Baseline coral disease surveys within three marine parks in Sabah, Borneo
- Published
- Accepted
- Received
- Academic Editor
- Robert Toonen
- Subject Areas
- Conservation Biology, Ecology, Marine Biology
- Keywords
- Coral disease, Marine park, White syndrome, Coral cover, Bleaching, Atramentous necrosis, Borneo
- Copyright
- © 2015 Miller et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2015. Baseline coral disease surveys within three marine parks in Sabah, Borneo. PeerJ 3:e1391 https://doi.org/10.7717/peerj.1391
Abstract
Two of the most significant threats to coral reefs worldwide are bleaching and disease. However, there has been a scarcity of research on coral disease in South-East Asia, despite the high biodiversity and the strong dependence of local communities on the reefs in the region. This study provides baseline data on coral disease frequencies within three national parks in Sabah, Borneo, which exhibit different levels of human impacts and management histories. High mean coral cover (55%) and variable disease frequency (mean 0.25 diseased colonies m−2) were found across the three sites. Highest disease frequency (0.44 diseased colonies per m2) was seen at the site closest to coastal population centres. Bleaching and pigmentation responses were actually higher at Sipadan, the more remote, offshore site, whereas none of the other coral diseases detected in the other two parks were detected in Sipadan. Results of this study offer a baseline dataset of disease in these parks and indicate the need for continued monitoring, and suggest that coral colonies in parks under higher anthropogenic stressors and with lower coral cover may be more susceptible to contracting disease.
Introduction
Tropical marine environments are faced with unprecedented natural and anthropogenic disturbances (Doney, 2006; Pratchett, Hoey & Wilson, 2014). Even optimistic scenarios for environmental change suggest mass declines in coral cover and species richness (Hoegh-Guldberg et al., 2007; Veron et al., 2009; Fabricius et al., 2011). It has been predicted that these ecosystems, which are vital to the economy and subsistence of approximately 500 million people, could be lost within the next century (Wilkinson, 2008; Brierley & Kingsford, 2009). The biggest threats to the longevity of coral reefs are often reported as being bleaching events and outbreaks of disease (Knowlton, 2001; Harvell et al., 2002; Gardner et al., 2003; Baker et al., 2004). Coral disease can lead to significant decreases in live coral cover (Miller et al., 2009), partial to whole-colony mortality (Willis, Page & Dinsdale, 2004) and changes in community composition (Bythell & Sheppard, 1993; Francini-Filho et al., 2008) consequently jeopardising the entire reef ecosystem (Bellwood et al., 2004; Sweet, Croquer & Bythel, 2014).
Disease outbreaks have been temporally and spatially linked to bleaching events (Muller et al., 2008; Bruckner & Hill, 2009; Croquer & Weil, 2009), and sea surface temperature (SST) anomalies have been correlated with increases in bleaching and disease prevalence (Bruno et al., 2007; Heron et al., 2010; Ruiz-Morenol et al., 2012). Current SST levels are at their highest on record (NOAA, 2006) and are predicted to increase in the future, particularly in the tropics (Hoegh-Guldberg et al., 2007) so the number of bleaching and disease outbreaks is also likely to increase. Additionally, increased coastal populations of humans and the associated anthropogenic stressors such as pathogen transportation (Harvell et al., 1999) pollution and eutrophication (Bruno et al., 2003), fishing and dredging (Nyström, Folke & Moberg, 2000) may increase coral susceptibility to diseases (Bourne et al., 2015).
The number of coral diseases identified worldwide varies between studies, ranging from 5 to 29 (Dalton & Smith, 2006), with 22 reported in the Atlantic (Weil, 2004) and 11 in the Indo-Pacific (Global Coral Disease Database; http://coraldisease.org/, accessed January 2015). There has been extensive research on the microbial pathogens associated with a variety of coral diseases; however the application of Koch’s postulates (Koch, 1982) is problematic in the marine environment. This may be partly due to the difficulty in confidently separating stress responses from signs of disease in corals under laboratory conditions and also the difficulty in defining coral disease signs in the laboratory in relation to those in the field. Therefore, causal agents of few coral diseases have been identified and are increasingly disputed (Harvell et al., 2007). Indo-Pacific reefs have almost ten times higher scleractinian species richness and coral cover than Caribbean reefs (Bruno & Selig, 2007). However, a lower disease prevalence and slower decline in coral cover is reported in the Indo-Pacific in most (Goldberg, Wilkinson & Wilkinson, 2004; Willis, Page & Dinsdale, 2004) but not all studies (Dinsdale, 2000). This difference could be explained by variation in coral community species composition and susceptibilities to diseases (Connell, 1997; Sutherland, Porter & Torres, 2004). Alternatively, a paucity of disease studies in the Indo-Pacific (Goldberg, Wilkinson & Wilkinson, 2004; Wilkinson, 2008; Harvell et al., 2007) and a focus on epizootics in the Caribbean (e.g., Croquer, Pauls & Zubillaga, 2003) may represent a significant bias.
Despite the biological and economic significance of coral reefs in the south-east Asian region, no systematic investigation of coral diseases has been conducted in Malaysia. Land clearance and future development plans for rapid increases in population sizes on the Malaysian coast (Comley et al., 2004) will augment pressures on marine ecosystems. This is especially so when mangrove and other coastline vegetation has been lost or degraded, removing natural barriers to coastal erosion and consequent land runoff (Wilkinson, 2008). Sabah in Borneo supports around 550 (Burke & Selig, 2002a; Burke & Selig, 2002b) of the Indo-Pacific’s 581 scleractinian coral species (Veron, 2000) and represents 75% of Malaysia’s coral reef area (WRI, 2010). However, these reefs are also included in the world’s 15% most critically threatened reefs (Wilkinson, 2008) and in 2011 97% of Malaysia’s reefs were categorised as threatened, and 50% as under high threat of severe degradation (Burke & Selig, 2002a; Burke & Selig, 2002b; Burke et al., 2011). The present study establishes the first baseline coral disease data-set within three national parks in Sabah, Malaysian Borneo, and therefore improves the geographical distribution of data of coral disease frequency in tropical reef environments.
Materials and Methods
Survey area
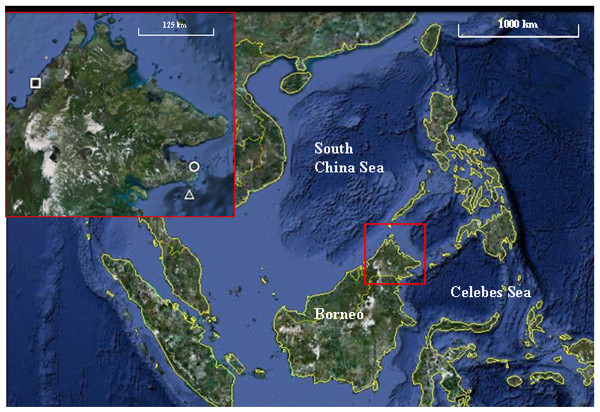
The study was conducted at three parks, Tunku Abdul Rahman Park (TARP), Tun Sakaran Marine Park (TSMP) and Sipadan Island Park (SIP) in Sabah, Malaysian Borneo (Fig. 1). All surveys were conducted between May and June 2010. The three parks were chosen for their varying levels of disturbance due to their proximity to the mainland and levels of fishing permitted. TARP covers 50 km2 area but is only 3 km from Kota Kinabalu (the capital city of Sabah) in the South China Sea (Fig. 1) and has had protected status since 1977. Indigenous communities of fishing villagers reside within the park boundaries and hook and line fishing is permitted throughout. TSMP covers 350 km2 area, is 10 km from the nearest city—Semporna (on the east coast of Sabah), and was gazetted in 2004 (Wood, 2001a; Wood, 2001b). Hook and line fishing is permitted in specific zones, and the park has its own enforcement staff to regulate this. SIP is a small island isolated by deep-water channels, 35 km from Semporna, covering an area of 168 km2 of water and reef. SIP has been partially protected since 1930 and fully gazetted since 1963. SIP remains a complete no take zone, enforced by the Sabah Parks National Park Authority. Access was permitted by the Economic Planning Unit of Malaysia (permit ID:TS/TMTS/UPM/P/4/4[48]).
Figure 1: Map of the three marine parks surveyed.
Tunku Abdul Rahman Park (TARP), Tun Sakaran Marine Park (TSMP) and Sipadan Island Park (SIP) in Sabah, Malaysian Borneo (inset) were surveyed May–June 2010. TARP (Inset, circle; grid references 6°02′N, 116°05′E) is a 50 km2 park in the south China Sea, 3 km from west Sabah’s coastline. TSMP (Inset, triangle; grid references 4°42′N, 118°51′E) is a 350 km2 park in the Celebes Sea, 10 km from the east Sabah’s coastline. SIP (Inset, square; grid references 4°7′N, 118°37.5′E) is a protected island 35 km south-east from Semporna, Sabah. Grid references were recorded at each transect site using Garmin GPS Map 76 handheld mapping equipment. Map data: Google Earth, DigitalGlobe. Scale bar = 1,000 km. Inset scale bar = 125 km.Disease surveys
At each of the 24 randomly selected survey sites, one 100 × 0.5 m was surveyed for occurrence of coral diseases and % hard coral cover. 8 transects were surveyed at TARP, TSMP and SIP 4 at 10 m and 4 at 5 m depth. In total an area of 1,200 m2 was surveyed. All cases of coral disease, bleaching and pigmentation response, affecting colonies in the transect area wereafter identified and categorised according to Coral Reef Targeted Research—Disease Working Group guidelines (Beeden et al., 2008, Table 1). Close-up photographs (using Canon Powershot G11 cameras), were taken of each disease enabling later verification and standardisation of diseaseidentification. Percent hard coral cover was recorded during the surveys and was later verified using examination of high resolution photographs taken at 0, 50 and 100 m distance points along the transect, approximately 2–3 m above the transect depth. Survey sites were selected using a random number generator choosing from possible GPS coordinates.
| Disease/syndrome | Notes for identification | References for identification methods |
|---|---|---|
| Atramentous Necrosis (AtN) | Patchy bleaching, distinct grey-blue microbial “mats” covering necrotic tissue lesions | Willis, Page & Dinsdale (2004) and Beeden et al. (2008) |
| Black Band Disease (BBD) | Distinctive dark band separating live tissue and exposed skeleton & relatively quick progression | Beeden et al. (2008), Miller (1996) and Richardson (2004) |
| Bleaching (BL) | Translucent tissue revealing white skeleton underneath & may be patchy, affecting whole colony or communities | Beeden et al. (2008) |
| Brown Band Disease (BrB) | Brown band of “active” disease delineating live and dead/absent tissue, sometimes bands of bleached tissue between disease and live tissue, rapid progression | Bourne et al. (2008) and Beeden et al. (2008) |
| Pigmentation Response (PR) | Fluorescent pink/purple/blue patches or spots, particularly around skeleton borers, parasites and algal overgrowth, unlikely mortality | Willis, Page & Dinsdale (2004) and Beeden et al. (2008) |
| White Syndrome (WS) | Distinct boundary of white–yellow (with time) exposed skeleton and live tissue, some bleached tissue may occur between live and diseased areas, rapidly progresses | Willis, Page & Dinsdale (2004), Bythell, Pantos & Richardson (2004) and Beeden et al. (2008) |
| Predation | Scarring from corallivorous fish, crown of thorns etc. leaving tissue loss and skeleton abrasion | Raymundo et al. (2008) |
Data analysis
The frequency of disease, bleaching and pigmentation response was calculated per unit area of surveyed reef (i.e., colonies affected per m2) for each transect before statistical analysis. The difference between the two depths of survey transects was tested with a nested t-test. Data from categories’ disease + bleaching + pigmentation response’, plus ‘bleaching’ and ‘pigmentation response’ alone satisfied a normal distribution so a nested site within park Analysis of Co-Variance (ANCOVA) was carried out to test for differences in frequency of these three categories between the three parks, with coral cover being a covariate. Additionally, the Kruskal–Wallis test was used to analyse differences in overall frequencies of all diseases, WS and AtN (these data did not satisfy a normal distribution) between TARP and TSMP. A linear regression was also used to investigate the correlation between % coral cover and disease frequencies and between bleaching and disease frequencies in TARP and TSMP. All analyses were carried out using Minitab 17 software, except the linear regression which was carried out in R using the MASS package (Venables & Ripley, 2002).
Results
Disease frequency
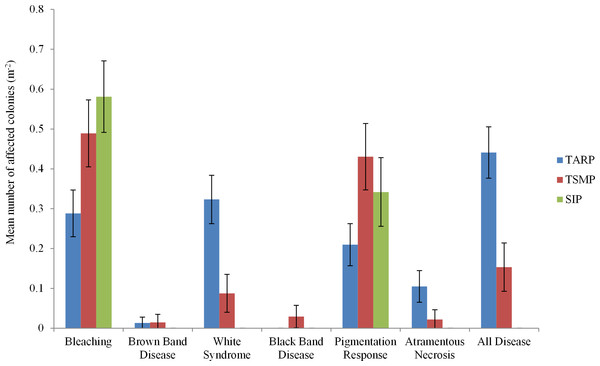
Three diseases commonly found in the Indo-Pacific region were not observed in Sabah during the present surveys: Skeletal Eroding Band, Porites Ulcerative White Spot and Porites Trematodiasis. There was no significant difference between the disease frequencies at 10 and 5 m depth at any park. A total of 443 colonies were recorded showing indications of immune responses or disease, including 95 with signs of a recognised coral disease (Atramentous Necrosis (AtN), Black Band Disease (BBD), Brown Band Disease (BrB) or White Syndrome (WS)), 201 partially or fully bleached colonies and 147 showing signs of a Pigmentation Response (PR) (Table 2). Diseases, bleaching and pigmentation responses pooled differed significantly between the three marine parks (nested ANCOVA: F = 7.08, P = 0.005, Table 2), and there was high variation in different frequencies of the different diseases observed (Fig. 2). The frequency of coral disease (excluding bleaching and pigmentation responses) was significantly higher at TARP than TSMP (Kruskal–Wallis: H = 20.23, P = < 0.0001), and none of the coral diseases widely recognised were observed at SIP (Table 2). WS was the most commonly observed disease, with a total of 74 and 12 colonies at TARP and TSMP respectively. The frequency of WS and AtN were both significantly higher at TARP than TSMP (Kruskal–Wallis: H = 18.6, P = < 0.001 and H = 11.09, P = < 0.001 respectively). Two other commonly observed diseases in the Indo-Pacific, Brown Band Disease (BrB) and Black Band Disease (BBD) were recorded during the surveys but at low frequencies, with BrB present at both TARP and TSMP, and BBD was only observed at TSMP. Bleaching and pigmentation responses were more frequent at SIP, although these differences were not significant, and bleaching was not significantly correlated with disease frequency at either TSMP or TARP.
Figure 2: The frequencies of all diseases, bleaching and pigmentation response (m−2) recorded at the three marine parks surveyed.
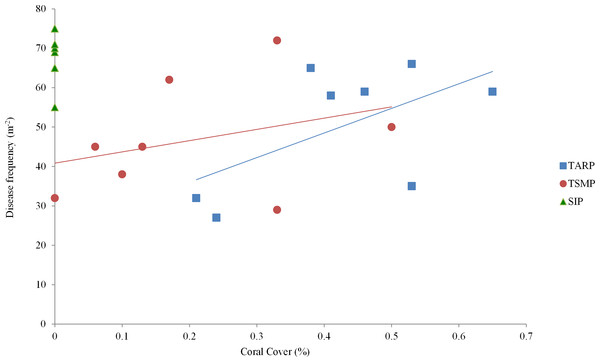
Tunku Abdul Rahman Park, TARP; Tun Sakaran Marine Park, TSMP and Sipadan Island Park, SIP; Disease, frequency of all recorded diseases (categories ‘White Syndrome,’ ‘Brown Band,’ ’Black Band,’ and ‘Atramentous Necrosis’). ±95% confidence intervals.Figure 3: The relationship between percent coral cover and disease frequency at three parks surveyed.
Tunku Abdul Rahman Park, TARP; Tun Sakaran Marine Park, TSMP and Sipadan Island Park, SIP. A positive correlation was found between coral cover and disease frequency at TARP (Linear regression: r2 = 0.48, p < 0.01) but not at TSMP (Linear regression: r2 = 0.57, p > 0.05). No disease was observed at SIP.| Park | Disease | Bleaching | WS | BrB | BBD | AtN | PR |
|---|---|---|---|---|---|---|---|
| TARP | 0.44* | 0.28 | 0.32* | 0.01* | 0 | 0.10* | 0.21 |
| TSMP | 0.15+ | 0.49 | 0.09+ | 0.01* | 0.02 | 0.02 | 0.43 |
| SIP | 0 | 0.58 | 0 | 0 | 0 | 0 | 0.34 |
| Mean | 0.25 | 0.42 | 0.18 | 0.01 | 0.008 | 0.06 | 0.30 |
Notes:
- TARP
-
Tunku Abdul Rahman Park
- TSMP
-
Tun Sakaran Marine Park
- SIP
-
Sipadan Island Park
- Disease
-
All diseases
- WS
-
White syndrome
- BrB
-
Brown band disease
- BBD
-
Black Band Disease
- AtN
-
Atramentous Necrosis
- PR
-
Pigmentation
Coral cover
Mean coral cover in TARP, TSMP and SIP was 50.1 ± 14.9%, 46.6 ± 13.7% and 68.1 ± 6.1% respectively (mean ± s.d.), with no significant difference between the three park sites in the relationship of coral cover and frequency of all diseases. A positive relationship was found between coral cover and disease at TARP (r2 = 0.48, p < 0.01, Fig. 3) but not at TSMP.
Discussion
Disease variation
White Syndrome (WS) was observed at the highest frequency of all diseases recorded, especially at TARP, where the number of colonies per m2 was four times higher than at TSMP. Some mis-categorisation of observed WS-like signs may explain some of this variance as predation, bleaching, white spot diseases, atramentous necrosis, brown band disease and skeletal eroding band are often mistaken for WS during rapid disease surveys (Willis, Page & Dinsdale, 2004; Lindop, Hind & Bythell, 2009). However, such significant differences between parks is unlikely to be explained by erroneous identification alone, given that the same observers conducted all surveys, photographs of diseased colonies were verified by one researcher after each survey and no diseases were observed at SIP.
The frequency of corals with bleaching and pigmentation response (PR) was higher in SIP than at either TARP or TSMP, although the differences were not significant. PR is thought to be an indicator of immune function in corals through the expression of a fluorescent protein linked to immune responses (Palmer, Mydlarz & Willis, 2008; Palmer, Roth & Gates, 2009), and frequently results from parasite infections and predation scars (Willis, Page & Dinsdale, 2004; Aeby, 2007). The higher PR frequency observed in SIP could therefore be explained by a greater predation on corals by fishes, whose populations are promoted due to the more effective park protection at SIP, possibly due to logistics of policing such a small park area. In comparison, despite being gazetted for over 30 years, there are still local fishing villages situated within TARP boundaries and illegal blast fishing and poaching does continue (particularly at the park edges and borders), in addition to the permitted hook-and-line fishing throughout the park (Wood, 2001a; Wood, 2001b). TSMP was established in 2005, and so relatively recently in terms of the reduction of fishing pressure. However, no fishing has been permitted, under strict enforcement in SIP, since the island became protected in 1963. The presence of the White Syndrome at TARP and TSMP is more concerning than the occurrence of Pigmentation Response, due to the recognized mortality associated with White Syndrome (Willis, Page & Dinsdale, 2004; Bourne et al., 2015). Although in the present study we did not have resources to carry out more than one survey, in future, surveys should be repeated to verify pigmentation responses observed is not a precursor for white syndrome or other disease.
Coral cover relationships
Coral cover and disease was positively correlated only at TARP. Some previous studies that have surveyed coral disease on a frequency per unit reef area basis did not record coral cover during field surveys (e.g., Haapkyl et al., 2007) whilst others did not test the relationship of coral cover and disease prevalence, either comparatively between surveyed sites or as an overall correlation (e.g., Kaczmarsky, 2006; Bruckner & Hill, 2009). Without such analyses, it is difficult to compare between studies and between sites/times since possible variations in host density are unknown. The variation in the relationship between host density (coral cover), and disease frequency has been shown in this study to be a useful indicator in itself. Higher disease frequency per unit coral cover at TARP indicates that factors other than host density are influencing the presence of coral disease at this park.
Inter-park environmental variance
Human disturbances, such as habitat fragmentation, pollution and sedimentation, are predicted to reduce reef resilience to natural stressors (Wilkinson, Souter & Goldberg, 2006), which subsequently may reduce the immune defence capacity of the coral holobiont against diseases (Reshef et al., 2006; Ritchie, 2006). Sedimentation and nutrient-rich effluent from intensive coastal-based agriculture (especially around the capital city of Sabah, Kota Kinabalu (Jakobsen et al., 2007)) just 3 km from TARP) and turbidity levels as high as 39.3 Nephelometric Turbidity Units (Anton et al., 2008), may explain the higher disease frequency observed at TARP compared to the other two sites. In addition to indirect stress effects, there is a potential for direct introduction of coral disease pathogens via human effluent, which has been recorded to be discharged into Kota Kinabalu’s rivers and therefore estuaries (Sakari et al., 2012), and has recently been associated with the onset of Caribbean White Spot disease in Elkhorn coral (Sutherland et al., 2010). Kaczmarsky, Draud & Williams (2005) also recorded around ten times higher disease prevalence of corals in waters in the US Virgin Islands with a sewage pollution indicator microbe species compared to those without (Kaczmarsky, Draud & Williams, 2005). The lack of an efficient sewage filtration system at Kota Kinabalu (Jakobsen et al., 2007) may therefore directly affect coral disease prevalence at sites close by. However, further investigation into the links between coral disease with quantified gradients of relevant pollutants, contaminants or indicator microbes, would need to be carried out to verify whether or not such influences are occurring in Sabah. Additionally, the more open access to TARP and lower ratio of staff to park area to police tourism and resident activity may increase damage to reefs from SCUBA divers, boat anchoring and other activities which have been linked to increased disease prevalence (Lamb et al., 2014), whereas SCUBA diving and boat anchoring is strictly regulated and controlled in SIP.
In conclusion, there were significantly greater coral disease frequencies in TARP, particularly WS and AtN compared to the other two sites, TSMP and SIP. The relationships between disease frequencies and coral cover was found to be different at TARP from TSMP and SIP inferring that inter-park variance in disease may be due to factors other than host density. The proximity of TARP to the developing coastline of Kota Kinabalu and associated anthropogenic pressures such as reduced water quality, along with high numbers of tourist and illegal fishing activity appear the most likely explanation for increased disease frequency at this site, but further research into these influences are needed. The apparent absence of recognised diseases at the most effectively protected, offshore site (SIP), is encouraging and the results suggest that bleaching and pigmentation responses are not necessarily indicative of overall less healthy reefs.