Prevalence of G6PD deficiency and G6PD variants amongst the southern Thai population
- Published
- Accepted
- Received
- Academic Editor
- Ipsita Mohanty
- Subject Areas
- Molecular Biology, Hematology, Medical Genetics
- Keywords
- G6PD deficiency, Hemolytic anemia, Neonatal hyperbilirubinemia, G6PD variants, Southern Thai population
- Copyright
- © 2022 Nuinoon et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Prevalence of G6PD deficiency and G6PD variants amongst the southern Thai population. PeerJ 10:e14208 https://doi.org/10.7717/peerj.14208
Abstract
Background
Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme essential for NADPH production and protecting cells, especially red blood cells, from free radicals. The oxidative stress from drugs, chemicals, and infections can induce red blood cell hemolysis in G6PD deficiency patients, causing a genetic disorder.
Objectives
This study aims to provide more information on G6PD deficiency prevalence and the G6PD variants in the southern Thai population.
Methods
Five hundred and twenty healthy subjects in 14 provinces in the southern part of Thailand participated in the study. EDTA-blood samples were collected for a hematological parameters study, G6PD deficiency screening, and a molecular study for G6PD mutation. G6PD deficiency screening was tested using a fluorescent spot test. The types of G6PD mutation were identified by the allele-specific PCR method.
Results
The prevalence of G6PD deficiency in southern Thailand was 6.1% (14/228) in males and 9.6% (28/292) in females. Two homozygous and 26 heterozygous G6PD deficiencies were found in females. G6PD Viangchan (871G>A) was the most common variant with 43%, followed by G6PD Mahidol (487G>A), 24% with an allele frequency of 0.025 and 0.012, respectively. Uncharacterized mutations existed in three samples. The study volunteers had anemia in 36.6% (107/292) females and 7.5% (17/228) males. Among G6PD deficiency subjects, only ten partial G6PD deficiency females had mild anemia.
Conclusions
This study suggests that the prevalence of G6PD deficiency in southern Thailand aligns with that of other parts of Thailand. Newborn screening for G6PD deficiency is recommended for personal information and medical reference to prevent acute hemolysis from oxidative stressors.
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) is an essential enzyme in the pentose phosphate pathway, a step of the nicotinamide adenine dinucleotide phosphate (NADPH) generation (Adu-Gyasi et al., 2015). NADPH reduces glutathione, critical for red blood cell (RBC) protection from oxidative stress (Cappellini & Fiorelli, 2008). G6PD deficiency is an X-linked recessive inherited disorder, and symptomatic patients are mostly hemizygous in males whereas predominantly heterozygous and less common homozygous in females (Chen et al., 2018). G6PD deficiency is the most common enzyme deficiency, affecting at least 400 million individuals worldwide (Suryantoro, 2003). Point mutations of the G6PD encoded gene is located on chromosome X. Approximately 140 mutations (Hue et al., 2009) cause G6PD deficiencies, resulting in an amino acid substitution. The mutations lead to protein variants with different enzyme activity levels associated with biochemical and clinical phenotypes (Eggleston & Krebs, 1974). Especially in G6PD-deficient newborns, hyperbilirubinemia from red blood cell lysis may cause brain damage (Wang et al., 2010). However, G6PD deficiency screening is not conducted in all newborns in Thailand. The G6PD deficiency in a person or heterozygous female, which no evidence of hyperbilirubinemia or hemolysis, has inadequate information on protecting themselves from inducing substances. The distributions of G6PD deficiency differ globally, with a high prevalence in malaria-endemic areas (Phompradit et al., 2011). Primaquine is required for malaria treatment, especially for the radical cure of Plasmodium vivax, and Plasmodium ovalae, and reduces the transmission of Plasmodium malariae infection. The G6PD deficiency in persons with a malarial infection cannot be treated with a certain amount of primaquine because it may cause hemolytic anemia (Recht, Ashley & White, 2018, World Health Organization, 2015). The study of prevalence and G6PD variant in all parts of Thailand benefits the planning of malarial prevention and the safety of Primaquine treatment among these persons. Moreover, as G6PD deficiency is a genetic disorder, genetic consultation for couples may include providing more understanding and awareness.
In Thailand, the prevalence of G6PD deficiency is in the range of 3–18% (Tanphaichitr, 1999). The most common mutations in G6PD-deficient Thais are G6PD Viangchan (871G>A) and G6PD Mahidol (487G>A), respectively (Charoenkwan et al., 2014). The prevalence of G6PD deficiency in the south of Thailand was 3.33% in Trang province (Pattaranggoon & Yimtiang, 2014). The G6PD Viangchan is the most common type found in G6PD patients in Suratthani and Songkhla provinces (Laosombat et al., 2005, Jitueakul et al., 2018). However, according to previous reports, the prevalence of G6PD deficiency and its variants was not explored in all southern Thai provinces. This study aims to determine the prevalence of G6PD deficiency and the types of G6PD mutation in the southern Thai population. The findings from this study should support the previous data and provide more information on G6PD deficiency distribution in Thailand.
Materials and Methods
Subjects and blood collection
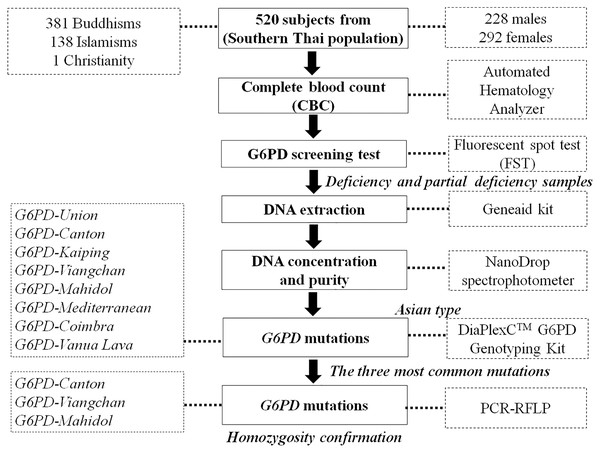
A cross-sectional study was conducted on 520 healthy volunteers (male:female = 228:292) in 14 provinces in the south of Thailand between July 2016 and August 2019. The interested persons were announced and informed of the recruitment criteria stating that the volunteer and their parents would be domiciled in southern Thailand. The number of volunteers from each province was calculated depending on the population in that province and the ratio of the total volunteers. Five hundred twenty volunteers were selected randomly. They were briefly informed and filled in their information in the questionnaire. They signed a consent form before the following processes. The 2.5 mL peripheral blood was drawn from each person and collected in ethylenediaminetetraacetic acid (EDTA) collection tubes without hemolysis. The blood sample of each subject was divided into 1.5 mL for G6PD deficiency screening and hematological parameters testing and 1.0 mL for DNA extraction for gene mutation analysis. The Human Research Committee of the Ethics Research Council, Walailak University (16/031 and WUEC-16-055-01) approved this research and informed consent. Figure 1 depicts the sample workflow of this study.
Figure 1: Sample workflow for laboratory testing.
G6PD, glucose-6-phosphate dehydrogenase; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism.Hematological parameters study
Hematological parameters were tested using Sysmex XN-1000 automated hematology analyzer (Sysmex Corporation, Kobe, Japan). The parameters included RBC count (RBC), hemoglobin (HBG), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red blood cell distribution width (RDW).
G6PD screening test by Fluorescent spot test (FST)
G6PD deficiency screening was tested using a Fluorescent spot test (FST) (R&D Diagnostics Ltd., Papagos, Greece). The protocol was clearly described in the previous study (Krithong et al., 2020). The G6PD variants further identified the G6PD deficiency and partial deficiency.
Detection of G6PD gene mutation
Genomic DNA was extracted from peripheral blood leukocytes using a Geneaid kit (Geneaid Biotech Ltd, New Taipei City, Taiwan). The DNA concentration of each sample was quantified with the NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA, USA). The G6PD mutations in G6PD-deficient and partially deficient samples were identified using multiplex allele-specific PCR, DiaPlexC™ G6PD Genotyping Kit (Asian type) (SolGent Co., LTD., Daejeon, Korea). The kit allows screening of eight mutations of Asian types, including Union (1360C>T), Canton (1376G>T), Kaiping (1388G>A), Viangchan (871G>A), Mahidol (487G>A), Mediterranean (563C>T), Coimbra (592C>T) and Vanua Lava (383T>C). The PCR reaction comprised 12.5 μL of 2X Multiplex PCR Smart mix (G6PD-Asian type), 2 μL of primer mixture (G6PD-Asian type), nuclease-free water, and DNA template (25–50 ng). PCR thermocycling conditions were as follows: initial denaturation at 95 °C for 15 min; followed by 30 cycles of 30 s at 95 °C, 30 s at 60 °C, and 40 s at 72 °C; followed by a final extension at 72 °C for 5 min. PCR products of each sample and DNA marker were analyzed by 3% agarose gel electrophoresis for the variants. After staining the gel with ethidium bromide for 5 min, the sizes of PCR products were detected under UV light with an ultraviolet transilluminator. Finally, PCR-RFLP was used for secondary confirmation of heterozygous female, hemizygous male, and homozygous female in the first three common G6PD variants (G6PD Viangchan, G6PD Mahidol, and G6PD Canton) (Nuchprayoon, Sanpavat & Nuchprayoon, 2002).
Statistical analysis
Data were analyzed using SPSS v.17 software. Hematological parameters were calculated for the mean, SD, median, and prevalence. The Kolmogorov-Smirnov test was used to test the normality of the data. The statistical difference in hematological parameters between the groups was calculated using the T-Test Independent statistics for normal distribution and the Mann-Whitney U test for non-normal distribution. The 100% stacked column was conducted using Infogram (https://infogram.com/).
Results
Demographic data and G6PD screening test
The data were collected from 520 subjects in 14 provinces of Southern Thailand, including 228 males and 292 females. The age of the subjects was in the range of 20–25 years old, with religious backgrounds of 73.3% being Buddhist and 26.5% being Muslim (Table 1). FST tested the G6PD deficiency. The prevalence of G6PD deficiency was 6.1% (14/228) for males. For females, the G6PD deficiency and partial or intermediate G6PD deficiency was detected in two and 26 persons, respectively. Thus, the prevalence in females was 9.6% (28/292). No significant differences (p > 0.05) existed in hematological parameters of G6PD deficiency, G6PD partial, and normal G6PD (Table 2). Mild anemia was found in 10 persons with partial G6PD deficiency in females but not in G6PD deficient males. Additionally, the prevalence of G6PD deficiency was 9.4% (13/138) in Muslims and 7.6% (29/381) in Buddhists.
| Provinces, n (%) | Participants | Religion | G6PD status | ||||
|---|---|---|---|---|---|---|---|
| Male, n (%) | Female, n (%) | Buddhism, n (%) | Islamism, n (%) | Christianity, n (%) | Partial G6PD deficiency, n (%) | G6PD deficiency, n (%) | |
| Nakhon Si Thammarat, 145 (27.9) | 65 (12.5) | 80 (15.4) | 127 (24.4) | 19 (3.7) | – | 6 (1.2) | 4 (0.8) |
| Trang, 40 (7.7) | 20 (3.8) | 20 (3.8) | 30 (5.8) | 9 (1.7) | 1 (0.2) | 4 (0.8) | 1 (0.2) |
| Songkhla, 53 (10.2) | 26 (5.0) | 27 (5.2) | 32 (6.2) | 20 (3.8) | – | 3 (0.6) | 3 (0.6) |
| Suratthani, 50 (9.6) | 24 (4.6) | 26 (5.0) | 41 (7.9) | 9 (1.7) | – | 3 (0.6) | 1 (0.2) |
| Krabi, 33 (6.4) | 12 (2.3) | 21 (4.0) | 21 (4.0) | 12 (2.3) | – | 2 (0.4) | 0 (0.0) |
| Phatthalung, 28 (5.4) | 11 (2.1) | 17 (3.3) | 20 (3.8) | 8 (1.5) | – | 1 (0.2) | 1 (0.2) |
| Chumphon, 24 (4.6) | 9 (1.7) | 15 (2.9) | 22 (4.2) | 2 (0.4) | – | 1 (0.2) | 0 (0.0) |
| Narathiwat, 37 (7.1) | 15 (2.9) | 22 (4.2) | 15 (2.9) | 22 (4.2) | – | 2 (0.4) | 0 (0.0) |
| Satun, 22 (4.2) | 10 (1.9) | 12 (2.3) | 14 (2.7) | 8 (1.5) | – | 1 (0.2) | 1 (0.2) |
| Pattani, 39 (7.5) | 14 (2.7) | 25 (4.8) | 21 (4.0) | 18 (3.5) | – | 1 (0.2) | 3 (0.6) |
| Ranong, 13 (2.5) | 5 (1.0) | 8 (1.5) | 12 (2.3) | 1 (0.2) | – | 0 (0.0) | 1 (0.2) |
| Phangnga, 13 (2.5) | 5 (1.0) | 8 (1.5) | 11 (2.1) | 2 (0.4) | – | 0 (0.0) | 1 (0.2) |
| Yala, 15 (2.9) | 8 (1.5) | 7 (1.3) | 8 (1.5) | 7 (1.3) | – | 1 (0.2) | 0 (0.0) |
| Phuket, 8 (1.5) | 4 (0.8) | 4 (0.8) | 7 (1.3) | 1 (0.2) | – | 1 (0.2) | 0 (0.0) |
| Total, 520 (100.0) | 228 (43.8) | 292 (56.2) | 381 (73.3) | 138 (26.5) | 1 (0.2) | 26 (5.0) | 16 (3.1) |
Notes:
The data indicates the general data of all participants including province, gender, religions and G6PD status.
Values are presented as n (%). G6PD, Glucose-6-phosphate dehydrogenase.
| Gender/G6PD status (n) | Prevalence (%) | RBC (106/µL) | HGB (g/dL) | HCT (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | RDW (%) |
|---|---|---|---|---|---|---|---|---|
| Male (228) | ||||||||
| – Normal (214) | 93.9 | 5.28 ± 0.54 | 14.5 ± 1.23 | 42.9 ± 3.40 | 81.7 ± 5.85 | 27.6 ± 2.32 | 33.8 ± 1.26 | 13.5 ± 1.66 |
| – Deficiency (14) | 6.1 | 5.09 ± 0.44 | 14.7 ± 1.08 | 43.0 ± 3.14 | 83.8 ± 4.29 | 27.8 ± 3.41 | 34.2 ± 0.84 | 12.9 ± 1.26 |
| P-valuea | 0.116 | 0.613 | 0.867 | 0.271 | 0.485 | 0.196 | 0.116 | |
| Female (292) | ||||||||
| – Normal (264) | 90.4 | 4.65 ± 0.45 | 12.1 ± 1.18 | 36.8 ± 3.15 | 79.6 ± 7.80 | 26.3 ± 3.03 | 32.9 ± 1.26 | 14.1 ± 1.93 |
| – Partial deficiency (26) | 8.9 | 4.60 ± 0.47 | 12.1 ± 1.33 | 36.6 ± 3.02 | 79.9 ± 7.03 | 26.4 ± 3.05 | 32.9 ± 1.66 | 13.8 ± 1.87 |
| – Deficiency (2) | 0.7 | 4.82, 4.02 | 13.2, 12.0 | 39.5, 36.6 | 82.0, 86.1 | 27.4, 28.9 | 33.4, 33.5 | 13.5, 14.7 |
| P-valueb | 0.598 | 0.510 | 0.526 | 0.998 | 0.996 | 0.675 | 0.527 | |
Notes:
The participants were grouped by sex and G6PD deficiency status. The data of the hematological parameters of each group were analyzed and were presented as mean ± SD. The different of each parameter between normal and G6PD deficiency was statistical analyzed.
Values are presented as n, %, mean ± SD, or raw data where appropriate. RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; fL, femtoliter; g/dL, gram per deciliter.
Characterization of G6PD mutations
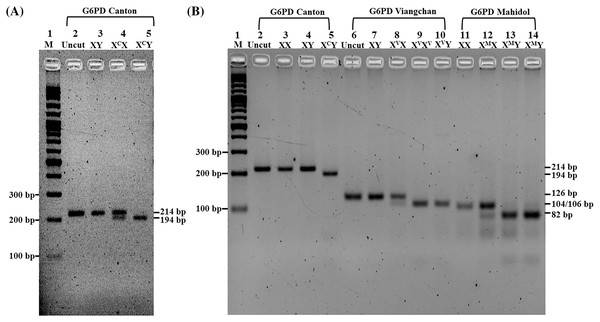
A total of 42 G6PD deficiency subjects (14 males and 28 females) were analyzed for eight types of G6PD gene mutation by DiaPlexC™ G6PD Genotyping Kit (Asian type) and further confirmation by PCR-RFLP in the first three common mutations. PCR-RFLP with AflII restriction enzyme revealed good discrimination among three genotypes (XY, XCX, and XCY) of the G6PD Canton (Fig. 2A) in addition to PCR-RFLP with XbaI and HindIII restriction enzymes for genotypic characterization of G6PD Viangchan and G6PD Mahidol, respectively (Fig. 2B). Figures S1 and S2 depict the product sizes of each variant in G6PD deficiency and partial deficiency.
Figure 2: Characterization of G6PD mutations by PCR-RFLP.
Two percent Agarose gel electrophoresis of PCR-RFLP from G6PD Canton (XC), G6PD Viangchan (XV), and G6PD Mahidol (XM). (A) Lane 1, standard marker (100 bp DNA ladder); lane 2, uncut normal control (XY); lane 3, cut normal control (XY); lane 4, heterozygous G6PD Canton (XCX); lane 5, hemizygous G6PD Canton (XCY). (B) Lane 1, standard marker (100 bp DNA ladder); lane 2, uncut normal control (XX); lane 3, cut normal control (XX); lane 4, cut normal control (XY); lane 5, hemizygous G6PD Canton (XCY); lane 6, uncut normal control (XY), lane 7, cut normal control (XY); lane 8, heterozygous G6PD Viangchan (XVX); lane 9, homozygous G6PD Viangchan (XVXV), lane 10, hemizygous G6PD Viangchan (XVY); lane 11, cut normal control (XX); lane 12, heterozygous G6PD Mahidol (XMX) lanes 13 and 14, hemizygous G6PD Mahidol (XMY).The frequency of G6PD variants in the southern Thai population
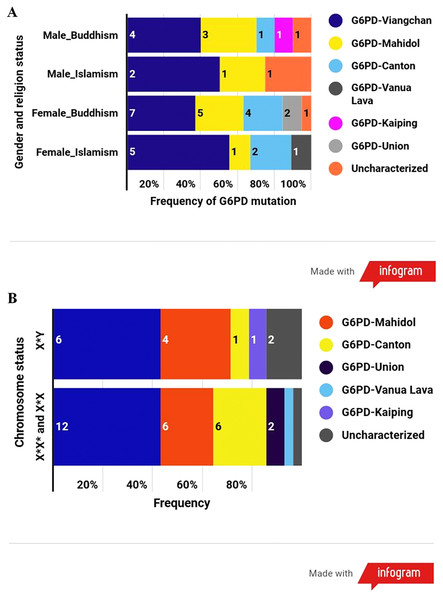
The results disclosed that the most common G6PD variant in this population was G6PD Viangchan (43%; allele frequency = 0.025), followed by G6PD Mahidol (24%; 0.012), G6PD Canton (17%; 0.009), G6PD Union (5%; 0.002), G6PD Vanua Lava (2%; 0.001), and G6PD Kaiping (2%; 0.001). Uncharacterized mutations existed in three samples (Table 3). Among G6PD-deficient samples, G6PD-Viangchan was the most common G6PD variant in every category according to the combination of gender and religion and followed by G6PD-Mahidol (Fig. 3A). G6PD mutational spectrum illustrated a similar pattern between males and females (Fig. 3B).
| G6PD variants | Male (228) | Female (292) | No. of X* Chr. | Total X or X* Chr. | Allele frequency | ||||
|---|---|---|---|---|---|---|---|---|---|
| Hemizygote (14) |
Normal (214) |
Homozygote (2) |
Heterozygote (26) |
Normal (264) |
|||||
| (X*Y) | (XY) | (X*X*) | X* | X | (XX) | ||||
| G6PD-Viangchan | 6 | 0 | 4 | 10 | 0 | 0 | 20 | 20 | 0.025 |
| G6PD-Mahidol | 4 | 0 | 0 | 6 | 0 | 0 | 10 | 10 | 0.012 |
| G6PD-Canton | 1 | 0 | 0 | 6 | 0 | 0 | 7 | 7 | 0.009 |
| G6PD-Union | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 0.002 |
| G6PD-Vanua Lava | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0.001 |
| G6PD-Kaiping | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.001 |
| G6PD-Uncharacterized | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 3 | 0.004 |
| G6PD-Normal | 0 | 214 | 0 | 0 | 26 | 528 | 0 | 768 | 0.946 |
| Total | 14 | 214 | 4 | 26 | 26 | 528 | 44 | 812 | 1.000 |
Notes:
G6PD variants of all G6PD deficiency samples were calculated for allele frequency.
G6PD, Glucose-6-phosphate dehydrogenase.
X* represented the G6PD gene mutation.
Figure 3: The frequency of G6PD variants according to different categories.
(A) Frequency of G6PD mutations according to the combination of gender and religion. (B) Frequency of G6PD mutations according to the number of X chromosome (gender).Red blood cell parameters in participants with anemia and different G6PD status
Table 4 demonstrates the mean ± SD of 228 males and 292 females of hematological parameters. Subjects were grouped into healthy (76.2%, 396/520) and anemia (23.8%, 124/520) by hemoglobin value; anemia has a hemoglobin value of less than 12 g/dL in females and less than 13 g/dL in males. Anemia was found in 36.6% (107/292) females and 7.5% (17/228) males. Interestingly, this study found two cases with homozygous G6PD-Viangchan (XVXV). In the first case, a 19-year-old from Ranong province, her complete blood count was as follows: red cell count 4.82 × 1012/L, Hb level 13.2 g/dL, hematocrit 39.5%, MCV 82.0 fL, MCH 27.4 pg, MCHC 33.4 g/dL, and RDW 13.5%. The second case was a 20-year-old from Songkhla province; her complete blood count was as follows: red cell count 4.02 × 1012/L, Hb level 12.0 g/dL, hematocrit 36.6%, MCV 86.1 fL, MCH 28.9 pg, MCHC 33.5 g/dL, and RDW 14.7 % as in Table 2.
| Gender | Anemia/Normal | RBCb (×106/µL) | HGBa (g/dL) | HCTa (%) | MCVa (fL) | MCHa (pg) | MCHCa (g/dL) | RDWa (%) |
|---|---|---|---|---|---|---|---|---|
| Male | Anemia (n = 17) | 4.86 ± 0.6 | 12.1 ± 0.8 | 37.8 ± 3.2 | 78.4 ± 7.5 | 25.6 ± 3.1 | 32.6 ± 1.6 | 14.8 ± 3.3 |
| Normal (n = 211) | 5.30 ± 0.5 | 14.7 ± 1.0 | 43.3 ± 3.0 | 82.1 ± 5.6 | 27.8 ± 2.2 | 34.0 ± 1.1 | 13.4 ± 1.4 | |
| P-value | 0.005 | 0.000 | 0.000 | 0.072 | 0.005 | 0.000 | 0.030 | |
| Female | Anemia (n = 107) | 4.58 ± 0.5 | 11.0 ± 0.9 | 34.0 ± 2.4 | 74.9 ± 8.9 | 24.3 ± 3.4 | 32.3 ± 1.5 | 15.2 ± 2.3 |
| Normal (n = 185) | 4.68 ± 0.4 | 12.8 ± 0.7 | 38.5 ± 2.2 | 82.4 ± 5.3 | 27.4 ± 2.0 | 33.3 ± 1.0 | 13.3 ± 1.1 | |
| P-value | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Discussion
G6PD deficiency is the most common enzyme deficiency worldwide (Bubp, Jen & Matuszewski, 2015). The prevalence of G6PD deficiency substantially varies across the world. In Thailand, the prevalence was 3–18%, with different percentages in each part of the country. Northeastern and northern Thailand have deficiencies of 22% and 17%, respectively (Charoenkwan et al., 2014; Kittiwatanasarn et al., 2003). The previous study of G6PD deficiency in neonatal for the Southern part showed a high prevalence of G6PD deficiency in male neonatal and hyperbilirubinemia in newborns mainly resulting from a G6PD deficiency (Pattaranggoon & Yimtiang, 2014; Tanphaichitr, 1999; Tuchinda et al., 1968). This study reported the prevalence of G6PD deficiency in healthy adults in the Southern area. The finding was consistent with other reports in which the prevalence was 6.1% and 9.6% in males and females, respectively. However, this report revealed a higher prevalence of G6PD deficiency in females than males because of the criteria set by the FST report. The World Health Organization (WHO) recommended the G6PD deficiency screening method of FST (Ley et al., 2017). This study used this test for G6PD deficiency screening and prevalence evaluation. The results of NADPH production included being fluorescent under the UV light, time to provide the NADPH synthesis in different G6PD levels, or activity in blood samples. These could divide the G6PD deficiency (in hemizygous male and homozygous female), and partial G6PD deficiency (heterozygous female) in healthy people’s blood samples (Thielemans et al., 2018). PCR-based methods confirmed all these deficiency samples.
The base substitution mainly causes gene mutation in G6PD deficiency. As the G6PD gene is 18 kb, the mutation in each point of the G6PD gene leads to a difference in severity (Gómez-Manzo et al., 2016). The class or severity of the G6PD deficiency is based on the activity of the remaining enzymes. For class I, the enzyme activity is less than 1% of the standard range and less than 10% in class II, whereas no abnormal signs were depicted in class V. Common G6PD mutations in Southeast Asia are G6PD Union (1360C>T), G6PD Canton (1376G>T), G6PD Kaiping (1388G>A), G6PD Viangchan (871G>A), G6PD Mahidol (487G>A), G6PD Gaohe (95 A>G), G6PD Chatham (1003 G>A), G6PD Coimbra (592C>T) and G6PD Vanua Lava (383T>C) (Iwai et al., 2001). In Thailand, many studies reported that G6PD Viangchan (871G>A) and G6PD Mahidol (487G>A) are the most commonly found (Laosombat et al., 2005; Nuchprayoon, Sanpavat & Nuchprayoon, 2002). This study also found that these two variants are the most common among the southern Thai population. The prevalence of G6PD deficiency in Thai Muslims and Buddhists in this study is 9.42% and 7.61%, respectively. The G6PD Viangchan is the most common type in both. The G6PD Viangchan was the most common type in Malaysian Malays (Ainoon et al., 2003; Yusoff et al., 2003). Most Thai Muslims have Malay ancestry (Kutanan et al., 2014), and the most common G6PD variant of Thai Muslims from this study was the same as in Malaysian Malays. Consistent with the Malaysian Chinese, the most common types are Canton and Kaiping. (Ainoon et al., 2004). Unidentified variants exist in three G6PD deficiency samples. Because the primers used in the multiplex allele-specific PCR in this study, cover only eight common mutations in Southeast Asia, the unidentified samples might be rare types in this area. Because the G6PD gene is located on chromosome X, the clinical signs are mainly present in hemizygous males and homozygous females when G6PD deficiency patients are exposed to oxidative inducing agents or infections (Hsieh et al., 2013). However, it mainly does not affect in heterozygous females (van den Broek, Heylen & van den Akker, 2016). This study’s limitation is the sample recruitment from certain provinces with small sample size. G6PD deficiency was classified by the level of residual enzyme activity. Thus, the classification will be revised based on the median residual enzyme activity (World Health Organization, 2022). G6PD Mahidol, the G6PD Canton, and the G6PD Viangchan were WHO Class III mutations, moderating deficiency, and were not so severe for primaquine treatment for P. vivax and P. ovale patients (Phompradit et al., 2011). However, the prevention from the oxidative agents should be informed to all. The prevalence of G6PD deficiency and G6PD variants has been reported in the southern Thai population. However, those reports recruited the samples from one province and five provinces with a high prevalence of malaria (Pattaranggoon & Yimtiang, 2014, Khammanee et al., 2022) with a retrospective study. The samples from all provinces located in southern Thailand were collected in a cross-sectional study in this study. However, the limitations of this study are still insufficient sample size in some provinces. It should be helpful in area-based research with differences in ethnicity. Therefore, this study could be confirmed with the evidence of the prevalence of G6PD deficiency in the southern Thai population, and G6PD-Viangchan and G6PD-Mahidol are the two most common G6PD mutations, consistent with previous reports. Our results provide valuable data for planning and informing people with G6PD deficiency, including their parents, to protect themselves from further exposure to hemolytic stimulants to achieve the well-being of G6PD deficiency persons.
Conclusions
The data reveal a high prevalence of partial G6PD deficiency in heterozygous females. This heterozygous group is the carrier of the abnormal gene to their child. Thus, G6PD screening should be performed for all family members of G6PD-deficient patients to provide information for self-protection from oxidative stress.
Supplemental Information
Raw data and statistical data.
Raw data of all participants including general data, G6PD deficiency screening results, hematological parameters.
PCR product of G6PD variant in G6PD deficiency samples.
The pattern of PCR products of G6PD deficiency cases was analyzed by 3% gel electrophoresis. Lane 1, standard marker; lane 2, mutant type control; lane 3, wild type control; lane 4, non-template control; lane 5, G6PD normal; lane 6, G6PD Canton (681 bp); lane 7 G6PD Kaiping (557 bp); lane 8 G6PD Viangchan (501 bp); lane 9 G6PD Mahidol (337 bp) and lane 10 no detectable mutation by PCR used in this study.
The pattern of PCR products of G6PD partial deficiency cases analyzed by 3% gel electrophoresis.
The pattern of PCR products of G6PD partial deficiency cases analyzed by 3% gel electrophoresis. Lane 1, standard marker; lane 2, mutant type control; lane 3, wild type control; lane 4, non-template control; lane 5, G6PD normal; lane 6, G6PD Vanua Lava (154 bp); lane 7, G6PD Viangchan (501 bp); lane 8, G6PD Mahidol (337 bp); lane 9, Canton (681 bp); lane 10, G6PD Union (803 bp) and lane 11, no detectable mutation by PCR used in this study.