Cerebral neurovascular alterations in stable chronic obstructive pulmonary disease: a preliminary fMRI study
- Published
- Accepted
- Received
- Academic Editor
- Feng Liu
- Subject Areas
- Neuroscience, Cognitive Disorders, Neurology, Psychiatry and Psychology, Radiology and Medical Imaging
- Keywords
- Cerebral blood flow, Degree centrality, Functional magnetic resonance, Cognitive impairment, Chronic obstructive pulmonary disease
- Copyright
- © 2022 Peng et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Cerebral neurovascular alterations in stable chronic obstructive pulmonary disease: a preliminary fMRI study. PeerJ 10:e14249 https://doi.org/10.7717/peerj.14249
Abstract
Purpose
Cognitive impairment (CI) is very common in patients with chronic obstructive pulmonary disease (COPD). Cerebral structural and functional abnormalities have been reported in cognitively impaired patients with COPD, and the neurovascular coupling changes are rarely investigated. To address this issue, arterial spin labeling (ASL) and resting-state blood oxygenation level dependent (BOLD) fMRI techniques were used to determine whether any neurovascular changes in COPD patients.
Methods
Forty-five stable COPD patients and forty gender- and age-matched healthy controls were recruited. Furthermore, resting-state BOLD fMRI and ASL were acquired to calculate degree centrality (DC) and cerebral blood flow (CBF) respectively. The CBF-DC coupling and CBF/DC ratio were compared between the two groups.
Results
COPD patients showed abnormal CBF, DC and CBF/DC ratio in several regions. Moreover, lower CBF/DC ratio in the left lingual gyrus negatively correlated with naming scores, lower CBF/DC ratio in medial frontal cortex/temporal gyrus positively correlated with the Montreal Cognitive Assessment (MoCA), visuospatial/executive and delayed recall scores.
Conclusion
These findings may provide new potential insights into neuropathogenesis of cognition decline in stable COPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is the most common chronic lung disease in the general population, and is manifested with chronic irreversible airway limitation. The prevalence rate of COPD is about 13.6% in China (Fang et al., 2018). With the increase of age more significant than 40, the mortality rate of chronic respiratory diseases showed the most apparent increasing trend. Notably, cognitive impairment is widespread in patients with COPD (Charbek et al., 2019). The prevalence of cognitive impairment is reported to be about 16%–85% in patients with COPD (Simargi et al., 2022; Yohannes et al., 2017). Furthermore, COPD increases the risk of cognitive impairment by about 2.5 times (Dodd, Getov & Jones, 2010). However, the pathophysiological mechanism underlying cognitive impairment in COPD remains elusive.
fMRI techniques have been widely used to investigate the cerebral dysfunctions in COPD patients and provided evidences of the cerebral structural and functional abnormalities and their potential neural associations with cognitive impairment. These changes included gray matter atrophy, abnormal brain activity and disrupted white matter integrity of brain regions relevant for the cognition, including the frontal cortex, cingulate cortex, anterior insula, and hippocampus et al. (Chen et al., 2016; Li et al., 2020; Wang et al., 2017; Yu et al., 2016; Zhang et al., 2016), which can partly explain the pathophysiological and psychological changes in COPD patients.
CBF is defined as the delivery rate of arterial blood to the capillary bed in the cerebral tissue. Without complex confounding factors, resting state CBF is relatively easy to conduct, and is closely coupled with brain metabolism, including glucose utilization, oxygen consumption, and aerobic glycolysis (Vaishnavi et al., 2010). The arterial spin labeling (ASL), with good reliability and reproducibility (Sigurdsson et al., 2015), has been validated against other perfusion methods, and has been utilized to measure CBF in many studies (Lan et al., 2019; Thamm et al., 2019). Previous studies have shown that cerebral blood flow (CBF) coupled with cerebral metabolism and oxygen consumption (Fan et al., 2022; Jezzard, Chappell & Okell, 2018; Liu & Brown, 2007). Furthermore, studies from Varkuti et al. (2011) and Hagmann et al. (2008) also identified positive correlation between cerebral structural hubs and CBF. In addition, degree centrality (DC) is generally measured by extracting the time series of one voxel, correlating it with the time series of all the other voxels in the brain, and then calculating the summation of the resulting correlation coefficients. The DC could represent functional relationships between a voxel or region and the rest within the entire cerebral connectivity matrix (connectome) at the voxel level without requiring a priori selection. Neurovascular coupling refers to the correlation between CBF and neuronal activity and metabolism in the brain region, is a marker of cerebral function (Vestergaard et al., 2016). A study has indicated a correlation between the CBF measured with ASL and functional connectivity measured with BOLD in several networks (Liang et al., 2013). The CBF-DC correlation represents the consistency of spatial distribution between blood supply and functional hubs. The CBF/DC ratio represents the cerebral blood supply per unit of connectivity hub, reflecting the neurovascular coupling. The two indices could be used to identify changes in the neurovascular coupling in COPD that cannot be detected by investigating the CBF and DC separately. As far as we know, very few studies have directly examined the relationship between functional network hubs and the cerebral blood supply in COPD patients. Therefore, it is unknown that whether resting state intrinsic functional network connectivity is closely related to CBF and whether cerebral neurovascular changes involve the potential mechanisms underlying cognitive impairment of COPD patients.
In our study, resting-state BOLD fMRI and ASL data of COPD patients and healthy subjects were acquired during resting state. resting-state BOLD fMRI data were used to identify functional hubs in the brain, and ASL data were exploited to measure CBF. The CBF-DC coupling and CBF/DC ratio were compared between the two groups, and the potential correlations between functional deficits and clinical characteristics were also investigated. In the present study, we hypothesized that abnormal cerebral neurovascular alterations may contribute to cognitive deficits in COPD patients.
Materials & Methods
Participants
A total of eighty-five right-handed subjects were enrolled into this study, including forty-five patients with stable COPD and forty age- and gender-matched healthy controls. The patients with stable COPD were recruited from the Respiratory departments from March 2018 to April 2019.
Inclusion criteria for COPD group were as follows: (1) diagnosed according to pulmonary function test (PFT) (Singh et al., 2019); (2) PFT findings indicating: FEV1/FVC <0.70, 30% ≤ FEV1 ≤ 80% predicted; (3) stable stage is defined as the absence of exacerbation (defined as hospital admission or prescription of antibiotics/systemic corticosteroids by a general practitioner) in the past six weeks (as same as the study of our prior study Wang et al., 2020). Exclusion criteria for all subjects were: (1) home O2 therapy; (2) other pulmonary diseases, neurological disease except cognition decline, history of other cardiovascular and metabolic diseases, psychiatric disorder known to affect cognition; (3) alcohol/substance abuse or dependence; (4) medicine, drinking or smoking within 24 h before MRI examination; (5) body mass index >30 kg/m2; (6) any contraindication to fMRI examination. The healthy controls with comparable age, sex and education level were asymptomatic, free from a known history of cerebrovascular accident, heart failure psychiatric or neurologic disorders, obstructive sleep apnea, any metabolic disease, or other diseases known to affect cognition, and they were taking no medications.
Each subject underwent a physical examination and pulmonary function test. The Medical Research Council (MRC) Dyspnea Scale was used to quantify dyspnea’s degree (Fletcher, 1960). Montreal Cognitive Assessment (MoCA) was used for cognitive screening. COPD severity was also assessed by using the GOLD stage. The protocol was approved by the ethics committee of the Changzheng Hospital (2018SL028). Our work was carried out following the Declaration of Helsinki.
Data acquisition
MRI examinations were performed at a 3T MR scanner (General Electrics) with an 8-channel head coil during resting state. 3D pseudo-continuous ASL images were acquired with the following parameters: time repetition/time echo = 4632/10.5 ms, slice thickness = four mm, the field of view = 240 × 240 mm2, NEX = 3.00, post-labeling delay=1525 ms. resting-state BOLD fMRI images were also acquired (time repetition /time echo = 2000/35 ms, flip angle = 90°, resolution = 64 × 64, field of view = 240 × 240 mm2, slice thickness = four mm, spacing = 0, slices = 38, 200 volumes). A high-resolution structural T1-weighted scan was performed with the following parameters: time repetition /time echo = 8.2/3.2 ms, flip angle = 12°, resolution = 256 × 256, slice thickness = one mm. Routine MRI examination images of all participants were acquired and checked by two experienced neuroradiologists to rule out anatomic abnormalities in the brain. For the resting state, all participants were instructed to relax and keep their eyes closed without thinking of anything in particular or falling asleep during scanning.
Data processing of CBF and DC
The processing steps were performed by using the MATLAB2016 (MathWorks, Natick, MA, USA) platform. CBF images underwent the following preprocessing steps, including normalization into the standard Montreal Neurological Institute (MNI) space, resampling into a three mm voxel size, smoothing with a 6 mm3 full-width-at-half maximum Gaussian kernel (FWHM) and removing signal of white matter and cerebrospinal fluid.
The resting-state BOLD fMRI images processing steps included format conversion, removing first ten time points, slice timing, realign estimation, spatially coregistration, normalization into the standard MNI space, resampling into a three mm voxel size, band filtering (0.01−0.08 Hz) and linear detrend. Several spurious variances (white matter, cerebrospinal fluid, head motion and global mean signals) were removed via linear regression. Data were discarded if the translation exceeded two mm or if rotation exceeded 2°. Using the Resting-State fMRI Data Analysis Toolkit (REST version 1.8) software (Song et al., 2011), the voxel-wise DC was computed with r (correlation threshold) set at 0.25. Only positive weighted Pearson’s correlation coefficients were considered in this study.
Voxel-Wise Comparisons in CBF and DC
By using REST software, the DC maps were z-transformed and smoothed (6 × 6 × 6 mm3 FWHM) to compare two groups. The intergroup comparisons of CBF and DC were performed voxel-wise while controlling for age, gender and education level (q < 0.05, FDR corrected).
Region of interest (ROI) analysis
CBF maps were normalized into z-scores within the whole grey mask to improve normality. The regions obtained from voxel-wise comparisons of CBF and DC were merged and defined as regions of interest (ROIs). The CBF and DC values of each voxel in the merged ROIs were extracted for each participant. Furthermore, correlational analyses between CBF and DC were performed for each ROI of each participant. There is a CBF-DC coefficient value for each ROI of each participant, reflecting the consistency of spatial distribution between CBF and DC at the ROI level. Then, a two-sample t test was used to compare the difference in CBF-DC correlation coefficients between the two groups.
To evaluate the amount of blood supply per unit of functional hubs, the CBF/DC ratio (both CBF and DC were original values without z-transformation) of each voxel were calculated and transformed into a z-score map to improve the normality. Based on the merged ROI, differences in CBF/DC ratio betw‘een the two groups were analyzed. For each participant, the mean CBF/DC ratio of each ROI was extracted by using REST software.
Statistical analysis
Two-sample t tests were performed to examine the differences in age, education and clinical symptoms scores between the two groups using SPSS 21.0 (SPSS Inc., Chicago, IL, USA), while Chi square test to analyze differences in gender. The statistical significance of group differences was set at p < 0.05. Comparisons of fMRI index (CBF, DC, CBF-DC coupling and CBF/DC ratio) were performed as methods abovementioned. The brain networks were visualized with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/) (Xia et al., 2013). Furthermore, A partial correlation analysis was performed to identify the association between CBF, DC, CBF/DC ratio and clinical characteristics (lung test index, MRC dyspnea severity, MoCA), with age, gender and education level as nuisance covariates; results with q < 0.05, FDR-corrected were considered statistically significant.
Results
Clinical characteristics differences
No significant differences were found in age (t = − 0.735, df = 83, p = 0.465), gender (χ2 = 0.262, df = 1, p = 0.609) and BMI (t = 1.989, df = 83, p = 0.667) between the COPD and control groups. Compared to HC, COPD showed lower lung function in FEV1% predicted (t = − 14.142, df = 83, p < 0.001) and FEV1/FVC % predicted (t = − 13.972, df = 83, p < 0.001). COPD patients showed lower MoCA (t = − 13.905, df = 83, p < 0.001) and subdomain scores (p < 0.05). Details were shown in Table 1.
| Group; mean (SD) | |||||
|---|---|---|---|---|---|
| Characteristics | COPD | Control | df | p value | t/χ2 |
| Number of subjects | 45 | 40 | – | – | |
| Age (years) | 67.93 (7.06) | 69.15 (6.15) | 83 | 0.465 | −0.735* |
| Age rang (years) | 52∼79 | 53∼78 | – | – | |
| Education (years) | 11.28 (3.39) | 11.78 (3.35) | 83 | 0.581 | −0.555 |
| Gender (Male/Female) | 25/20 | 20/20 | 1 | 0.609 | 0.262* |
| Body Mass Index (kg/m2) | 23.3 (3.1) | 23 (3.3) | 83 | 0.667 | 1.989 |
| Duration of disease (years) | 7.2 (5.12) | N/A | – | – | |
| Number of Smokers | 23 | 19 | 1 | 0.74 | 0.11* |
| FEV1/FVC (%predicted) | 58.63 (11.47) | 85.18 (3.81) | 83 | <0.001 | −13.972 |
| FEV1 (%predicted) | 54.78 (16.40) | 96.68 (9.59) | 83 | <0.001 | −14.142 |
| MoCA | 18.27 (4.68) | 28.78 (1.03) | 83 | <0.001 | −13.905 |
| Visuospatial/Executive | 2.2 (1.32) | 4.68 (0.57) | 83 | <0.001 | −10.939 |
| Naming | 2.18 (0.68) | 2.83 (0.55) | 83 | 0.011 | −4.77 |
| Attention | 4.31 (1.47) | 5.75 (0.49) | 83 | <0.001 | −5.883 |
| Language | 2.2 (0.94) | 2.85 (0.36) | 83 | <0.001 | −4.094 |
| Abstraction | 1.49 (0.63) | 1.93 (0.27) | 83 | <0.001 | −4.086 |
| Delayed Recall | 1.51 (1.25) | 4.93 (0.27) | 83 | <0.001 | −16.865 |
| Orientation | 4.38 (1.89) | 5.88 (0.34) | 83 | <0.001 | −4.948 |
| Dyspnea (mMRC) | 2.31 (0.51) | N/A | |||
Notes:
- COPD
-
chronic obstructive pulmonary disease
- FEV1
-
forced expiratory volume in 1 second (#8175)
- FEV1/FVC
-
forced expiratory volume in 1 s/ forced vital capacity
- mMRC
-
modified Medical Research Council scale for dyspnea
- MoCA
-
Montreal Cognitive Assessment
- N/A
-
Not applicable
- SD
-
standard deviation
Differences in CBF and DC
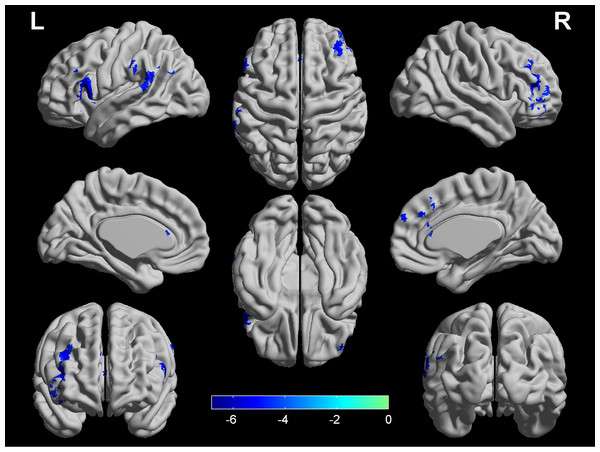
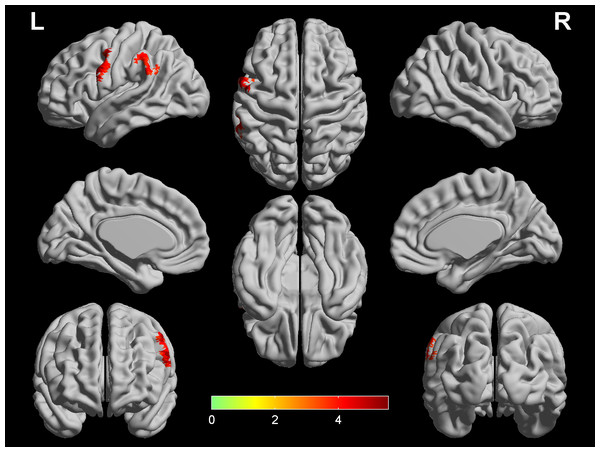
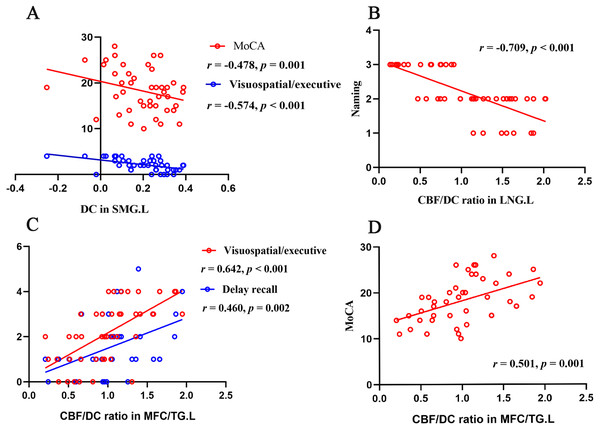
As depicted in Fig. 1, the COPD group showed decreased CBF in the left dorsolateral prefrontal cortex (DLPFC), right DLPFC, left supramarginal gyrus (SMG) and bilateral anterior cingulum gyrus (ACG). Further correlational analyses showed no significant correlations between any regions with CBF and any clinical variables in the COPD group (p > 0.05). As depicted in Fig. 2, the COPD group showed greater DC in the left precentral gyrus (PreCG) and left SMG. The mean DC values in the left SMG showed significantly negative correlation with MoCA score (r = − 0.478, p = 0.001) and visuospatial/executive score (r = − 0.574, p < 0.001), with age, gender, education level as covariates, and FDR corrected for multiple comparisons. Details were shown in Table 2.
Figure 1: Significant differences in CBF between COPD patients and HC.
Four regions (left DLPFC, right DLPFC, left SMG and bilateral ACG) show lower CBF in COPD patients (q < 0.05, FDR corrected) while controlling for the age, gender and education level. The color bar represents the t value from two-sample t-test. ACG, anterior cingulum gyrus; CBF, cerebral blood flow; COPD, chronic obstructive pulmonary disease; LPFC, dorsolateral prefrontal cortex; HC, healthy controls; SMG, supramarginal gyrus; L, left; R, right.Figure 2: Significant differences in DC between COPD patients and HC.
Two regions (left PreCG and left SMG) show higher DC in COPD patients (q < 0.05, FDR corrected) while controlling for the age, gender and education level. The color bar represents the t value from two-sample t-test. CBF, cerebral blood flow; COPD, chronic obstructive pulmonary disease; HC, healthy controls; PreCG, precentral gyrus; SMG, supramarginal gyrus; L, left; R, right.CBF-DC coupling analysis
The merged maps with significantly intergroup clusters in CBF and DC comparisons covered four brain clusters, including the left PreCG/DLPFC, right DLPFC, left SMG and bilateral ACG. The CBF has significantly correlated DC in the four merged regions in all participants. There were no significant intergroup differences of the correlation coefficients in the left PreCG/PLPFC (t = 1.59, df = 83, p = 0.12), right DLPFC (t = − 0.72, df = 83, p = 0.48), left SMG (t = − 1.04, df = 83, p = 0.30) and bilateral ACG (t = − 1.84, df = 83, p = 0.07).
Comparison of CBF-DC ratio
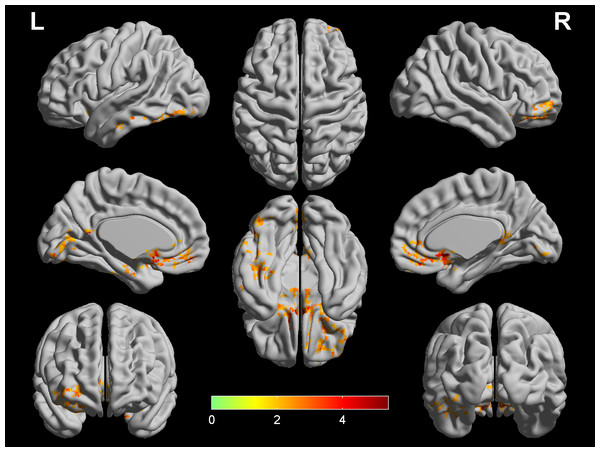
At the voxel level, the COPD group showed increased CBF/DC ratio in the bilateral medial frontal cortex (ventromedial prefrontal cortex, orbitofrontal cortex and rectus gyrus), bilateral caudate nucleus, left middle temporal gyrus (parahippocampus gyrus and fusiform gyrus), and left lingual gyrus (LNG), seen in Fig. 3. Furthermore, in the COPD group, the correlational analyses revealed that the CBF/DC ratio in the left LNG showed significantly negative correlations with the naming score (r = − 0.709, p < 0.001), and the CBF/DC ratio in the medial frontal cortex/temporal gyrus showed significantly positive correlations with MoCA score (r = 0.501, p = 0.001), visuospatial/executive score (r = 0.642, p < 0.001) and delayed recall score (r = 0.460, p = 0.002), with age, gender, education level as covariates, and FDR corrected for multiple comparisons, seen in Fig. 4. Furthermore, the mean CBF and DC values in the above regions were extracted for intergroup comparison. In the left LNG, COPD patients showed lower CBF (t = − 2.174, df = 8, p = 0.035) and lower DC (t = − 2.610, df = 83, p = 0.012) than the healthy controls; lower CBF (t = − 3.744, df = 83, p = 0.001) and lower DC (t = − 3.818, df = 83, p < 0.001) were also observed in the medial frontal cortex/temporal gyrus in COPD group.
In ROI analyses, compared to healthy controls, COPD patients exhibited decreased CBF/FCS ratio in the left PreCG/DLPFC (t = − 2.711, df = 83, p = 0.01) and left SMG (t = − 2.452, df = 83, p = 0.018). However, no significant correlation between the CBF/DC ratio of any ROI and any clinical characteristics was found in the COPD group. Further validation of CBF and DC changes showed that COPD patients exhibited lower CBF and higher DC in the two regions (p < 0.001).
Discussion
As far as we know, this is the first study to use both resting-state CBF and DC approaches to investigate cerebral neurovascular coupling changes related to the potential neurological mechanisms underlying cognitive impairment in stable COPD patients. Compared with healthy controls, COPD patients showed decreased CBF in the bilateral DLPFC, ACG and left SMG, and increased DC in the left PreCG and left SMG. More importantly, COPD showed increased CBF/DC ratio in the bilateral medial frontal cortex, bilateral caudate nucleus, left temporal gyrus and left LNG, and CBF/DC ratio in several brain regions significantly correlated with MoCA, visuospatial/executive and delay recall scores. These findings may provide neuroimaging evidence and improve our understanding of neural mechanisms underlying cognitive impairment in COPD patients from the perspective of neurovascular coupling.
| Brain region | MNI Coordinates | Number of Voxels | Peak t value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| CBF | |||||
| DLPFC.L | −57 | 21 | 12 | 167 | −6.839 |
| DLPFC.R | 39 | 39 | 12 | 176 | −5.923 |
| SMG.L | -66 | -33 | 24 | 113 | −5.827 |
| ACG.B | 3 | 36 | 30 | 90 | −5.161 |
| DC | |||||
| PreCG.L | −60 | 9 | 33 | 130 | 5.81 |
| SMG.L | −63 | −51 | 39 | 105 | 4.508 |
Notes:
- ACG
-
anterior cingulum gyrus
- CBF
-
cerebral blood supply
- COPD
-
chronic obstructive pulmonary disease
- DC
-
degree centrality
- DLPFC
-
dorsolateral prefrontal cortex
- HC
-
healthy control
- PreCG
-
precentral gyrus
- SMG
-
supramarginal gyrus
- L
-
left
- R
-
right
- B
-
bilateral
Figure 3: Comparison of CBF/DC ratio between COPD patients and HC.
Two regions (bilateral media frontal cortex/temporal gyrus and left supramarginal gyrus) show higher CBF/DC ratio in COPD patients (q < 0.05, FDR corrected) while controlling for the age, gender and education level. The color bar represents the t value from two-sample t-test. CBF, cerebral blood flow; COPD, chronic obstructive pulmonary disease; HC, healthy controls.Figure 4: Correlation analyses of functional parameters and clinical variates.
(A) DC in left SMG negatively correlated with MoCA score ( r = − 0.478, p = 0.001) and visuospatial/executive score ( r = − 0.574, p < 0.001); (B) CBF/DC ratio in left LNG negatively correlated with naming score (r = − 0.709, p < 0.001); (C) CBF/DC ratio in MFC/TG.L positively correlated with visuospatial/executive score (r = 0.642, p < 0.001) and delay recall score (r = 0.460, p = 0.002); (D) CBF/DC ratio in MFC/TG.L positively correlated with MoCA (r = 0.501, p = 0.001). CBF, cerebral blood flow; COPD, chronic obstructive pulmonary disease; HC, healthy controls; LNG, lingual gyrus; MFC, medial frontal gyrus; SMG, supramarginal gyrus; TG, temporal gyrus; L, left; R, right.Voxel-wise analyses revealed that COPD patients showed higher CBF/DC ratio in several regions. Subsequent ROI-based analyses revealed lower CBF and lower DC values of these regions in the COPD patients. But, by using voxel-wise analyses, there was no overlap between the merged regions generated in intergroup comparisons of CBF, DC and regions with intergroup different CBF/DC ratio; we speculated that CBF/DC ratio could enlarge the differences between the COPD and healthy group. Moreover, COPD patients showed a significantly decreased CBF/DC ratio in some merged ROIs. We demonstrated that a combination of CBF, DC and CBF/DC ratio based on voxel- and ROI-wise analyses might be a comprehensive and reliable method to explore the underlying mechanism of COPD patients. In the present study, COPD patients showed increased CBF/DC ratio in the left LNG, which was driven by disproportionally attenuated CBF and DC. The LNG involves in encoding of complex images (Machielsen et al., 2015), identification and recognition of words (Mechelli et al., 2000). Supporting this, the LNG is functionally associated with decreased naming performance (Deverdun et al., 2019) and visual processing (Chee et al., 2009) and visual hallucination (Goldman et al., 2014). In the COPD group, the negative correlation between the higher CBF/DC ratio of the LNG and the naming score indicated that the attenuation degree of DC is greater than that of CBF in this region, resulting in decompensated increase of CBF per unit of functional connectivity in the left LNG and poor naming function. In addition, COPD patients also showed higher CBF/DC ratio in the bilateral medial frontal cortex, bilateral caudate nucleus, and left middle temporal gyrus. Several COPD studies have provided evidence of reduced thickness and volume in these regions (Chen et al., 2016; Zhang et al., 2013). Increased CBF/DC ratio of these regions may play a compensatory role for the reduced grey matter volume. In the present study, the increased CBF/DC ratio in bilateral medial frontal cortex and left middle temporal gyrus is driven by decreased CBF and DC. Consist with our study, hypoperfusion in the frontal cortex and cognitive abnormalities have been found in COPD patients (Hulya & Seniha, 2006). Based on the finding of positive correlation between higher CBF/DC ratio in this region and MoCA, visuospatial/executive and delayed recall. A possible explanation for these increased CBF-DC ratio could be the compensation mechanism that acts to counterbalance regional deficits in cognitive function. Compensatory mechanisms accompany the cognitive impairments progress in COPD patient. In addition, synaptic loss is associated with cognitive decline and compensatory mechanisms, which can alleviate cognitive impairment caused by synaptic loss by maintaining the activity level of neural circuits. Here, the finding of increased CBF-DC ratio in COPD suggests that COPD patients could use additional blood supply per function hub unit for cognitive function, presumably suggesting the plasticity of human brain compensating for cognition decline.
In the ROI analyses, COPD patients exhibited decreased CBF/DC ratio in the left PreCG/DLPFC and left SMG which involved in somatic motor function and spatial working memory. Decreased grey matter density and neural activation in the left PreCG have been reported in COPD patients (Zhang et al., 2012; Zhang et al., 2013; Zhang et al., 2016). The DLPFC is a crucial part of dorsal visual processing stream regions involved in visual reproduction impairment in COPD. A surface-based morphometry study suggested that the thinner DLPFC is a predictive factor of poorer visual reproduction performance (Chen et al., 2016). They also found reduced cortical thickness and surface in the PreCG and SMG in COPD. In our study, these regions showed lower CBF and higher DC, suggesting that the decreased CBF/DC ratios were driven by the CBF decrease and DC increase, which might relate to reduced cortical thickness and surface, rendering to cognitive impairment in COPD.
In voxel-wise analyses, several brain regions showed significant intergroup differences in CBF and DC between COPD patients and healthy controls. The brain constitutes only about 2% of the body weight, but easily receives up to 15–20% of the total cardiac output as CBF. CBF changes may be ascribed to the following reasons. The hypothesis of neurovascular coupling suggested that neural activity changes govern CBF through complex coordinated mechanisms involving neurons, glial cells, and vascular components (Venkat, Chopp & Chen, 2016). Moreover, the neural stimuli may be involved in controlling the diameter of the cerebral vessel and brain blood supply, resulting in CBF changes. Finally, chemical mediators, such as neuroinflammation factors, adenosine, nitric oxide (Lourenço et al., 2014), hydrogen, potassium, calcium and lactate (Dienel, 2012), may trigger hemodynamic responses resulting in vasodilation/vasoconstriction and CBF changes. In the present study, COPD patients showed decreased CBF in bilateral DLPFC, ACG and left SMG. Meanwhile, higher DC values were found in the left PreCG and left SMG in COPD. The higher DC represents more correlations between the given voxel and the rest voxels, indicating that neurons in this voxel are more critical and active. Tomasi & Volkow (2010) suggested that voxels with high DC serve as the interconnection hubs, meaning effective and fast brain communication with minimal energy cost. Widespread evidence of increased functional activation has been reported in patients with COPD (Dodd et al., 2012; Xin et al., 2019; Yu et al., 2016; Zhang et al., 2016). Increased resting state connection could be interpreted as reducing precise control over functional networks that are not beneficial, indicating a disrupt network. DLPFC is a region involved in visual reproduction and spatial working memory (Courtney et al., 1998), which is sensitive to hypoxia (Jayalakshmi et al., 2007). Furthermore, Chen et al. (2016) suggested that the thinner DLPFC is a predictive factor of poorer visual reproduction performance. And Kravitz et al. demonstrated that DLPFC is a key neural locus for short-term visual memory (Kravitz et al., 2011). Previous dyspnea-related fMRI studies had reported activation in the ACG (Herigstad et al., 2015). In addition, by using breathlessness-related word-cue task, another task study showed that baseline activity in the ACG and prefrontal cortex correlated with improvements in breathlessness and breathlessness-anxiety (Herigstad et al., 2017). Moreover, the SMG is involving in a multimodal complex that integrates somatosensory inputs to the brain and is associated with attention processing. Meanwhile, higher DC values were found in the left PreCG and left SMG in COPD. The higher DC represents more correlations between the given voxel and the rest voxels, indicating that neurons in this voxel are more critical and active. Tomasi & Volkow (2010) suggested that voxels with high DC serve as the interconnection hubs, meaning effective and fast brain communication with minimal energy cost. Widespread evidence of increased functional activation has been reported in patients with COPD (Dodd et al., 2012; Xin et al., 2019; Yu et al., 2016; Zhang et al., 2016). Increased resting state connection could be interpreted as reducing precise control over functional networks that are not beneficial, indicating a disrupt network. Our study showed a significantly negative correlation between the mean DC value of the left supramarginal gyrus and the visuospatial/executive function score, suggesting that the left SMG may be associated with visuospatial/executive dysfunction in COPD patients. Taken together, the CBF and DC DLPFC, ACG, SMG and PreCG were impaired in COPD patients, which might provide new neuroimaging evidence contributing to the neural basis of cognitive impairment in COPD patients.
We found significant correlations between CBF and DC in the four merged ROIs in both COPD and healthy groups. Further comparison analyses showed no significant differences in the correlation coefficients between the two groups which meant normal neurovascular coupling in the four ROIs in all subjects. To minimize interference factors, we excluded participants with other diseases or disorders that may affect the cerebral function and structure, such as cardiovascular, metabolic diseases, neurosis and psychosis. We speculated that this finding might be associated with current status of COPD patients in this study. More COPD patients with different stages may be needed to explore in the further studies. Unexpectedly, no associations between abnormal brain functions and pulmonary-specific disease markers were found. We conceived that the extrapulmonary manifestations of COPD may not be strongly related to pulmonary-specific disease markers. In addition, a large body of literature provided evidence that cigarette smoking involves in the cerebral functional or structural abnormalities in COPD (Baeza-Loya et al., 2016; Dodd et al., 2012; Gons et al., 2011; Taki et al., 2013). However, no correlation between the duration or the amount of smoking and the cerebral abnormalities were found in all smokers.
Several limitations should be taken into account when interpreting our findings. Firstly, relatively small sample size may influence our interpretations. More COPD patients are needed in the further investigations. Secondly, CBF and DC are indirect indices, preventing us from direct and more reliable measurements of cerebral perfusion and neural activity. Thirdly, analysis of grey matter volume should be performed to further support our present findings. Finally, COPD patients after oxygen therapy are not enrolled in this study, a follow-up investigation with longitudinal comparison is needed to validate the present findings.
Conclusion
Our study revealed disrupted neurovascular coupling in COPD patients via a combination of resting-state BOLD fMRI and ASL techniques. Specifically, increased CBF/DC ratio in the left lingual gyrus, medial frontal cortex/temporal gyrus were involved in cognitive processing in COPD patients. These findings presented novel evidence that abnormal neurovascular coupling may contribute to a potential neural mechanism of cognitive impairment in COPD patients.