Growth promotion and mycorrhizal colonization of Argan (Argania spinosa (L.) Skeels) inoculated with the edible desert truffle Tirmania nivea (Desf.) Trappe
- Published
- Accepted
- Received
- Academic Editor
- Curtis Daehler
- Subject Areas
- Agricultural Science, Biotechnology, Mycology, Plant Science
- Keywords
- Desert truffles, Tirmania, Argania spinosa, Endomycorrhiza, Host plant growth
- Copyright
- © 2022 Khrizi et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Growth promotion and mycorrhizal colonization of Argan (Argania spinosa (L.) Skeels) inoculated with the edible desert truffle Tirmania nivea (Desf.) Trappe. PeerJ 10:e13769 https://doi.org/10.7717/peerj.13769
Abstract
This study presents the first evidence of the mycorrhizal compatibility between the edible desert truffle Tirmania nivea and the valuable fruit tree Argania spinosa. Seed germination trials demonstrated that soaking pre-treatment of argan seeds in hydrogen peroxide (9%) for five days combined with the application of a fungicide treatment on an inert sowing material maximized the seed germination of this tree species. The mycorrhizal synthesis was conducted under greenhouse conditions by inoculating, in vivo, the host plant seedlings with spores of T. nivea. The growth and mycorrhizal status of A. spinosa was assessed 15.5 months after inoculation. The desert truffle mycorrhization significantly promoted all the investigated morphological parameters of growth and improved the physiological performances of the host plant through enhancing plant water status and chlorophyll concentration. The mycorrhizal symbiosis led to the formation of typical desert truffle endomycorrhizae with intracellular coils. The resistance of A. spinosa to the harsh environmental conditions of desert habitats makes it a potential candidate for cultivation of desert truffles.
Introduction
Desert truffles are a vast family of hypogeous ascomycetes fungi delimited to taxa members of the genera Terfezia, Tirmania, Picoa, Carbomyces, Elderia, Eremiomyces, Kalaharituber, Mattirolomyces, Mycoclelandia, Stouffera and Ulurua (Kovács & Trappe, 2014). They are endemic to semi-arid and arid areas, mostly the countries around the Mediterranean basin, North-Africa and the Middle East. Desert truffles constitute an important part of the mycological flora of Algeria where they are represented by three genera Terfezia, Tirmania and Picoa (Fortas & Chevalier, 1992; Zitouni-Haouar, Fortas & Chevalier, 2014; Zitouni-Haouar et al., 2015; Zitouni-Haouar et al., 2018). These seasonal mushrooms cannot be regarded as a predictable crop since their fructification is strongly conditioned by a certain threshold of precipitation. Their ascomata (fruiting bodies) usually appear in December and they can still be harvested until the end of June.
Species of the genus Tirmania belong to a commercially important group of edible mushrooms with considerable socio-economic interest. Due to their delicate flavor, musky smell, and soft white tissues, these mushroom species are considered as the most highly prized desert truffles in the Middle East where they are better known as white truffles or Zubaidi (Al-Rahmah, 2001; Mandeel & Al-Laith, 2007). In good desert truffles seasons, Tirmania crops are marketed at prices comparable to those of meat, and served, indeed, instead of meat. However, in poor harvesting seasons, they become a very costly delicacy (Alsheikh & Trappe, 1983). Furthermore, Tirmania species have, undoubtedly, always been an important staple of the diet of several populations not only for their gastronomic value but also for their nutritional interest. In fact, they proved to be a rich source of proteins, amino acids, carbohydrates, fatty acids and minerals (Sawaya et al., 1985; Bokhary, 1987; Bokhary, Suleiman & Basalah, 1989; Hussain & Al-Ruqaie, 1999; Al-Ruqaie, 2009; Hamza, Jdir & Zouari, 2016; Bouatia et al., 2018). Nutritionally, digestible and non-digestible carbohydrates make Tirmania nivea a very healthy food providing both energy and fiber which are essential for the function of the intestinal track (Al-Laith, 2014). Tirmania also has a prominent place in the ethnomedicine of the Arabian countries as it is frequently used by Bedouins for the treatment of some ophthalmic diseases (Alsheikh & Trappe, 1983). These traditional medicine claims were then validated by several works which highlighted the therapeutic potential of Tirmania species through their interesting antimicrobial, antiviral, anticancer, antioxidant and antiradical activities (Chellal & Lukasova, 1995; Al-Laith, 2010; Gouzi et al., 2011; Gouzi, Leboukh & Bouchouka, 2013; Neggaz & Fortas, 2013; Stojkovic et al., 2013; Hamza, Jdir & Zouari, 2016; Gargano et al., 2017; Schillaci et al., 2017; Owaid, Muslim & Hamad, 2018; Elsayed et al., 2019a; Elsayed et al., 2019b).
Tirmania spp. are obligatory symbiotic mycorrhizal fungi. Their life cycle is only achieved through colonization of a suitable host plant, which is mainly an annual or perennial Cistaceae species belonging to the genus Helianthemum. Some affinities for a specific pH preferred by the host plant were also observed for this group of desert truffles. This is the case of the alkaline preference of T. nivea in accordance with its host plant Helianthemum salicifolium, while Tirmania pinoyi displays a preference for the acidophilous Helianthemum species, H. guttatum (Chevalier, 2014).
Several types of mycorrhizae have been described in previous desert truffles mycorrhizal associations investigated under natural or experimental conditions. Most of these involved Terfezia or Picoa species and emphasized the mycorrhizal plasticity of desert truffles to form ectomycorrhizae, endomycorrhizae and even ectendomycorrhizae on roots of different phytosymbionts (Awameh, Alsheikh & Al-Ghawas, 1979; Awameh, 1981; Alsheikh, 1984; Chevalier et al., 1984; Dexheimer et al., 1985; Leduc, Dexheimer & Chevalier, 1986; Roth-Bejerano, Livne & Kagan-Zur, 1990; Fortas & Chevalier, 1992; Kagan-Zur et al., 1999; Gutiérrez, Morte & Honrubia, 2003; Kovács, Vágvölgyi & Oberwinkler, 2003; Zaretsky et al., 2006; Slama et al., 2010; Navarro-Ródenas et al., 2012; Navarro-Ródenas et al., 2013; Zitouni-Haouar, Fortas & Chevalier, 2014; Dafri & Beddiar, 2018). However, mycorrhizae of Tirmania species have been the least studied so far and only one work was conducted on the mycorrhizal status of T. nivea, reporting the formation of endomycorrhizae on Helianthemum ledifolium and H. salicifolium roots inoculated with this Tirmania species (Awameh, Alsheikh & Al-Ghawas, 1979). In light of these many experiments, several factors such as the fungal species (Chevalier et al., 1984), phosphorus substrate fertility (Fortas & Chevalier, 1992; Navarro-Ródenas et al., 2012), culture conditions (Gutiérrez, Morte & Honrubia, 2003), common mycorrhizal type of the host plant (Kovács, Vágvölgyi & Oberwinkler, 2003), auxin–phosphate interaction (Zaretsky et al., 2006), irrigation water availability (Navarro-Ródenas et al., 2013) or the host plant (Zitouni-Haouar, Fortas & Chevalier, 2014) were suggested to help explain structural variability of desert truffles mycorrhizae.
The symbiotic relationship between desert truffles and their host plants was the key for the domestication of these wild edible mushrooms through the production and field transplantation of the desert truffles-Helianthemum mycorrhizal seedlings (Slama et al., 2010; Morte, Honrubia & Gutiérrez, 2008; Morte et al., 2009; Morte et al., 2012; Morte et al., 2017; Morte, Gutiérrez & Ródenas, 2020). Since the first Terfezia ascomata were produced from a plantation of Helianthemum almeriense mycorrhizal plants in Spain, an increasing demand for this crop has prompted research into new strategies to enhance the production of these fungi. This will be possible by the selection and propagation of suitable mycorrhizal seedlings adapted to the cultivation sites (Morte & Andrino, 2014).
The cultivation of Terfezia spp. and Tirmania spp. is also compatible with woody crops like almond, cherry tree and olive trees, among others, to optimize land use and irrigation systems. This allows a double crop in the same plot (one tree-borne the other—hypogeous truffles) (Honrubia, Andrino & Morte, 2014). Although desert truffles were mostly harvested under Helianthemum species, Tirmania ascomata were frequently collected in the desert plains of Algeria near to Argania spinosa trees (A. Khrizi, 2017, personal communication). This woody species is a fruit tree with an enormous economic importance related especially to oil production destined for nutritional, medicinal and cosmetic purposes (Zunzunegui et al., 2010). It is perfectly suited to arid environments and can grow on poor, shallow soils. Furthermore, owing to its deep rooting system, it is considered as having a strong effect against erosion and desertification (Nouaim et al., 2002) which are the main environmental problems encountered in desert truffles natural habitats. Thus, the main objective of the present study was to assess and characterize the mycorrhizal potential of T. nivea for establishing an effective mycorrhizal symbiosis with A. spinosa with the aim of involving this tree host species in desert truffle mycorrhizal plant production for cultivating these prized edible fungi. Moreover, this symbiotic interaction opens the possibility of a double-cropping (desert truffles—Argan fruits) in the same field. The mycorrhization with desert truffles is also a precious tool for improving the growth and survival of the nursery-grown Argan seedlings which is still a challenge in the reforestation programs of this valuable tree species within its area of distribution.
Materials and Methods
Origin of the fungal material
Mature desert truffles ascomata were collected in April 2011 from Bechar province (a Saharan area, Southwest of Algeria), near their natural host plant Helianthemum lippii. The ascocarps were first morphologically characterized and then sun-dried for 2 months. The desert truffles exsiccata were finally preserved at room temperature in paper bags.
Morphological and molecular identification of the fungal material
Macromorphological characteristics including color, form and size of peridium and gleba were recorded from fresh ascomata. Micromorphological studies of asci and spores were conducted on rehydrated gleba sections cut from dried samples. Microscopic observations were performed in distilled water and Melzer’s reagent (Langeron, 1952). Ascospore and asci shape and dimensions were measured in distilled water mounts on at least 50 randomly selected mature spores using an Olympus CX22 microscope equipped with an ocular micrometer.
The most representative ascoma was chosen for the molecular characterization which was performed by sequencing the ITS region of the nuclear ribosomal DNA. The genomic DNA was extracted from 20 mg of exsiccata powder obtained after gleba grinding in liquid nitrogen using GF-1 Plant DNA Extraction Kit (Vivantis Technologies, USA) following the manufacturer’s instructions.
The polymerase chain reaction (PCR) amplification was carried out on the ITS1-5.8S-ITS2 region of the rDNA as described by Zitouni-Haouar et al. (2015), using the primers ITS1 and ITS4 (White et al., 1990). The amplification success was checked on 1% agarose gel stained with ethidium bromide. PCR products were sequenced with the same ITS primers at Eurofins Genomics (Ebersberg, Germany).
The sequence generated in this study was first compared with sequences deposited in public databases using the BLAST algorithm (Altschul et al., 1997), for its taxonomic affiliation and then deposited in GenBank (http://www.ncbi.nlm.nih.gov) under accession number MZ379289.
The morphological characterization and the BLAST analysis of the rDNA ITS sequences revealed the strong affiliation of the studied ascomata to the Tirmania nivea taxon.
Plant material
The seeds used in the present study came from ripe fruits of similar size harvested in July 2015 from Argan trees “A. spinosa” in the region of Tindouf (Southwest of Algeria, 27°40′00″N, 8°09′00″W and 450 m altitude).
Substrat used for the mycorrhizal synthesis
The soil used for the experiment was collected from Bechar (Southwest Algeria, 31°37′00″N, 2°13′00″W). It has been analyzed and found to present the following physico-chemical characteristics: pH = 7.12, organic matter = 0.563%, assimilable phosphorus = 83.47 ppm, N = 0.02%, AC = 0.5%, TC = 6.36%, EC = 0.201 ds/m. The soil texture analysis revealed its loamy-sand texture with mean composition of 80.6%, 15.16% and 4.24% for sand, silt and clay respectively. The collected soil was dried in the open air, sieved through a 2-mm mesh sieve to eliminate pebbles and large debris and then autoclaved for 1 h at 120 °C (Fortas, 1990).
Mycorrhizal synthesis and assessment
Seed germination tests of Argania spinosa
The Argan nuts containing the seeds were obtained by drying the fruits in the sun and then removing their pulp by hand (Nouaim et al., 2002; Benaouf, Miloudi & Belkhodja, 2014). The nuts were then stored in paper bags in the laboratory at ambient temperatures until they were sown. The argan seeds present hard shells that make the germination process complicated. In order to remedy this phenomenon, we have tested different combined seed treatments, inspired from several previous works, and based mostly on hydrogen peroxide and water soaking treatments which were applied directly on the nuts without removing their shells (Khelifi, Morsli & Khelifi-Slaoui, 1996; Berka & Harfouche, 2001; Nouaim et al., 2002; Miloudi & Belkhodja, 2009; Ikinci, 2014). The efficiency of the germination assays was also evaluated with the application of a fungicide SUMICO®, which was kindly provided by Dr. Gérard CHEVALIER. The different experiments conducted to enhance the Argan seed germination are indicated in Table 1. The germination was checked daily and evaluated on 100 seeds used for each experiment.
| Experiments | Prior chlorine disinfection treatment | Soaking solution | Soaking time | Soaking temperature | Sowing material | Sowing Temperature | Germination period | Cumulative seed germination % | Observations |
|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Disinfection of seed coat with chlorine bleach for few seconds and washing | Sterile water | 96 h | Room temperature (∼20 °C) | Sterile moistened cotton | 30 °C | 15 days | 30% | Fungal contamination |
| Experiment 2 | No prior disinfection | H2O2 (9.9%) | 72 h | 25 °C | Water agar medium | 25 °C | 10 days | 7% | Seed rot |
| Experiment 3 | No prior disinfection | H2O2 (9.9%) | 96 h | 25 °C | Water agar medium | 30 °C | One week | 18% | Seed rot |
| Experiment 4 | No prior disinfection | H2O2 (9.9%) | One week | 30 °C | Sterile moistened mold | Greenhouse temperature (∼20 °C–25 °C) | One month | 48% | Seed rot |
| Experiment 5 | No prior disinfection | H2O2 (9%) | 120 h | 30 °C | Sterile surgical compress humidified in permanence with SUMICO® fungicide treatment | 30 °C | 10 days | 80% | Maximum germination rate without any rot or seed contamination |
Experimental mycorrhizal inoculation
The mycorrhizal synthesis was realized in 400 cm3 polyethylene pots filled with the sterilized soil and amended with well-germinated seeds (seed rootlet length ≈ one cm) sown directly on the surface of the substrate and covered with a thin soil layer equaling the seeds volume. Inoculation was realized with a mature aqueous spore suspension of T. nivea prepared by blending rehydrated carpophore pieces. For each pot (containing 1 seedling), an ascospore suspension containing 1 g of rehydrated fruiting body in 50 ml of sterilized distilled water and with a concentration of approximately 1–2.8 × 109 spores was applied directly in contact with the seedling roots inside the pot according to the technique of Zitouni-Haouar, Fortas & Chevalier (2014). The production of mycorrhized plants was performed following the technique of Chevalier, Grente & Pollacsek (1973), Chevalier & Grente (1979) and Fortas & Chevalier (1992) applied for the mycorrhizal synthesis of Tuber melanosporum and desert truffles in genera Terfezia and Tirmania respectively. The pots containing uninoculated, control seedlings received 50 mL of distilled water instead of the ascospore suspension. Seedlings were then grown in non-conditioned greenhouse under natural lighting from March until their harvest in June of the next year (15 months and a half). The plants were watered during the first month of the experimentation with sterilized water and then were periodically irrigated when necessary with tap water.
Mycorrhiza observation and characterization
After 15.5 months of culture, both the inoculated seedlings and non-inoculated controls were carefully removed from their pots and their root systems gently freed from soil particles by their immersion for a half-hour in individual water containers in order to minimize the loss or the physical disturbance of the mycorrhizal roots. The whole root systems were then examined using a stereomicroscope to identify the macro-morphological pattern of T. nivea mycorrhizae. The mycorrhizal type was subsequently determined under a light microscope on root pieces of 1-cm length randomly sampled from the root systems of inoculated seedlings. The root segments were prepared according to Phillips & Hayman (1970) protocol, modified slightly, with 2 h heating in 10% KOH clearing solution and 1 h staining at 90 °C in lactophenol-0.1% trypan blue. A randomly selected aliquot of fifty stained root fragments was then lightly rinsed with distilled water and mounted in lactoglycerol (v/v) on each of five glass microscope slides (10 per slide). The percentage of the mycorrhizal colonization was determined according to the frequency of infection (F (%)) which is expressed by: F % = 100 (N − N0)/N; where N is the total number of observed root fragments and N0 is the number of root fragments uninfected (Trouvelot, Kough & Gianinazzi-Pearson, 1986).
Seedling growth assessment
At the end of the experimental period, ten seedlings randomly selected from each treatment were harvested for the evaluation of seedling growth. The effect of mycorrhization on plant growth was estimated by measuring the following morphological traits: plant height, leaf number, leaf length, shoot fresh weight, shoot dry weight and root fresh weight. The shoot dry weights were determined after oven drying the material at 60 °C in an air-forced oven for 72 h.
Mycorrhizal growth response and mycorrhizal growth dependency
Mycorrhizal growth response and mycorrhizal growth dependency were estimated from the shoot dry weight of the inoculated and control plants. The mycorrhizal growth response (MGR) was calculated according to Hetrick, Wilson & Cox (1992) with the following equation: MGR % = [(dry weight of mycorrhizal plants − dry weight of non-mycorrhizal plants)/dry weight of non-mycorrhizal plants] × 100. The mycorrhizal growth dependency (MGD) was determined by expressing the difference between the average dry weight of mycorrhizal plants and the average dry weight of the non-mycorrhizal plants as a percentage of the dry weight of the mycorrhizal plants (Plenchette, Fortin & Furlan, 1983).
Photosynthetic pigments concentrations
Chlorophyll a and b were estimated from the freshly harvested plants according to Navarro-Ródenas et al. (2013). The chlorophyllous pigments were extracted by grinding 0.05 g of leaf tissue in 5 ml of 80% aqueous acetone using a mortar and pestle at 4 °C. The resulting extracts were centrifuged at 10,000 g for 10 min and the absorbance of the solution was measured by UV–Visible spectrophotometry at 646 and 663 nm. The chlorophylls concentrations were then calculated using the equations of Wellburn (1994):
Chlorophyll a (Ca) = 12.21 × A663 − 2.81 × A646
Chlorophyll b (Cb) = 20.13 × A646 − 5.03 × A663.
The content of the total chlorophyll was expressed in milligram per gram (mg g−1) of fresh weight.
Analysis of plant water status
Leaf relative water content (RWC) was estimated according to Barrs (1968). A composite sample of 10 leaf discs is taken and the fresh weight is recorded. The turgid weight is then determined after saturation of leaf tissue in distilled water for 24 h at 4 °C. The dry mass weight is subsequently evaluated after oven-drying the leaf samples to a constant weight at 80 °C for 24 h. Relative water content is calculated using the following formula: RWC (%) = (fresh weight − dry weight)/(turgid weight − dry weight) × 100.
Leaf hydration was determined according to Alessio et al. (2008) as H (%) = (FW − DW)/DW × 100; where FW is leaf fresh weight and DW is leaf dry weight. Dry weight was measured after drying leaves in an oven at 70 °C for 72 h (until constant weight was reached).
Statistical analyses
The Morpho- and physiological data were analyzed using a Student’s t-test. Computations were carried out using the statistical software STATISTICA (version 10.0, StatSoft) for Windows. Differences among treatments were considered significant at p < 0.05.
Results
Evaluation of seed germination of Argania spinosa
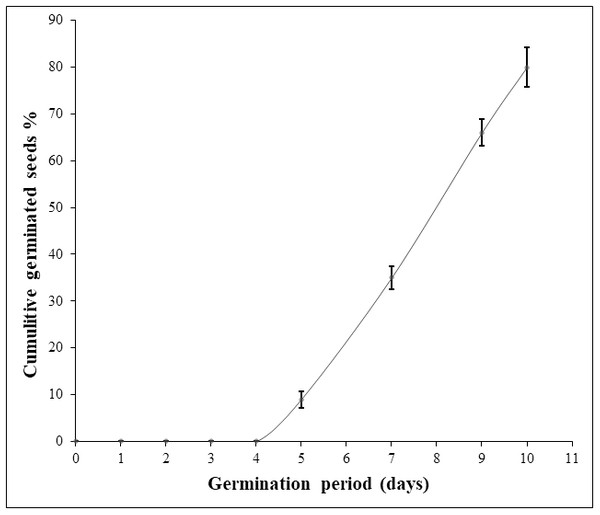
Considering the first experiment, disinfection of seed coat with chlorine bleach followed by sterile water soaking and sowing on sterile moistened cotton led to a mediocre germination percentage (30%) with fungi-seed contamination. Applying hydrogen peroxide (9.9%) for seed surface-sterilization and as a soaking solution gave better results in terms of inhibition of fungal contamination. Nevertheless, this treatment resulted in seed rot except when a fungicide was applied. In the second and the third experiments, soaking seeds in hydrogen peroxide (9.9%) for 72h-96 h on water agar medium gave a very low germination rate (7%, 18%). Hydrogen peroxide soaking time was a significant source of variation for germination percentage and germination period. For experiment 4, the increasing of hydrogen peroxide soaking time to one week raised the germination percentage to 48%. However, the change in sowing conditions including mold as a sowing material and unstabilized ambient temperature extended the germination period to one month (Table 1). In the last experiment, the germination rate was significantly enhanced to the maximum (80%), ten days after seed soaking in hydrogen peroxide (9%) for 120 h at 30 °C combined with SUMICO® fungicide treatment on sterile moistened surgical compress. Thus, the use of a fungicide treatment suppressed seed deterioration and fungal contamination (Table 1; Fig. 1). Moreover, when both hydrogen peroxide (9%) and fungicide treatments were combined, seed dormancy was reduced considerably to five days (Fig. 1). It is important to notice that a germination rate of 100% was not achieved in any treatments, probably due to non-viability of some seeds.
Figure 1: Daily Argan seed germination rate of the best experiment (experiment 5).
The germination assay was performed on two seed batches (replicates) of 50 seeds each and the germination percentage values were used to calculate the standard deviation (SD).Morphology and frequency of the mycorrhizal colonization
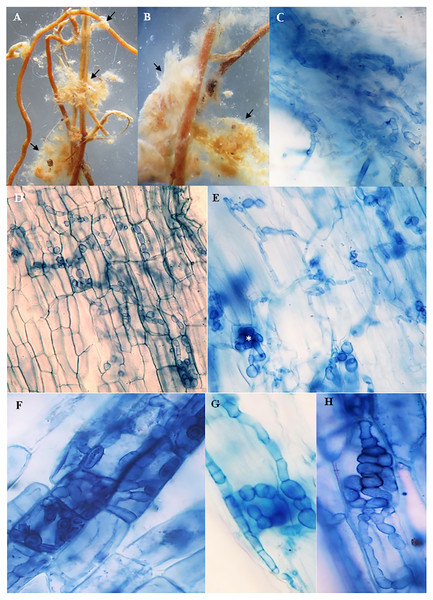
The roots of inoculated and non-inoculated seedlings of A. spinosa were examined fifteen months and a half after in vivo inoculation with T. nivea. Under stereomicroscope, the freshly collected inoculated roots appeared slightly swollen at the tips compared with uncolonized ones. Compact yellowish mycelium masses were also observed exclusively around the surface of the mycorrhizal roots (Figs. 2A and 2B). At the light microscope level, the cortical cells of the stained inoculated roots were colonized by hyphal coils and desert truffles endomycorrhizae. Indeed, T. nivea hyphae developed densely on the host root surface (Fig. 2C) and colonized intracellularly the inside of A. spinosa cortex cells by producing coiled hyphae (Figs. 2D and 2H). The A. spinosa inoculated roots were highly colonized; T. nivea mycorrhizae were detected in 74% of A. spinosa infected root samples (Table 2) whereas the control roots showed no evidence of mycorrhizal infection.
Figure 2: Mycorrhizal colonization of Argania spinosa roots inoculated with Tirmania nivea.
(A, B) Macromorphological characters of mycorrhizal roots covered with compact yellowish mycelium masses, observed under stereomicroscope (arrows). (C) Inoculated roots invaded by dense T. nivea hyphae. (D–H) Intracellular colonization of cortical cells by T. nivea swelling hyphae forming coils (asterisks). All microscopic images ×640.Effects of mycorrhization on morpho-physiological growth parameters of the host plant
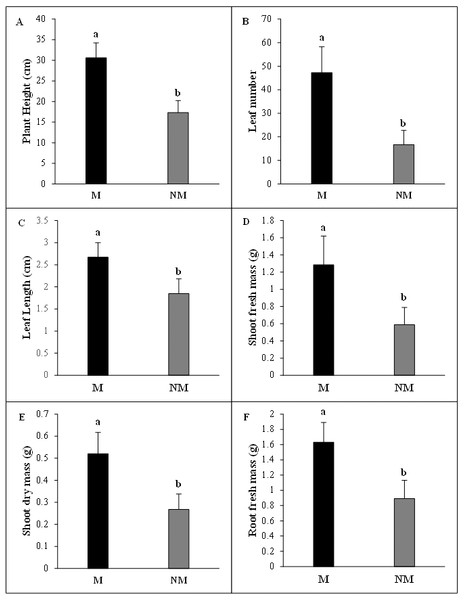
The growth of A. spinosa mycorrhizal plants inoculated with T. nivea was significantly increased relative to that of uninoculated seedlings. The improvement in plant height was notably greater (P < 0.001) in inoculated plants than the controls. The number of leaves and mean leaf length were substantially increased in mycorrhizal plants by 2.83 and 1.45 folds, respectively, compared to the non-mycorrhizal ones. Moreover, the fresh green and the dry biomasses of the shoot and the density of the fresh root systems were sharply promoted by the twofold (P < 0.05) in inoculated plants in comparison with uninoculated control seedlings (Fig. 3). The high mycorrhizal growth response index (94.32%) highlighted the growth-stimulating effect of the mycorrhization by T. nivea on A. spinosa seedlings. In addition, the calculated value of the mycorrhizal growth dependency index (48.53%) reflected the positive response of A. spinosa to desert truffle mycorrhizal symbiosis (Table 2).
| Combination | MC (%) | MGR (%) | MGD (%) |
|---|---|---|---|
| AS/TN | 74 | 94.32 | 48.53 |
Figure 3: Effect of inoculation with Tirmania nivea on morphological growth parameters of mycorrhizal (M) and non-mycorrhizal plants (NM) of Argania spinosa.
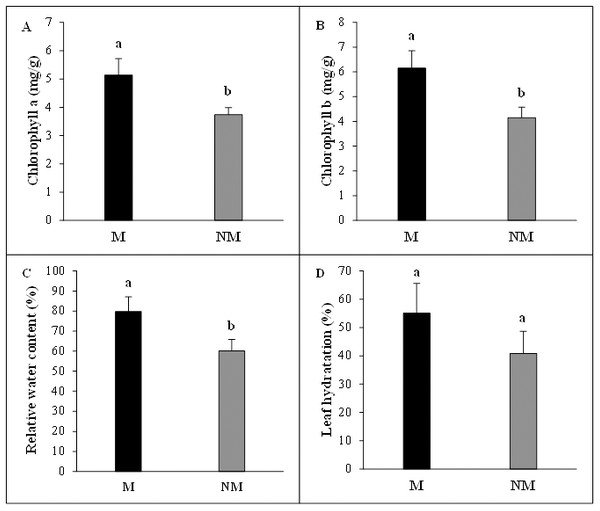
(A) Plant height, (B) leaf number, (C) leaf length, (D) shoot fresh weight (FW), (E) root fresh weight (FW), (F) shoot dry weight (DW).The mycorrhizal status had vital beneficial effects on photosynthetic pigments concentrations of A. spinosa seedlings. Chlorophyll a concentration per leaf fresh weight was significantly higher (P < 0.05) and increased by 37% in mycorrhizal plants compared to non-mycorrhizal plants. The chlorophyll b content in mycorrhizal plants presented a 48% increase above non-mycorrhizal ones, showing clearly once more the considerable contribution of mycorrhization to the improvement of photosynthesis activity in mycorrhizal seedlings. Moreover, the desert truffle mycorrhizal infection positively affected the tissue water status of A. spinosa. In fact, the leaves relative water content (RWC) values showed that the mycorrhizal plants contained ≈ 20% more water than controls and the leaf hydration amounts were higher by 14% in inoculated seedlings in comparison with non-inoculated ones (Fig. 4).
Figure 4: Effect of inoculation with Tirmania nivea on physiological growth parameters of mycorrhizal (M) and non-mycorrhizal plants (NM) of Argania spinosa.
(A) Chlorophyll a content, (B) chlorophyll b content, (C) relative water content, (D) leaf hydration.Discussion
The present study highlighted the importance of the use of hydrogen peroxide (9%) instead of water as soaking pre-treatment at 30 °C combined with the application of an effective fungicide (SUMICO®) treatment on an inert sowing material for the success of Argan seed germination. The results of this work presented a simple, efficient and repeatable method ensuring a high argan seed germination rate and an enhanced seedling growth. It is important to point out that the success of seed germination and the establishment of vigorous seedlings in nurseries are crucial factors for the multiplication and propagation of A. spinosa, a multipurpose tree species of great economic, agronomic and ecological interests, but nevertheless, known for the complexity of its seed germination process.
The findings of this study clearly indicated that argan seed dormancy can be broken and germination is hastened using hydrogen peroxide (9%–9.9%) as a soaking pre-treatment at 30 °C. Furthermore, the seed germination increase seems to correlate well with the duration of soaking. Hydrogen peroxide plays a vital role in seed germination and plant growth development as well as the acquisition of stress tolerance (Li, Gong & Liu, 2012). Berka & Harfouche (2001) showed that hydrogen peroxide (3%) and warm water appear to be the best soaking pre-treatments to suppress the tegumentary inhibition of A. spinosa seeds. These authors demonstrated also that temperature appears to be a limiting factor in argan seed germination. Indeed, at high temperatures (25–28 °C), germinative rate and speed of germination are higher than at temperatures below 25 °C. The stimulating effect of hydrogen peroxide on seed germination has been also observed in several tree species such as Pseudotsuga menziesii (Ching, 1959), Pinus roxburghii (Ghildiyal, Sharma & Khanduri, 2007) or Prunus scoparia (Imani et al., 2011). On the other hand, the application of SUMICO® fungicide in this work appears to promote and speed up germination avoiding the deceleration and inhibition of germination engendered by the exposure of seeds to contamination. Moreover, the use of surgical compress seems to minimize fungal contamination and reinforce the fungicidal action. Our findings are in good agreement with the results of Bani-Aameur & Alouani (1999) who found that treatment with the fungicide thiram increased argan seed germination and its application is necessary to protect the nut, when the shell is scarified.
The mycorrhizal response of A. spinosa seedlings to infection by the desert truffle species T. nivea was assessed fifteen months and a half after in vivo inoculation. The mycorrhizal roots of A. spinosa were invaded by the irregular lobed septate hyphae of T. nivea which formed intracellular coils. These endomycorrhizal structures were discontinuously present throughout the whole colonized roots. The previous works investigating the mycorrhizal systems produced by desert truffles have reported their exceptional mycorrhizal plasticity. In fact, these fungi can form the three major types of mycorrhizae, i.e., ectomycorrhizae, endomycorrhizae and ectendomycorrhizae either in vivo or in vitro culture conditions. However, this structural mycorrhizal variability was observed in response to biotic factors related to the mycosymbiont partner (Chevalier et al., 1984) or the phytosymbiont species involved in the mycorrhizal interaction (Zitouni-Haouar, Fortas & Chevalier, 2014) and its common mycorrhizal type (Kovács, Vágvölgyi & Oberwinkler, 2003). In addition, the desert truffles transition between ecto-, endo-, and ectendomycorrhizal types was also influenced by abiotic elements relevant to the level and the source of phosphorus in the culture substrate (Fortas & Chevalier, 1992; Navarro-Ródenas et al., 2012), the culture conditions of the mycorrhizal synthesis (Gutiérrez, Morte & Honrubia, 2003), the interdependency between exogenous phosphate and auxin levels (Zaretsky et al., 2006) or drought stress conditions (Navarro-Ródenas et al., 2013). This last factor is consistent with our suggestion that the bioclimatic origin of the host plant may determine the mycorrhizal type formed by desert truffle. Indeed, our experiment was conducted on A. spinosa, a host plant native to arid and desert bioclimatic niche well known by water scarcity conditions. Adaptation and surviving to water-stress episodes imply the adoption of a suitable mycorrhizal type allowing resistance to drought. Navarro-Ródenas et al. (2013) reported a morpho-physiological adaptation of Terfezia claveryi-H. almeriense symbiosis to drought conditions by forming endomycorrhizae. The results of these authors demonstrated that irrigation water scarcity induced a change in the mycorrhizal type formed by desert truffle, which was more intracellular under drought stress.
The high mycorrhizal colonization rate obtained in the present study provides strong evidence of the intimate interaction and the good mycorrhizal compatibility between T. nivea and A. spinosa. The common harvest of this desert truffle species close to A. spinosa trees in desert lands of Algeria also supports a probable mycorrhizal relationship between these two symbiotic partners in their natural biotope.
The T. nivea inoculation conferred significant developmental benefits to A. spinosa mycorrhizal seedlings compared to non-mycorrhizal ones. The results of the present work provide the first evidence of the positive effect of T. nivea mycorrhizae on the growth and development of a valuable fruit tree. Desert truffle mycorrhization successfully increased A. spinosa plant height, leaves number and length and green and dry biomasses. The data presented in this study are in perfect congruence with previous work performed on desert truffle mycorrhizal associations. The results of these investigations clearly demonstrated the positive correlation between root colonization by desert truffles mycorrhizae and enhanced growth attributes of the respective host plant most notably Helianthemum spp. In fact, Awameh (1981) reported an important increase in number of leaves and plant dimensions of the herbaceous Helianthemum, H. salicifolium and H. ledifolium plants inoculated by the desert truffle species Terfezia boudieri. In a similar work, Helianthemum sessiliflorum-T.boudieri mycorrhizal plants gained significantly more biomass, based on both fresh and dry weights, than did non mycorrhizal ones. The shoot to root ratio, (based on dry weights) was about twofold higher. Inoculation with T. boudieri enabled larger canopy development relative to root development, an outcome of the mycorrhizal support in the mycorrhized plants (Turgeman et al., 2011). Moreover, Zitouni-Haouar, Fortas & Chevalier (2014) demonstrated the significant beneficial effect exerted by the in vivo mycorrhization of Terfezia leptoderma, T. boudieri and T. claveryi on plant height, leaf number and length, and shoot dry matter values of several annual and perennial Cistaceae species from the genera Helianthemum, Cistus and Fumana, in addition to the forest tree species Pinus halepensis. However, some abiotic factors such as the phosphorus source or fungal secreted auxin seem to influence the growth response of the mycorrhizal host plants. Indeed, Navarro-Ródenas et al. (2012) investigated the effect of T. claveryi inoculation on the in vitro growth of H. almeriense with different phosphorus sources. A significant effect of the phosphorus source on the shoot growth was detected. Both phytate and inorganic phosphorus treatments reduced the shoot growth of the mycorrhizal plants compared to medium without any source of phosphorus. Turgeman et al. (2015) demonstrated moreover that H. sessiliflorum exposure to high levels of auxin secreted by T. boudieri at early stages of host development produced considerable slowing of seedling growth during the mycorrhizal establishment.
On the other hand, the high rate of the mycorrhizal growth response index obtained in the present study for A. spinosa mycorrhizal seedlings is a pure reflection of the nutritional benefits gained from mycorrhizae which are recognized as improved access to limiting soil resources, most notably immobile nutrients (e.g., P, Cu, Zn, and ammonium) (Johnson, Graham & Smith, 1997). The abilities of mycorrhizal species and strains to promote plant growth opened new perspectives for the use of these fungi inoculations in nurseries and forestry (Martins, 2008). The mycorrhizal growth dependency (MGD) index value derived from this work (48.53%) is in good agreement with previous results obtained by Zitouni-Haouar, Fortas & Chevalier (2014) for a similar forest tree species P. halepensis (47.27%) mycorrhized by T. leptoderma. However, these authors found much higher values of MGD for other herbaceous and woody Cistaceae species. The mycorrhizal dependency or “responsiveness” was defined earlier by Gerdemann (1975) as the degree to which a plant species is dependent on the mycorrhizal condition to produce its maximum growth at a given level of soil fertility.
Inoculation with the desert truffle T. nivea and subsequent mycorrhizal formation contributed effectively to the improvement of the physiological performance of A. spinosa. Chlorophyll contents were considerably enhanced in the leaves of mycorrhizal plants compared to controls. This is particularly observed for chlorophyll b concentrations which were much higher in mycorrhizal plants than those of chlorophyll a. These results are in agreement with previous studies showing the significant enhancement in chlorophyll concentrations of desert truffles-Helianthemum spp. mycorrhizal seedlings than in controls (Turgeman et al., 2011; Navarro-Ródenas et al., 2013) with a specific increase in chlorophyll b content compared to chlorophyll a (2.4- and 1.52-fold, respectively) (Turgeman et al., 2011). The enhanced competence of mycorrhizal plants to persevere in the harsh environmental conditions of deserts is the outcome of special adaptations, including increased Chl b content, lower photosynthetic activation energy, and enhanced stomatal conductance, that alter and improve the physiological performances of the host plant (Turgeman et al., 2011).
Water status plays a major role in the growth and physiological processes of plants. Mycorrhizal and non-mycorrhizal plants frequently exhibit differences in water status (Augé, 2001). RWC and leaf hydration represent important criteria for the evaluation of plant water status. RWC is a useful indicator of the state of water balance of a plant essentially because it expresses the absolute amount of water, which the plant requires to reach artificial full saturation (González & González-Vilar, 2001). In this study, mycorrhizal plants exhibited better RWC values than their corresponding non-mycorrhizal plants. This finding showed that T. nivea fungal hyphae increased the water uptake of A. spinosa mycorrhizal roots which could further substantiate the potential of this host plant to grow and survive in the severe environmental conditions of its desert habitat. The improvement of water status via mycorrhizal symbiosis could play an indirect role in enhancing nutrient uptake, osmotic adjustment and the capacity for gas exchange (Zhu, Song & Xu, 2010). Our data are consistent with an earlier work performed by Turgeman et al. (2011) who reported higher rates of transpiration in H. sessiliflorum plants inoculated by T. boudieri than in control plants reflecting, thus, an increase in water uptake of mycorrhizal seedlings. Desert truffle mycorrhizae are also well known for increasing effective root hydraulic conductivity and survival rate of mycorrhizal plants under drought-stress conditions (Morte, Lovisolo & Schubert, 2000), providing the plant a greater capacity to tolerate limited soil water availability (Morte, Navarro-Ródenas & Nicolas, 2010).
Conclusion
This work demonstrated the success of the in vivo mycorrhizal association between the highly prized edible mushroom T. nivea and the valuable host tree A. spinosa. The symbiotic association exerted a beneficial effect on the growth and physiological parameters of the host plant. The mycorrhizal compatibility of Argan tree with desert truffles and its strong adaptation to the hostile conditions of the desert truffles arid biotopes make it a potential phytosymbiont for the cultivation projects of these widely appreciated edible mushrooms while it can contribute simultaneously to afforestation and ecosystem restoration of its native degraded habitats.
Supplemental Information
Tirmania nivea ITS_Sequence MZ379289
This is an ab1 file (ABI sequencer data file) of the Tirmania nivea rDNA ITS sequence. This file can be viewed with the chromas software which is freely available on the link: http://technelysium.com.au/wp/chromas/
Raw data
Morphological growth parameters (height, leaf number, leaf length, shoot fresh weight “FW”, shoot dry weight “DW” and root fresh weight “FW”), and physiological growth parameters (chlorophyll a content, chlorophyll b content, relative water content, leaf hydration,) of A. spinosa (AS) plants after 15.5 months of inoculation with T. nivea (TN) (Raw Data)