attachment may cooperatively function during host infection remains to be determined.
Conclusions
This study demonstrates that P. syringae pv. tomato DC3000 attaches to physical surfaces in the presence of host plant exudates. Plant metabolic signals that induce type III secretion by P. syringae, including citric acid and aspartic acid, were identified as inducers of surface attachment. Our findings demonstrate that surface attachment and T3SS gene expression by P. syringae are co-activated by the same plant metabolites, suggesting that these two processes may be co-regulated during infection.
Supplemental Information
P. syringae attaches to the surface of assay plate wells in response to tomato leaf exudates.
P. syringae DC3000 were cultured in minimal medium (MM) alone, or MM supplemented with 50 mM fructose and/or tomato leaf exudate. After 16 h, assay plate wells were washed with water to remove unattached bacteria, then stained with crystal violet (CV). Graphed are means of Absorbance (A562) measurements of CV-stained microtiter plate wells. Small case letters denote statistical significance groupings based on pairwise t-tests, p < 0.05. Error bars are standard error; n = 4. Data are representative of three independent experiments.
Crystal violet staining correlates with the amount of viable surface attached P. syringae cells.
P. syringae DC3000 were cultured for 16 h in minimal medium (MM) alone or MM supplemented with 50 mM fructose and Arabidopsis seedling exudate. Attached and planktonic bacteria were enumerated by serial dilution plating on KB agar and counting of colony-forming units (cfus). (A) Graphed are means of percent attached cfus vs total cfus (attached plus planktonic). Error bars are standard error; n = 4. (B) Graphed are means of log total cfus (planktonic and attached). Error bars are standard error, n = 4. Asterisks indicate statistical significance based on pairwise t-tests, *** p < 0.001. Data are representative of at least three independent experiments.
P. syringae surface attachment induced by different sugars.
DC3000 were cultured for 16 h in (A) Arabidopsis seedling exudate or (B) water in either minimal medium (MM) only or MM supplemented with 50 mM fructose (fruc), glucose (gluc), galactose (gal), sucrose (suc), or mannitol (man). Graphed are means of Absorbance (A562) measurements of crystal violet (CV)-stained wells after incubating cultures. Error bars are standard error; n = 6. (C) DC3000 were cultured with or without Arabidopsis seedling exudate in MM supplemented with varying concentrations of fructose. Graphed are means of A562 measurements of CV-stained wells after incubating cultures for 16 h. Error bars are standard error; n = 4. Asterisks indicate statistical significance based on pairwise t-tests, ** is p < 0.01, ns is not significant.