Genome-wide identification and expression analysis of calmodulin-like proteins in cucumber
- Published
- Accepted
- Received
- Academic Editor
- Sapna Langyan
- Subject Areas
- Agricultural Science, Bioinformatics, Molecular Biology, Plant Science
- Keywords
- Cucumis sativus L, Calmodulin-like protein, Genome-wide, Phylogenetic analysis, Expression pattern
- Copyright
- © 2023 Liu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Genome-wide identification and expression analysis of calmodulin-like proteins in cucumber. PeerJ 11:e14637 https://doi.org/10.7717/peerj.14637
Abstract
Background
The calmodulin-like (CML) protein is a crucial Ca2+-binding protein that can sense and conduct the Ca2+ signal in response to extracellular stimuli. The CML protein families have been identified and characterized in many species. Nevertheless, scarce information on cucumber CML is retrievable.
Methods
In this study, bioinformatic analyses, including gene structure, conserved domain, phylogenetic relationship, chromosome distribution, and gene synteny, were comprehensively performed to identify and characterize CsCML gene members. Spatiotemporal expression analysis in different organs and environment conditions were assayed with real-time quantitative polymerase chain reaction (qRT-PCR).
Results
Forty-four CsCMLs family members were well characterized, and the results showed that the 44 CsCML proteins contained one to four EF-hand domains without other functional domains. Most of the CsCML proteins were intron-less and unevenly distributed on seven chromosomes; two tandemly duplicated gene pairs and three segmentally duplicated gene pairs were identified in the cucumber genome. Cis-acting element analysis showed that the hormone, stress, and plant growth and development-related elements were in the promotor regions. In addition, spatiotemporal expression analysis revealed distinctive expression patterns for CsCML genes in different tissues and environmental conditions, and a putative protein interaction network also confirmed their potential role in responding to various stimuli. These results provide a foundation for understanding CsCMLs and provide a theoretical basis for further study of the physiological functions of CsCMLs.
Introduction
Calcium ions (Ca2+) are essential nutrients in plant growth and development and are also the primary second messengers of all eukaryotic cells in cell signal transduction (Zeng et al., 2017). When plants suffer stress from external stimuli (salt, temperature, drought, heavy metal toxicity, light, plant hormones, and pathogenic microorganism infection, among others), changes in the concentration of intracellular and extracellular Ca2+ occur, evoking a calcium signature (Perochon et al., 2011). The downstream conduction of calcium signals requires the involvement of calcium-binding proteins, which are referred to as calcium sensors. The major sensors include calmodulins (CaMs), CaM-like proteins (CMLs), Ca2+-dependent protein kinases (CDPKs), and calcineurin B-like proteins (CBLs) (Ranty et al., 2016; Yang & Poovaiah, 2003). They commonly contain elongation factor hand (EF-hand) motifs. The EF-hand is a helix-loop-helix structure capable of binding Ca2+, following which it undergoes a conformational change, interacting with downstream proteins or modulating its own catalytic activity (Kim et al., 2009; Perochon et al., 2011; Zeng et al., 2017). The CML is a Ca2+ sensor protein similar to CaM and contains several EF-hands that bind to Ca2+ andact on downstream targets (Mohanta, Kumar & Bae, 2017). These sensors can be divided into two groups: sensor relays, such as CaM, CML, and CBL, which do not have catalytic activity themselves and need to interact with downstream target proteins and form similar Ca2+/CaM complexes; and sensor responders, which contain other effector domains except for EF-hands, directly relaying the signal to downstream targets, such as CDPKs (Perochon et al., 2011).
As a typical Ca2+ sensor in higher plants, CMLs possess one to six EF-hand motifs without any other functional domain (McCormack, Tsai & Braam, 2005). To date, CML genes have been analyzed in various species; such as, 50 have been identified in Arabidopsis (McCormack & Braam, 2003), 32 in rice (Boonburapong & Buaboocha, 2007), 68 in grape (Konstantin et al., 2021), 79 in Chinese cabbage (Nie, Zhang & Zhang, 2017), 52 in tomato (Munir et al., 2016a), 50 in alfalfa (Sun, Yu & Guo, 2020), 21 in ginkgo (Zhang et al., 2022), 82 in chrysanthemum (Fu et al., 2022), 41 in soybean (Yadav et al., 2022), and 58 in apple (Li et al., 2019a; Li et al., 2019b). Although the cucumber genome has been well assembled and annotated (Cavagnaro et al., 2010; Li et al., 2019a; Li et al., 2019b; Osipowski et al., 2020), the characteristics and functions of CML gene members are still unknown. Previous research showed that CMLs play a critical role in plant growth and development, stress, and hormone responses. In Arabidopsis, CML23 and CML24 are associated with plant flowering (Tsai et al., 2007); AtCML4/5 functions in vesicle transport within the plant endomembrane system (Ruge et al., 2016); and promoter analysis showed that AtCML15 and AtCML16 might have a role in floral development (Ogunrinde et al., 2017). AtCML8 (Zhu et al., 2017) and AtCML9 (Leba et al., 2012) were verified to participate in the plant immune response against Pseudomonas syringae; AtCML37 and AtCML38 were induced by wounding, osmotic stress, and drought; while AtCML39 was dramatically expressed when stimulated by methyl jasmonate (MeJA) (Vanderbeld & Snedden, 2007). In soybean, GsCML27 was induced by salt, bicarbonate, and osmotic stress, and the ectopic expression of GsCML27 decreased by changing the content of cell ions and osmotic regulation (Chen et al., 2015). In Vitis amurensis, CML41b, CML71, CML54, and CML85 were induced by UV-C and plant hormones, such as MeJA and salicylic acid (SA) (Konstantin et al., 2021). The CML family members thus have diverse functions in plant development and stress resistance.
As sequencing technology has become increasingly accessible, the whole-genome identification of functional genes is no longer limited to model plants. Cucumber (Cucumis sativus L.) is an economically important vegetable worldwide (Ali, Maryam & Seyed, 2016). As the cucumber genome sequence has been completed (Huang et al., 2009), the CML gene family can be comprehensively analyzed and characterized. Although several literatures have reported the certain CsCMLs, to date, systematical analysis of cucumber CML have not been conducted. The aim of this study was thus to identify the putative CML family members in the cucumber genome and analyze the structure, evolutionary relationships, chromosomal distribution, and promoter elements. Furthermore, the expression patterns of the CsCMLs were also detected under phytohormone and abiotic stress treatments in different organs. The findings of this study provide a foundation for understanding the functional characteristics of CsCMLs at the physiological and molecular levels.
Material and Methods
Genome-scale identification of CML in cucumber
To identify CML family members in cucumber, the cucumber, Arabidopsis, and tomato genome database were downloaded from EnsemblPlant (Yates et al., 2022) (http://jul2018-plants.ensembl.org/index.html), and for Arabidopsis, 50 CML proteins were retrieved from UniProt (https://www.uniprot.org/) (McCormack & Braam, 2003); additionally, 32 rice CML proteins were downloaded from TIGR (http://rice.plantbiology.msu.edu/) (the protein sequences were shown in Data S1). Fifty CML protein sequences of Arabidopsis were used as queries to search against the cucumber peptides for the first blast by TBtools. BlastP was used for the second blast to obtain candidate cucumber CMLs with an e-value lower than 10−5, and the redundant and repetitive sequences were removed manually. NCBI-CDD (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and Interproscan (http://www.ebi.ac.uk/interpro/search/sequence/), and SMART (http://smart.embl-heidelberg.de) were used to predict EF-hand domains, eliminating the protein sequences without EF-hands or with other functional domains. And blasted with AtCaM2(Accession number: NP_850344.1) which acted as typical CaMs (McCormack & Braam, 2003) as well as the amiono acid identity less than 80% to ensured the CMLs. The identified genes were named CsCML1 to CsCML44, and the nucleotide and putative amino acid sequences were used for further analysis.
Sequence analysis
The physicochemical parameters of CsCMLs, including the molecular weight (MW), theoretical point (pI), instability index, grand average of hydropathicity (GRAVY), aliphatic index, and number of amino acids, were predicted using ExPASyProtParam (http://web.expasy.org/protparm/). N-terminal myristoylation and S-palmitoylation were analyzed by GSP-Lipid (http://lipid.biocuckoo.org/webserver.php), and the subcellular location was predicted by Wolf PSORT (http://www.genscript.com/psort/wolf_psort.html). To predict the number of EF-hands, SMART (http://smart.embl-heidelberg.de/smart/set_mode.cgi?NORMAL=1) was used.
Structure analysis and phylogenetic tree construction of CsCMLs
MEME (http://meme-suite.org/index.html) was used to analyze the conserved domains, and the number of motif was set to 6. The exon-intron structure was analyzed by GSDS (http://gsds.gao-lab.org/). The CsCML nucleotide sequences were retrieved from the cucumber genome. Two-kilobase upstream sequences were considered to be promoters, and PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Lescot et al., 2006) was used to analyze the cis-acting elements in the promoter region, and visualized by Simple BioSequence Viewer in TBtools (Chen et al., 2020). The phylogenetic tree was constructed using the neighbor-joining method in MEGA7 (Kumar, Stecher & Tamura, 2016) with 1000 bootstrap replicates. Classification of the CsCML proteins was performed based on the phylogenetic relationships with 50 Arabidopsis AtCMLs, 32 rice OsCMLs.
Chromosomal distribution and syntenic analysis
The CsCML genes were mapped to the cucumber genome database based on physical location information. CsCML gene duplication was analyzed according to Multiple Collinearity ScanX (MCSanX). Synteny was analyzed among Arabidopsis and tomato using TBtools (Sun et al., 2018).
Plant materials and treatments
Cucumber (‘Fengshou 3 hao’) seeds were germinated on two layers of moist gauze in a light incubator (RXZ type, Ningbo, China) at 28 °C for 24 h. The germinated seeds were transplanted into soil (Jiffy substrates, Jiffy International AS, Kristiansand, Netherlands) under a 16 h light/8 h dark cycle and 85–90% humidity. Three-week-old seedlings were used to assay the effects of phytohormone and abiotic stress treatments (low temperature and drought). For phytohormone analysis, the seedlings were sprayed with ABA (abscisic acid, 100 µmol/L) or GA3 (gibberellic acid, 100 µmol/L). For low-temperature stress, the seedlings were placed at 5 °C for 3 h in an incubator; for drought stress, the seedlings were removed from the soil, the soil was removed from the seedlings, and then the seedlings were left at room temperature for 3 h (Munir et al., 2016a; Munir et al., 2016b). Seedlings without any treatment were used as control. All treatments were performed using three biological replicates, and two mature leaves were collected, immediately frozen in liquid nitrogen, and stored at −80 °C for further analysis.
RNA extraction and gene expression pattern analysis
Total RNA was extracted from the leaves, stems, flowers, and peels. Frozen samples were well ground to powder in liquid nitrogen before extracting according to the manufacturer’s instructions of the RNA extraction kit (Sangon Biotech, Shanghai, China). RNA integrity was electrophoresed on 1% (w/v) agarose gel, and then RNA was quantified using a micro spectrophotometer (KAIAO, Guongdong, China). Total RNA (1 µg) was used for cDNA synthesis using the 5X All-In-One RT MasterMix (Abm, Richmond, BC, Canada). Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using EvaGreen 2X qPCR MasterMix-No Dye (Abm, Richmond, BC, Canada) with a fluorescence qPCR instrument (BioRad, Hercules, CA, USA). The specific primers used for qRT-PCR are listed in Table S1. The cucumber Actin gene (accession number: XM_011659465.2) was used as an internal control. Real-time PCR was executed for triplicates. Relative expression was analyzed using the 2−ΔΔCt method (Livak & Schmittgen, 2001). The data expressed represent the average of three biological replicates.
Protein interaction network prediction
The 44 CsCML protein sequences were submitted to the online server STRING (https://cn.string-db.org/cgi/input?sessionId=bvfXM8z80Gzw&input_page_show_search=on, version 11.5).
Statistical analysis
Each experiment contained three independent biological replicates. The gene expression assayed was conducted three technical replicates and the data was processed by Excel and represented as the mean ± standard error (SE).
Results
Genome-wide identification and characterization of CML in cucumber
As a result, 44 putative CML family members in cucumber were obtained and subjected to Pfam, InterProScan, and SMART to verify the EF-hand conserved domain. The gene name, gene ID, number of amino acids, amono acids identity to AtCaM2, MW, pI, number of EF-hand domains, GRAVY, predicted subcellular location, N-terminal myristoylation, S-palmitoylation, instability index, and aliphatic index were listed in Table 1. The number of amino acids in the CsCML proteins ranged from 81 to 251. The CsCMLs shared 24%–77% identity with AtCaM2. The MW of CsCML1–44 varied from 9.187 (CsCML20) to 26.355 kDa (CsCML16), and the pI ranged from 3.78 (CsCML11) to 9.17 (CsCML32). Most CsCML proteins contained two to four EF-hand domains, except CsCML4, which possessed only one.
| Gene name | Gene ID | Number of amino acids | % of amono acids identity to AtCaM2 | Molecular weight (KDa) | pI | Number of EF-hand domain | Predicted subcellular location | GRAVY | N-terminal myristoylation site | S-Palmitoylation site | Instability index | Aliphatic index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CsCML1 | KGN43936 | 184 | 33 | 21.06 | 8.83 | 4 | nucl: 7, cyto: 3, mito: 3 | −0.666 | unstable | 68.32 | ||
| CsCML2 | KGN46235 | 178 | 38 | 20.92 | 5.5 | 4 | chlo: 8, nucl: 3, extr: 2 | −0.560 | unstable | 70.11 | ||
| CsCML3 | KGN47684 | 165 | 28 | 19.03 | 4.22 | 3 | cyto: 9, chlo: 4 | −0.312 | + | unstable | 85.64 | |
| CsCML4 | KGN50423 | 174 | 33 | 20.63 | 4.54 | 1 | cyto: 7, nucl: 5, chlo: 1 | −0.630 | unstable | 75.57 | ||
| CsCML5 | KGN51467 | 182 | 46 | 19.71 | 4.34 | 4 | chlo: 5, mito: 5, nucl: 3 | −0.388 | stable | 75.49 | ||
| CsCML6 | KGN51513 | 145 | 31 | 15.90 | 4.34 | 4 | cyto: 6, chlo: 4, extr: 3 | −0.079 | unstable | 89.38 | ||
| CsCML7 | KGN52027 | 161 | 41 | 17.74 | 4.36 | 4 | nucl: 6, cyto: 5.5, nucl_plas: 4.5, cyto_E.R.: 3.5 | −0.234 | stable | 84.29 | ||
| CsCML8 | KGN52351 | 185 | 48 | 20.12 | 4.33 | 4 | chlo: 7, mito: 5, nucl: 2 | −0.332 | unstable | 77.95 | ||
| CsCML9 | KGN52470 | 180 | 46 | 20.07 | 4.5 | 2 | nucl: 5, chlo: 4, extr: 4 | −0.168 | unstable | 76.83 | ||
| CsCML10 | KGN54524 | 192 | 37 | 21.26 | 4.85 | 3 | mito: 6, nucl: 4, cyto: 3 | −0.566 | unstable | 58.39 | ||
| CsCML11 | KGN54599 | 157 | 35 | 16.82 | 3.78 | 2 | cyto: 9.5, cyto_ER: 5.5, chlo: 1, mito: 1, plas: 1 | 0.138 | stable | 102.55 | ||
| CsCML12 | KGN55185 | 90 | 32 | 10.62 | 9 | 2 | mito: 12, chlo: 1 | −0.768 | stable | 76 | ||
| CsCML13 | KGN55186 | 97 | 29 | 11.00 | 5.29 | 2 | chlo: 9, nucl: 3, cyto: 1 | −0.375 | stable | 83.51 | ||
| CsCML14 | KGN55728 | 163 | 46 | 18.12 | 4.48 | 4 | nucl_plas: 5.5, plas: 5, nucl: 4, cyto: 2, mito: 2 | −0.361 | stable | 83.31 | ||
| CsCML15 | KGN55913 | 147 | 48 | 16.73 | 4.82 | 3 | cyto: 7, plas: 3, nucl: 2, extr: 1 | −0.526 | + | stable | 78.3 | |
| CsCML16 | KGN56046 | 229 | 28 | 26.36 | 4.52 | 3 | cyto: 7, nucl: 3, chlo: 2, extr: 1 | −0.359 | + | + | unstable | 78.3 |
| CsCML17 | KGN56734 | 227 | 25 | 26.05 | 5.51 | 4 | chlo: 8, cyto: 3, extr: 2 | −0.482 | + | unstable | 78.15 | |
| CsCML18 | KGN56931 | 150 | 77 | 17.06 | 4.08 | 4 | cyto: 6, chlo: 3, extr: 2, cysk: 1.5, cysk_plas: 1.5 | −0.381 | stable | 84.47 | ||
| CsCML19 | KGN57816 | 156 | 41 | 17.01 | 4.62 | 4 | chlo: 6, extr: 6, nucl: 1 | −0.397 | + | stable | 78.14 | |
| CsCML20 | KGN57835 | 83 | 38 | 9.19 | 4.38 | 2 | nucl: 11, mito: 3 | −0.496 | stable | 57.83 | ||
| CsCML21 | KGN58193 | 141 | 34 | 15.94 | 4.72 | 4 | nucl: 7, chlo: 4, cyto: 1, mito: 1 | −0.495 | + | unstable | 70.5 | |
| CsCML22 | KGN58678 | 201 | 33 | 22.72 | 4.42 | 3 | nucl: 9, nucl_plas: 6.5, plas: 2, chlo: 1 | −0.518 | unstable | 76.12 | ||
| CsCML23 | KGN59507 | 167 | 29 | 18.27 | 4.77 | 2 | mito: 7, nucl: 4, chlo: 3 | −0.375 | unstable | 65.45 | ||
| CsCML24 | KGN59556 | 210 | 40 | 24.51 | 4.79 | 2 | nucl: 10, cyto: 3 | −0.285 | stable | 86.29 | ||
| CsCML25 | KGN59929 | 180 | 36 | 20.39 | 4.86 | 3 | chlo: 8, mito: 2, vacu: 2, cyto: 1 | −0.398 | unstable | 67.17 | ||
| CsCML26 | KGN60706 | 227 | 45 | 25.47 | 4.59 | 4 | chlo: 11, cyto: 2 | −0.247 | unstable | 73.79 | ||
| CsCML27 | KGN60815 | 146 | 64 | 16.84 | 4.07 | 4 | cyto: 12, cysk: 1 | −0.390 | unstable | 85.48 | ||
| CsCML28 | KGN61657 | 162 | 60 | 18.79 | 4.53 | 4 | nucl: 5, cyto: 4, pero: 2, plas: 1, extr: 1 | −0.686 | + | stable | 72.22 | |
| CsCML29 | KGN62011 | 168 | 46 | 19.23 | 4.72 | 4 | cyto: 6, nucl: 3, chlo: 2, extr: 2 | −0.860 | stable | 63.33 | ||
| CsCML30 | KGN62012 | 190 | 38 | 21.16 | 5.59 | 4 | mito: 7, cyto: 4, chlo: 3 | −0.578 | + | stable | 64.11 | |
| CsCML31 | KGN62512 | 87 | 30 | 9.81 | 5.85 | 2 | cyto: 7, mito: 4, chlo: 3 | −0.316 | stable | 66.21 | ||
| CsCML32 | KGN62513 | 81 | 33 | 9.42 | 9.17 | 2 | cyto: 11, nucl: 2 | −0.536 | + | + | stable | 87.9 |
| CsCML33 | KGN62517 | 137 | 29 | 15.45 | 4.88 | 3 | mito: 5, cyto: 4, chlo: 2, nucl: 2 | −0.499 | unstable | 82.63 | ||
| CsCML34 | KGN62522 | 100 | 31 | 11.68 | 5.16 | 2 | chlo: 6, extr: 3, nucl: 2, cyto: 2 | −0.658 | + | stable | 70.1 | |
| CsCML35 | KGN62524 | 161 | 26 | 18.13 | 5.39 | 3 | extr: 6, nucl: 4, cyto: 2, chlo: 1 | −0.681 | unstable | 76.4 | ||
| CsCML36 | KGN62525 | 87 | 35 | 10.11 | 5.09 | 2 | cyto: 9, nucl: 2, mito: 1, extr: 1 | −0.569 | unstable | 71.84 | ||
| CsCML37 | KGN62526 | 171 | 23 | 19.19 | 9.11 | 3 | nucl: 7.5, nucl_plas: 4.5, cyto: 3, chlo: 1, mito: 1 | −0.651 | unstable | 79.88 | ||
| CsCML38 | KGN62844 | 174 | 38 | 19.33 | 4.58 | 2 | cyto: 9, nucl: 2, chlo: 1, mito: 1 | −0.128 | stable | 96.84 | ||
| CsCML39 | KGN63180 | 140 | 31 | 15.66 | 4.37 | 4 | cyto: 6, chlo: 4, nucl: 3 | −0.325 | stable | 78.57 | ||
| CsCML40 | KGN63491 | 142 | 33 | 15.88 | 4.51 | 3 | cyto: 6, chlo: 3, mito: 3, nucl: 1 | −0.409 | unstable | 87.25 | ||
| CsCML41 | KGN63786 | 160 | 41 | 17.37 | 4.11 | 4 | chlo: 7, nucl_plas: 3.5, plas: 3, nucl: 2, cyto: 1 | 0.059 | stable | 93.37 | ||
| CsCML42 | KGN63870 | 188 | 39 | 20.57 | 4.33 | 4 | nucl: 4, nucl_plas: 4, chlo: 3, mito: 3, cyto: 2, plas: 2 | −0.562 | stable | 76.91 | ||
| CsCML43 | KGN64007 | 204 | 42 | 23.39 | 4.34 | 2 | cyto: 6, ER: 2, cysk: 2, golg: 2, nucl: 1 | −0.070 | unstable | 91.67 | ||
| CsCML44 | KGN64933 | 251 | 24 | 28.31 | 5.26 | 2 | nucl: 9.5, cyto_nucl: 5.5, chlo: 3 | −0.550 | unstable | 58.29 |
Notes:
- Cyto
-
cytosol
- ER
-
endoplasmic reticulum
- Vacu
-
vacuolar; membrane
- Chlo
-
chloroplast
- Nucl
-
nuclear
- Extr
-
extracellular
- Mito
-
mitochondria
- Cysk
-
cytoskeleton
- Plas
-
plasmamembrane
- MW
-
molecular weight
- pI
-
theoretical isoelectric point of proteins
- GRAVY
-
grand average of hydropathicit
+ means presence.
Moreover, most CsCMLs were cytosolic and nuclear proteins, but some were plastid ones, such as chloroplast and mitochondrial proteins. The GRAVY values of most CsCML proteins were negative, indicating that CML proteins in cucumber are hydrophilic. The analysis of myristoylation and palmitoylation indicated that only CsCML15, CsCML16, CsCML28, and CsCML32 had N-terminal myristoylation sites, and eight CsCMLs had S-palmitoylate sites, which implies that these CsCML proteins may have membrane-protein interactions. Half of the CML proteins were unstable.
Structure analysis, conserved motif and cis-acting elements in promotor of CMLs in cucumber
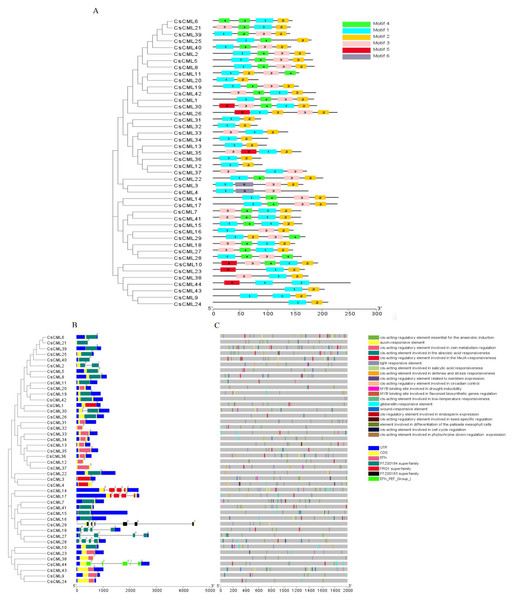
To further investigate the features of the CsCML proteins, conserved motifs were identified using the MEME program, and six distinct motifs were identified (Fig. 1A). Motifs 1 and 2 were present in all 44 CsCML family members, except CsCML44 and CsCML28, which lacked motif 2. Some paralogous proteins contained different motifs, such as CsCML6/21, CsCML11/20, CsCML1/30, CsCML33/34, and CsCML3/4, while CsCML5/8, CsCML14/17, CsCML19/42, CsCML7/41, and CsCML31/32 shared a similar motif. Motif 6 was only found in CsCML3/4. These results might indicate that these paralogous CsCMLs are more diverse than the nonhomologous proteins. Therefore, further analysis should explore the function of the CsCML protein members.
Figure 1: Phylogenetic relationship, gene structure, conserved protein motifs, and putative cis-acting elements in CsCMLs.
Phylogenetic relationship, gene structure, conserved protein motifs, and putative cis-acting elements in CsCMLs. (A) Distribution of the motifs in the CsCML proteins. Motifs 1–6 are displayed with different colored boxes. (B) Exon-intron structure and EF-hand domain. The grey dashed line represents the intron. (C) Putative cis-acting elements.Exon-intron analysis showed that 35 of the total CsCML members had no introns, while 9 had one to six introns (only CsCML29 had six introns; Fig. 1B). Intron phases concerning codons were also investigated. The numbers 0, 1, and 2 indicated that splicing occurred after the first, second, and third nucleotide in the codon, respectively. Most of the first introns were phase 0 introns, suggesting that the splicing phase is highly conserved in cucumber. The CsCML proteins contained five conserved EF-hand (Fig. 1B). Most of them belonged to EF-hand domain and the PTZ00184 superfamily. Only CsCML29 and CsCML44 belonged to PTZ00183 superfamily and EFh_PEF_Group 1, respectively.
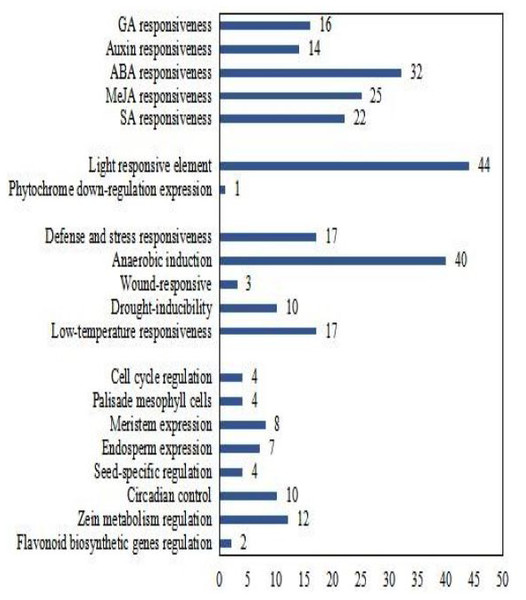
To better understand the transcriptional regulation of CsCMLs, the cis-acting elements were investigated in the upstream 2000-bp sequences for CsCML s using PlantCARE2.0 (Fig. 1C, Data S2). The major elements were related to plant hormone responsiveness, light responsiveness, defense and stress responsiveness, and plant growth and development, typically including circadian control, meristem expression, and seed-specific regulation. Figure 2 shows the number of CsCMLs containing cis-acting elements. All CsCML gene promoter regions contained G-box/GT1-motif, which is related to light responsiveness. Of the CsCML gene promoter regions, 32 contained the ABRE motif, which is the cis-acting element involved in ABA responsiveness. Moreover, the promoter region of the CsCML contained MeJA (CGTCA-motif, TGACG-motif), SA (TCA element), GA (P-box and GARE-motif), and auxin (TGA element) responsiveness elements. Of the CsCML gene promoter regions, 40 contained the anaerobic induction regulatory element. The TC-rich repeats involved in defense and stress responsiveness were present in 17 CsCML genes. Additional stress responsiveness elements, such as the wound-responsive element WUN-motif, drought-inducibility element MBS, and low-temperature response element LTR, were also found. Some motifs involved in plant growth and development, such as circadian control, meristem expression (CAT-box), seed-specific regulation (RY-element), and endosperm expression (GCN4-motif), were observed in a few genes. Besides, some CsCMLs promoter regions contained the element which involved in zein metabolism regulation and flavonoid biosynthetic genes regulation. Overall, the analysis of cis-acting elements suggested that the family members of CsCML genes play different and complex roles in plant growth and development and stress responsiveness.
Figure 2: Number of CsCML genes containing cis-acting elements.
Phylogenetic relationships of CML proteins in cucumber, Arabidopsis and rice
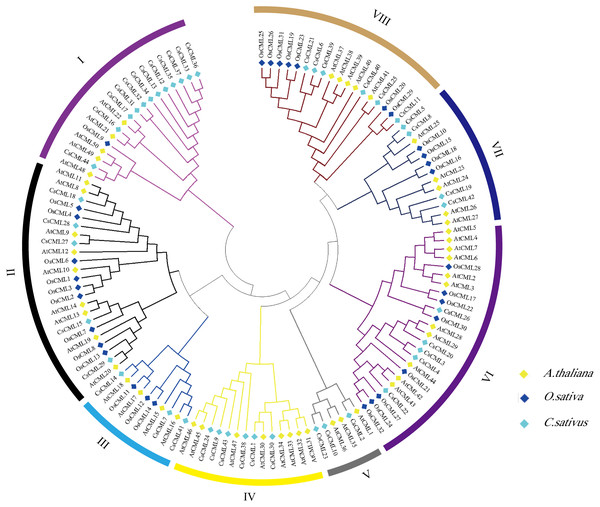
To explore the the systematic evolution of CsCMLs, we constructed the phylogenetic tree with 44 CsCMLs, 50 AtCMLs, and 32 OsCMLs (Fig. 3). The tree showed that the CML were classified into eight subgroups (I–VIII) based on the classification of AtCMLs, and most CMLs were in subgroups II (23 CMLs) and VI (24 CMLs). The smallest subgroup was V which consisted of six CMLs while without any OsCML. Notably, in subgroup I, several CsCMLs monopolized a small branch, which might result in the special function of these CsCMLs.
Figure 3: Phylogenetic tree of CML proteins in cucumis sativus, Arabidopsis thaliana, Oryza sativa based on the neighborhood-joining method.
Different colors represent different groups.Chromosomal location and duplication of CsCML and synteny analysis in cucumber, Arabidopsis thaliana, and tomato
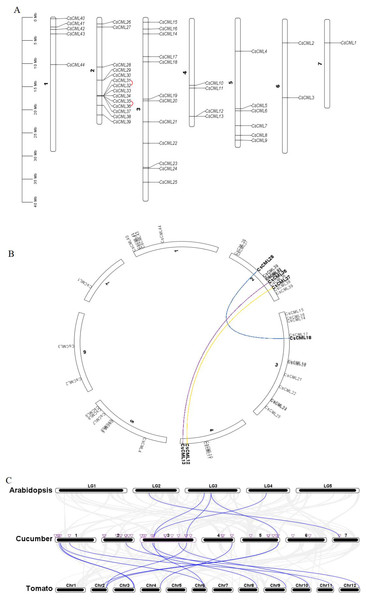
The identified CsCML genes were mapped to the seven chromosomes of the cucumber genome database. The results showed that 44 CsCML family members were diversely spread across all seven chromosomes (Fig. 4A). The highest number of genes (14) was located on chromosome 2, followed by chromosome 3 with 12 genes. Chromosomes 1, 4, and 5 contained five, four, and six CsCML genes, respectively. However, there were only one and two genes located on chromosomes 7 and 6, respectively.
Figure 4: Chromosome distribution, gene duplication, and synteny of CsCMLs.
(A) Chromosome distribution and CsCML gene tandem duplication. The red lines indicate tandemly duplicated gene pairs. (B) Interchromosomal relationships. Different colored lines represent the different segmentally duplicated gene pairs of CsCML. (C) Synteny analysis of CsCML in cucumber, Arabidopsis, and tomato. The blue lines represent the synteny of the CML gene in cucumber, Arabidopsis, and tomato. The purple triangles represent CsCML genes.To determine the gene duplication of the 44 CsCMLs, segmental duplication was analyzed using TBtools (Figs. 4A and 4B). Two tandemly duplicated gene pairs CsCML31/CsCML32 and CsCML35/CsCML36 (Fig. 4A) and three segmentally duplicated gene pairs CsCML28/CsCML18, CsCML35/CsCML13, and CsCML37/CsCML12 (Fig. 4B) were identified in the cucumber genome. To further understand the evolution of CML genes, collinear analysis related to the Arabidopsis, tomato, and cucumber genomes was performed (Fig. 4C Data S4). Collinear gene pairs were present between cucumber and both Arabidopsis and tomato. There were many more CsCMLsyntenic gene pairs between cucumber and potato than between Arabidopsis and cucumber. CsCML9 and CsCML24 were derived from the same gene in Arabidopsis, and CsCML9 and CsCML43 were associated with two syntenic genes in tomato. Moreover, some CsCML genes were not syntenic gene pairs in Arabidopsis or tomato, illustrating that these genes might be unique to cucumber.
Expression patterns of CsCML genes in different tissues and under different conditions
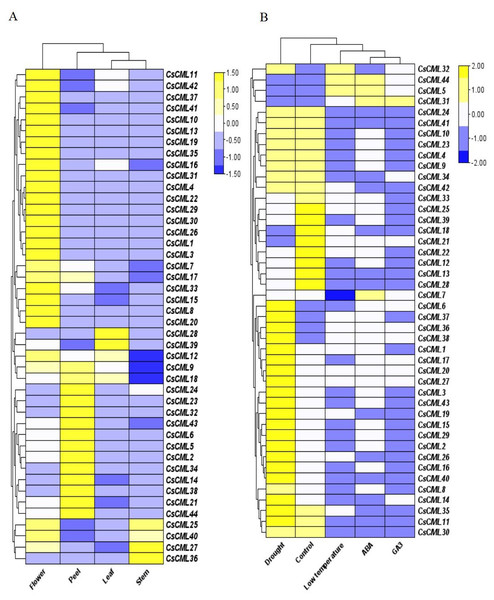
To identify the potential physiological role of CsCMLs, the expression patterns of 44 CsCML genes were investigated by qRT-PCR in the leaves, stems, flowers, peels, and under different stress conditions or different hormone treatments (Data S3), and a heatmap was used to represent the results (Figs. 5A and 5B). The results presented a distinct expression pattern in different organs and conditions.
Figure 5: The relative expression patterns of CsCML genes in different tissues of cucumber under different conditions.
Heatmap showing the relative expression patterns of CsCML genes in different tissues of cucumber under different conditions. The heatmap was constructed based on the relative expression of CsCML genes determined by qRT-PCR in various tissues (A) and under different conditions (B) and was performed using TBtools. The relative expression was log2 transformed. Each value represents the mean of the relative expression of three replicates. Genes highly or weakly expressed are colored yellow to blue, respectively.The CsCML genes showed relatively high expression mainly in the flowers, especially CsCML31, CsML3, and CsML13. Several CsCML genes were highly expressed in the cucumber peel tissue, such as CsCML 23, 32, 38, and 44. CsCML28 and CsCML39 were highly expressed in the leaf, while CsCML27 and CsCML36 were strongly expressed in the stem (Fig. 5A). The gene expression differences demonstrated that gene expression specificity exists in different tissues and the CsCMLs may be more involved in floral organ morphogenesis.
Plants are subject to a variety of environmental conditions during growth and development. In this study, 44 CsCML displayed disparate expression patterns under different conditions. Thirty-one CsCML genes were highly up-regulated under drought, while CsCML5, CsCML21, CsCML31, CsCML44, and CsCML18 were down-regulated. While the expression pattern under ABA, GA3, and low temperature showed the similar trend. In Fig. 5B, one gene (CsCML31) was strongly induced by GA3, which was also induced by ABA. Several CsCML genes remarkably up- regulated under ABA and low temperature, such as CsCML5 and CsCML44. In addition, CsCML32 was simultaneously up-regulated under low temperature. These results indicated that CsCML genes might play a pivotal role in hormone signal transduction and the response to biotic stress.
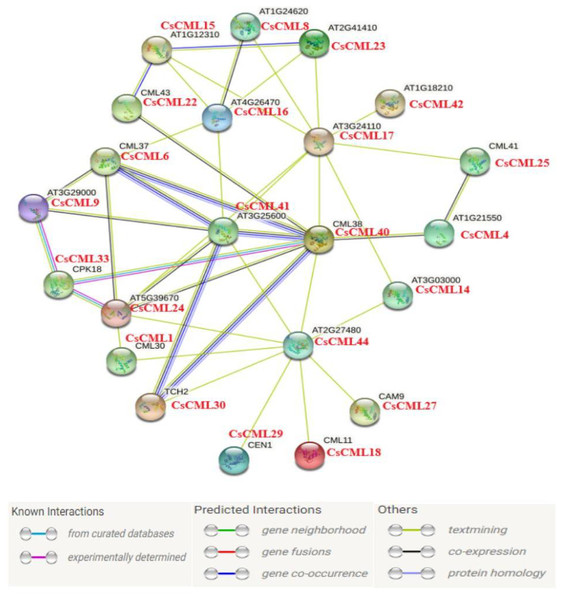
The protein interaction network for CsCMLs
In this study, 44 CsCML proteins were subjected to STRING to predict the protein interaction network in cucumber. Whereas 21 CsCML proteins were involved in the interaction network, and eight CML proteins correlated with more than four other CML proteins, AT1G18210, CML38, and AT2G27480 were associated with nine CsCML proteins. As shown in Fig. 6, CsCML40, CsCML6, CsCML41, and CsCML30 formed the close interaction and represented hypothetic co-occurrence and co-expression. The analysis of the protein interaction network indicated that CsCML regulated the expression of downstream genes by interacting with other proteins and provided a useful resource for further research.
Figure 6: Protein interaction network of CML proteins.
The homologous genes from cucumber and Arabidopsis are in red and black, respectively.Discussion
Identification and characterization of CMLs in cucumber
Forty-four CsCML members were identified in cucumber using 50 Arabidopsis CML proteins as queries, which is smaller than tomato (Munir et al., 2016a; Munir et al., 2016b), wheat (Liu et al., 2022), papaya (Ding et al., 2018), and ginkgo (Zhang et al., 2022). The number of CMLs in cucumber is lower than that in other species, which is likely due to the low number of gene duplications in the cucumber genome (Asano et al., 2012); as exhibited by our results (Figs. 4B and 4C). Our bioinformatic analysis indicated that the CsCML molecular weight ranged from 9.42 KDa to 28.31 KDa, and most CsCML proteins tended to be acidic and hydrophilic (Table 1), which was consistent with alfalfa, whose molecular weight varied from 7.37 KDa to 29.98 KDa, and most of MtCMLs were acid (Sun, Yu & Guo, 2020). The previous study pointed out that CaM shared a high conserve with CML (Snedden et al., 2015). In the Arabidopsis (McCormack & Braam, 2003) and papaya (Ding et al., 2018), the CML shared the 16.1% to 74.5%, 22.4% to 88.1% identity with AtCaM2, respectively. To ensure our prediction accuracy, we adopted the amino acid identity with 16–80% as the selection criterion. At present study, the 44 CsCML shared 24% to 77% amino acid identity with AtCaM2.
Protein post-translational modifications have diverse biological functions related to signal transduction, protein transport, protein regulation, protein localization, and extracellular communication (Mohanta, Kumar & Bae, 2017; Shi & Du, 2020; Xu et al., 2015). Myristoylation and palmitoylation are two major posttranslational modifications. Studies have revealed that proteins possessing myristoylation motifs tend to be in the plasma membrane (Mehlmer et al., 2010). Our study indicated that a few CML genes (CsCML3, 15, 16, 17, 19, 21, 28, 30, 32, and 34) contained myristoylation and/or palmitoylation sites, which may cause CML conformation changes and promote protein–membrane and protein–protein interactions. CMLs were reported localized in various parts; in this study, the CsCML proteins were mainly localized in the nucleus and cytoplasmic, which was in consonance with lotus (Gao et al., 2022). CMLs mediate Ca2+ signal transduction by binding with Ca2+ to form CML/Ca2+ complex compounds. CML proteins also contain an EF-hand conserved domain, which can bind to Ca2+. Therefore, the number of EF-hands may affect the role of CMLs. In Arabidopsis, there are typically two to six EF-hands (McCormack & Braam, 2003), while cucumber possesses one to four. This may be the result of differences in the sequence and structure of the CMLs. Previous research found that most CML genes are intron-less, while some contain no more than nine introns (Ding et al., 2018; Mohanta, Kumar & Bae, 2017; Sun, Yu & Guo, 2020). Our results showed that most of the cucumber CMLs are intron-less, while others have fewer than six introns (Fig. 1). These introns may have evolved with CaMs, thus possibly exerting positive pressure for CML evolution (Mohanta, Kumar & Bae, 2017). These results are similar to those reported for Medicago truncatula Gaertn. (Sun, Yu & Guo, 2020). Besides, the variance of the conserved motif in the CsCML (Fig. 1A) will contribute to the function divergence (Li et al., 2019a; Li et al., 2019b).
The CsCMLs were divided into eight subgroups (Fig. 3), which is similar to that in apple (Li et al., 2019a; Li et al., 2019b) and Medicago truncatula (Sun, Yu & Guo, 2020), while four subgroups are present in wild tomato (Shi & Du, 2020) and seven are present in Chinese cabbage (Nie, Zhang & Zhang, 2017). These results demonstrated that CMLs are highly conserved among species and perform the similar function. Gene location analysis showed that 44 CsCML genes were not uniformly distributed on the seven chromosomes (Fig. 4A). Of the CsCML genes, 14 and 12 were on chromosomes 2 and 3, respectively. Gene duplication is considered to play an important role in gene expansion (Kong et al., 2007). In our study, three segmentally duplicated gene pairs and two tandemly duplicated gene pairs were found in the cucumber CsCMLs (Figs. 4A and 4B). And many CsCML genes showed an extensive synteny relationship with Arabidopsis and tomato (Fig. 4C). These results were subjected to less duplication in cucumber during evolution, which explains why the CML genes in cucumber are less numerous than in Arabidopsis. Moreover, this may be due to the long-term adaptation of species to different growth environments (Guo, 2013).
Expression patterns of CsCML genes
It has been widely reported that CMLs play an important role in plant development and stress responses (McCormack, Tsai & Braam, 2005; McCormack & Braam, 2003; Reddy et al., 2011). In our study, the spatio-temporal expression pattern demonstrated a distinct tissue specificity (Fig. 5A). CsCML27 and CsCML36 were only strongly expressed in the stem. Likewise, CsCML28 and CsCML39 were only strongly expressed in leaves. As paralogous genes may perform similar functions, the evolutionary relationships and potential functions of the CMLs were explored in a phylogenetic tree of cucumber and Arabidopsis (Fig. 3). Microarray data showed that AtCML21 had pollen-specific expression (Becker et al., 2003). In addition, AtCML15 was depicted as involving floral development (Ogunrinde et al., 2017). Our results indicated that the homologous genes CsCML41 and CsCML7 were highly expressed in the flowers (Fig. 5A). Moreover, CsCML13 and CsCML31 were strongly upregulated in the flowers, which are in the same subgroup with AtCML21 (Fig. 3), indicating that they may participate in flowering and fruit growth. This result corroborates findings in Arabidopsis (McCormack, Tsai & Braam, 2005).
CMLs play a vital role in response to biotic and abiotic stress. In Arabidopsis, CML8 (Zhu et al., 2017) and CML9 (Leba et al., 2012) was strongly induce by Pseudomonas syringae. Besides, AtCML9 involved in salt tolerancce through its effects on the ABA-mediated pathways (Magnan et al., 2008). One study also reported that AtCML37 was positively regulated by drought, while AtCML42 exhibited opposite function (Heyer et al., 2022). Additionally, the overexpression of ShCML44 enhanced tolerance during cold and drought (Munir et al., 2016a; Munir et al., 2016b).
In the present study, CsCML5, 32, and 44 were strongly induced under low temperature stress, and high numbers of CsCMLs such as CsCML1, 6, 17, and 20 were highly expressed under drought(Fig. 5B). As cucumber is a cold-sensitive vegetable, these genes might be good candidates for stress tolerance. It has been reported that CML genes are induced inordinately by different hormones (McCormack, Tsai & Braam, 2005; Midhat et al., 2018). Some CsCML genes exhibited similar expression patterns under ABA and GA3 treatment; for example, CsCML31 was up-regulated under both ABA and GA3, whereas, CsCML41 was down-regulated (Fig. 5B), suggesting that these genes may be commonly involved in response to ABA and GA. Moreover, Fig. 6 showed the protein interaction network in Arabidopsis and cucumber, deduced that CsCML6, CsCML30, CsCML40, and CsCML41 were co-expressed to participate in cucumber response to drought. The CsCML genes in this study were distinctively expressed in different tissues and induced and/or suppressed by different hormones and stressors. These results were consistent with previous findings that the CML genes are involved in hormone and other stress responses because they contains cis-acting elements.
Conclusions
Forty-four CsCML genes were identified from the cucumber genome. Gene structure and sequence analysis showed that these CsCML genes containing one to four highly-conserved EF-hand functional domains were unevenly located on seven chromosomes. Cis-acting element analysis indicated that these genes might respond to multiple hormones and stresses. Spatiotemporal expression analysis results confirmed that CsCML genes play a vital role during plant development and stress resistance. Altogether, this study provides a good foundation for further studies of the functions of CsCML genes in cucumber.