Diversity of an uncommon elastic hypersaline microbial mat along a small-scale transect
- Published
- Accepted
- Received
- Academic Editor
- Michael Beman
- Subject Areas
- Biodiversity, Biogeography, Ecology, Microbiology
- Keywords
- Cuatro Ciénegas Coahuila, Microbial communities, Phylogenetic diversity, Rare biosphere, Metabolome analysis
- Copyright
- © 2022 Espinosa-Asuar et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Diversity of an uncommon elastic hypersaline microbial mat along a small-scale transect. PeerJ 10:e13579 https://doi.org/10.7717/peerj.13579
Abstract
We evaluated the microbial diversity and metabolome profile of an uncommon hypersaline elastic microbial mat from Cuatro Ciénegas Basin (CCB) in the Chihuahuan Desert of Coahuila, México. We collected ten samples on a small scale transect (1.5-m) and described its microbial diversity through NGS-based ITS and 16S rDNA gene sequencing. A very low number of taxa comprised a considerable proportion of the mat and were shared across all sampling points, whereas the rare biosphere was more phylogenetically diverse (Faith’s Phylogenetic Diversity (FPD) index) and phylogenetically disperse (using a null model distribution of Phylogenetic Species Clustering (nmdPSC)) than the abundant (high read count) taxa for both analyzed libraries. We also found a distinctive metabolome profile for each sample and were able to tentatively annotate several classes of compounds with relevant biological properties.
Introduction
Microbial mats are tightly interacting communities, usually self-sustaining, that are horizontally stratified with multilayered biofilms embedded in a matrix of exopolysaccharides and inorganic substances that bind cells together (Bolhuis, Cretoiu & Stal, 2014; Prieto-Barajas, Valencia-Cantero & Santoyo, 2018; Stolz, 2000). These stratified structures, as well as stromatolites, are the oldest communities found in the fossil record, informing our understanding of ancient metabolic interactions (Gutiérrez-Preciado et al., 2018; Nutman et al., 2016).
Although modern microbial mats and stromatolites are geographically widespread (Gerdes, 2010), their distribution is restricted to extreme environmental conditions where multicellular algae and grazing organisms cannot grow (Bebout et al., 2002). One such site, with a high diversity of microbial mats and stromatolites, is the desert oasis of the Cuatro Ciénegas Basin (CCB), found in the Chihuahuan Desert of the state of Coahuila in northern México. This small site (150,000 km2) is extremely biodiverse—in particular for microbes (Souza, Olmedo-Álvarez & Eguiarte, 2018)—and is considered of international importance by the RAMSAR convention (www.ramsar.org). CCB is characterized by an imbalanced stoichiometry (i.e., very low concentration of phosphorus) (Elser et al., 2005, 2006; Papineau, 2010; Planavsky et al., 2010), which appears to be a strong selective pressure driving microbial lineages to adapt locally (e.g., the reported Bacillus coahuilensis genes involved in phosphorous utilization efficiency (Alcaraz et al., 2008)). The uniqueness of microbial life in CCB—deemed as an “Astrobiological Precambrian Park” (Souza et al., 2012)—resides in the presence of taxa related to marine lineages (Rebollar et al., 2012; Souza et al., 2006; Souza, Olmedo-Álvarez & Eguiarte, 2018), rich prokaryotic communities (López-Lozano et al., 2013; Pajares et al., 2012, 2013; Souza, Olmedo-Álvarez & Eguiarte, 2018), and diverse fungal (Velez et al., 2016) and viral communities (Taboada et al., 2018), as well as a high microbial diversity within the microbial mats and stromatolites (Bonilla-Rosso et al., 2012; De Anda et al., 2018; Peimbert et al., 2012).
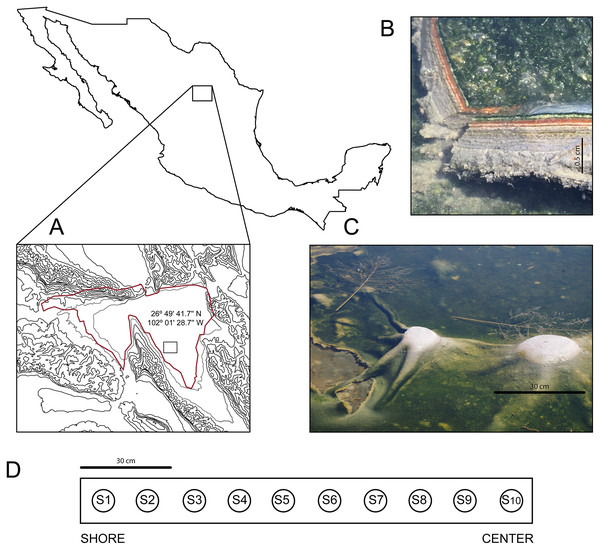
In March 2016, we discovered an outermost elastic layer microbial mat in CCB, which enables the formation of dome-like structures under wet conditions (Fig. 1).
Figure 1: Site and sample.
(A) The Cuatro Ciénegas Basin within México and geographic location of the Archaean Domes in Pozas Azules Ranch. (B) Details of an Archaean Domes slice. (C) Domes when the sampling was conducted (note the size scale). (D) Sampling design along border toward center 1.5-m transect (sample 1 (S1) to sample 10 (S10)). Photo credit: David Jaramillo.The internal microbial layer, insulated by an outer aerobic community, thrives under an anaerobic environment, rich in methane, hydrogen sulfide, and small volatile hydrocarbons that recreate the atmosphere of the Archaean Eon, hence we named these mats “Archaean Domes” (Medina-Chávez et al., 2019). Similar types of elastic mats have previously been described in marine tidal zones (Gerdes et al., 1993).
Microbial mats are ideal systems for the study of microbial community structure under extreme conditions (Paerl & Yannarell, 2010; Prieto-Barajas, Valencia-Cantero & Santoyo, 2018). In this sense, there are approaches that can improve our understanding of microbial mat communities, such as determining the phylogenetic diversity of communities under extreme environmental conditions where, for example, abiotic filtering and competitive exclusion have been reported as drivers of phylogenetic clustering (Goberna et al., 2014); another possible approach is to describe microbial subcommunities, e.g., those of rare taxa, a less-studied topic than the whole community (Liu et al., 2019) but nevertheless important to explore (Jia, Dini-Andreote & Falcão Salles, 2018); other form is to analyze the metabolome of communities, which is one of the functional expressions of diversity and an underexplored aspect of microbial communities that can help complete our understanding of the observed diversity.
The investigation of microbial mats has gained special relevance in recent years with regards to the interpretation of structuring processes in microbial communities. However, hypersaline elastic mats remain poorly studied, limiting our understanding of microbial diversity and functioning under extreme environmental conditions (e.g., high salinity concentrations, elevated evaporation and frequent desiccation cycles). In this study, we evaluated the microbial diversity and metabolome profile within this elastic hypersaline microbial mat from CCB, across ten samples collected on a 1.5-m transect. Specifically, we aimed: (1) to explore microbial diversity by analyzing amplicons of the 16S rDNA and ITS regions, (2) to determine whether rare (low read count) and abundant (high read count) taxa are phylogenetically structured; and (3) to evaluate the microbial metabolome.
Materials and Methods
Sampling site and sample collection
The Archean Domes (26°49′41.7″N, 102°01′28.7″W) is a seasonal, water-fluctuating oval pond (50 m × 25 m (Madrigal-Trejo, 2022)). During the dry season (i.e., most of the year at CCB; Montiel-González et al., 2018), the microbial mats of the Archaean Domes remain humid under a salty crust, which is then dissolved during the rainy season, when the pond fills with water up to ≈20 cm, allowing for the development of the dome-like structures.
The microbial mats of the Archaean Domes were sampled during the rainy season of 2018 (October), with ten evenly spaced samples (S1–S10) along a 1.5-m transect, from the border toward the center, in the ‘Pozas Azules’ ranch of Pronatura Noroeste within CCB (Fig. 1). Salinity and pH were constant along the 1.5-m transect (salinity 5.25%, pH 9.8), and the average nutrient content showed only a slight stoichiometric imbalance (C:N:P 122:42:1), as Medina-Chávez et al. (2019) reported.
Microbial mats were collected and transferred to sterile conical tubes (50 mL), stored at 4 °C, and subsequently frozen in liquid nitrogen until processing in the laboratory. Sampling was under the collection permit SGPA/DGVS/03188/20 issued by Subsecretaría de Gestión para la protección Ambiental, Dirección General de Vida Silvestre.
Total DNA extraction and amplicons sequencing
Prior to DNA extraction, approximately 0.5 g of each mat sample was rinsed with sterile water, removing associated sediments. Total DNA extraction was performed following the protocol reported in De Anda et al. (2018).
DNA was sequenced at the Laboratorio de Servicios Genómicos, LANGEBIO (http://langebio.cinvestav.mx/labsergen/) for the V3 region of the 16S rDNA gene (357F universal primer 5′-CTCCTACGGGAGGCAGCAG-3′/519R universal primer 5′-GWATTACCGCGGCKGCTG-3′; (Lane, 1991)), and the ITS DNA region (ITS1F 5′-TCCGTAGGTGAACCTGCGG- 3′/ITS4R 5′-TCCTCCGCTTATTGATATGC-3′ for ITS; (White et al., 1990)). For each sample, 3 µg of genomic DNA (DO 260/280 1.8) was used; 16S amplicons were sequenced within Illumina Next-Seq 500 platform (2 × 150 PE, using forward reads; reverse reads were discarded from the analysis due to low quality), and the ITS amplicons were sequenced with a Mi-Seq platform (2 × 300 PE).
Bioinformatics analyses
The 16S rDNA amplicon reads were quality filtered and dereplicated using the QIIME 2 bioinformatics platform version 2019.7 (Bolyen et al., 2018). Divisive Amplicon Denoising Algorithm 2 (dada2) denoise-single was used; sequences were demultiplexed and truncated at the 20th bp from the left, and the 120th bp from the right. ASVs were then taxonomically classified using the silva_132_99_v3v4_q2_2019-7 database (https://www.arb-silva.de/). For all diversity analyses the final ASV table was rarefied to 760,000 reads per sample.
The ITS amplicon reads were processed using the ITS-specific workflow of the dada2 v1.13.1 package (Callahan et al., 2016) in R (R Core Team, 2020). Briefly, cutadapt was used to remove primer and adapter contamination from the raw reads (Martin, 2011). Prior to determining ASVs, reads with inconsistent bases were excluded, and reads were required to have less than two expected errors based on their quality scores. ASVs classification was inferred from forward and reverse reads using the run-specific error rates.
Due to the wide range of sizes of the ITS amplicons reported for fungal species in CCB (485–782 bp; (Velez et al., 2016)), two different strategies were implemented to define taxonomic diversity. First, the post-denoised ASVs were merged after excluding reads with an overlap of less than 12 bp and reads with mismatches in the overlapping region. Chimeras were removed using the consensus method of “removeBimeraDenovo” implemented in dada2. The taxonomic assignment was performed with the UNITE database (Nilsson et al., 2019), using the RDP naive Bayesian classifier (Wang et al., 2007) available in the dada2 package with a minimum bootstrap value of 80. ASVs with a minimum of 85% identity and with E-values lower than e−45 were retained (Merge ITS data: Table S1). ASVs without taxonomic assignment (mostly non-fungi organisms) were classified using blastn (non-redundant nucleotide collection (nr/nt) database) (Altschul et al., 1990), where only those ASVs within identities greater than 90% were enlisted (Table S2). The remaining ASVs were considered non-classified.
As a great proportion of the reads failed to merge (~56%), a parallel classification was implemented. The RDP naive Bayesian classifier was used on the two sets of reads (forward and reverse) to check for congruence in the classification between the ASVs. ASVs that had an assignment bootstrap value lower than 50, or showed inconsistent classifications among forward and reverse reads, were aligned to the NCBI database (non-redundant nucleotide collection (nr/nt) database), to corroborate their taxonomic assignment. A more robust classification of ASVs was reached when using forward ASVs, thus all subsequent analyses were performed using the Forward-Only ASVs; even though some ASVs reached a genus level assignment, the taxonomic classification of forward reads was limited to the phylum and kingdom level (ITS table on Tables S3 and S4); the Eukaryotic classification followed the UNITE database. The ITS community composition table (Forward-Only data) was rarefied to 164,820 reads using the vegan package (Oksanen et al., 2013) in R (R Core Team, 2020).
In sum, the filtered 16S rDNA amplicon sequences included 760,326–1,973,982 high quality reads per sample, with a total of 6,063 ASVs (Table S5). The filtered ITS amplicon sequences included 164,822–339,394 high quality reads per sample, with a total of 923 ASVs (Table S4). ITS amplicons were obtained for only six samples (S1, S2, S3, S4, S6 and S7) due to low DNA amount that remained in four samples (S5, S8, S9 and S10), after 16S rDNA amplicon sequencing.
Alpha and beta diversity estimates
To evaluate all samples from 16S (S1–S10) and six samples (S1–S4, S6, S7) from the Forward-Only ITS data, we generated rarefied diversity plots using the vegan package (Oksanen et al., 2013) in R (R Core Team, 2020).
In order to visualize and count the number of overlapping ASVs within the ten samples from the 16S rDNA gene and the six samples from the ITS region, we constructed an UpSet plot using the UpSetR package (Conway, Lex & Gehlenborg, 2017) in R (R Core Team, 2020).
Alpha diversity (Shannon index) and beta diversity (Bray-Curtis, Jaccard distances) were estimated using the 16S rDNA ASV community composition table (Table S5), as implemented in QIIME 2. For the Forward-Only ITS ASV community composition table (Table S4), alpha and beta diversity were estimated with the phyloseq package (McMurdie & Holmes, 2013) in R (R Core Team, 2020). The beta diversity distance matrix was used to construct an UPGMA dendrogram of samples (Carteron et al., 2012). Mantel tests (999 permutations) (Legendre & Legendre, 2012) were conducted with the vegan package (Oksanen et al., 2013) to unveil the relationships between beta diversity and geographic distance, as well as between beta diversity and the metabolomic profile distance matrix (see below).
Phylogenetic diversity
We estimated phylogenetic diversity within samples using Faith’s Phylogenetic Diversity index (Faith, 1992) and the Phylogenetic Species Clustering index (Helmus et al., 2007) using the picante package (Kembel et al., 2010) in R (R Core Team, 2020). Given a phylogenetic tree, phylogenetic diversity (PD) estimates the total length of branches encompassing a subset of taxa (e.g., sampled communities), where PD increases as the subset of taxa is more phylogenetically diverse (Faith, 1992). Phylogenetic Species Clustering (PSC) is a measure of the degree of clustering of taxa across the phylogeny (Helmus et al., 2007), where a PSC value approaching zero indicates that the taxa analyzed were phylogenetically clustered.
To estimate the two metrics, we used the rarified diversity matrices and the corresponding phylogenetic trees for bacterial and fungal ASVs, separately. We tested the observed patterns of PSC across samples against 1,000 replicates under a null model and estimated standardized effect sizes (SES) for this index (null model Phylogenetic Species Clustering, nmPSC). This allowed us to identify samples that had more or less phylogenetic clustering than expected by chance alone. To construct the null model, we randomized the entries of the rarified diversity matrices while keeping total sample diversity constant. In addition, we estimated PD and PSC for the most abundant and rare ASVs within each site. To define rare (low proportionated or low read count) and abundant (high proportionated or high read count) ASVs, we estimated the first and third quartiles (1Q, 3Q) of the distribution of ASV (organized by low to high read count), for each site separately. We used the sites’ 1Q and 3Q as the thresholds to define rare and abundant ASVs; the sites’ 1Qs ranged from 3 to 16 reads per ASV, whereas the 3Qs ranged from 37 to 306 reads per ASV; rare and abundant ASV numbers using this method were equivalent per site. We estimated SES for PD and PSC as defined above, using matrices for rare and abundant ASV.
The phylogenetic tree was generated by QIIME 2 (Bolyen et al., 2018) for 16S data. For the ITS data (Forward-Only data), a phylogeny was obtained by applying an equal phylogenetic QIIME 2 methodology, using Maftt (Katoh & Toh, 2010) and FastTree (Price, Dehal & Arkin, 2009) within Cipres Science Gateway (Miller, Pfeiffer & Schwartz, 2010) with default XSEDE parameters.
Metabolomics
Soluble compounds extraction and LC-MS/MS analysis
The samples (10 g each) were extracted with 60 mL of CHCl3-MeOH (1:1) in an orbital shaker at 150 rpm for 24 h. The mixture was filtered, and the solvent was evaporated under reduced pressure. The dried extracts were reconstituted in 60 mL of CH3CN-MeOH (1:1) and defatted with hexanes. Then the CH3CN-MeOH layer was dried under a vacuum and resuspended in MeOH (LC-MS grade) to yield a concentration of 1 mg mL−1. All samples were filtered with a 0.22 μm membrane and analyzed using a Waters Acquity Ultraperformance Liquid Chromatography (UPLC) system (Waters Corp., Milford, MA, USA) coupled to a Thermo Q Exactive Plus (Thermo Fisher, Waltham, MA, USA) mass spectrometer. The UPLC separations were performed using an Acquity BEH C18 column (50 mm × 2.1 mm I.D., 1.7 μm; Waters) equilibrated at 40 °C and a flow rate set at 0.3 mL min−1. The mobile phase consisted of a linear CH3CN-H2O (acidified with 0.1% formic acid) gradient starting at 15% to 100% of CH3CN over 8 min. The mobile phase was held for another 1.5 min at 100% CH3CN before returning to the starting conditions. High-resolution mass spectrometry (HRMS) data and MS/MS spectra were collected in the positive/negative switching electrospray ionization (ESI) mode at a full scan range of m/z 150−2,000, with the following settings: capillary voltage, 5 V; capillary temperature, 300 °C; tube lens offset, 35 V; spray voltage 3.80 kV; sheath gas flow and auxiliary gas flow, 35 and 20 arbitrary units, respectively.
Untargeted metabolomics
The HRMS-MS/MS raw data was individually aligned and filtered with MZmine 2.17 software (http://mzmine.sourceforge.net/) (Pluskal et al., 2010). Peak detection was achieved as follows: m/z values were detected within each spectrum above a baseline, a chromatogram was constructed for each of the m/z values that spanned longer than 0.1 min, and finally, deconvolution algorithms were applied to each chromatogram to recognize the individual chromatographic peaks. The parameters were set as follows for peak detection: noise level (absolute value) at 1 × 106, minimum peak duration 0.5 s, tolerance for m/z variation 0.05, and tolerance for m/z intensity variation 20%. Deisotoping, peak list filtering, and retention time alignment algorithm packages were employed to refine peak detection. The join align algorithm compiled a peak table according to the following parameters: the balance between m/z and retention time was set at 10.0 each, m/z tolerance at 0.05, and retention time tolerance size was defined as 2 min. The spectral data matrix (comprised of m/z, retention time, and peak area for each peak) was imported to Excel (Microsoft) for heatmap analysis using R (R Core Team, 2020).
Molecular networking was performed from the mzML files using the standard Global Natural Products Social (GNPS) molecular networking platform workflow with the spectral clustering algorithm (http://gnps.ucsd.edu; Aron et al., 2020). For spectral networks, parent mass of 0.01 Da and fragment ion tolerance of 0.02 Da were considered. For edges construction, a cosine score over 0.70 was fitted with a minimum of four matched peaks, and two nodes at least in the top 10 cosine scores (K) and maximum of 100 connected the components. After that, the network spectra were searched against GNPS spectral libraries, and graphic visualization of molecular networking was performed in Cytoscape 3.8.0 (Shannon et al., 2003). Chemical structural information within the molecular network was obtained using the GNPS MolNetEnhancer workflow (Ernst et al., 2019), which incorporated in silico structure annotations from the GNPS library search (metabolomic_support_info.zip in Supplemental Files). The chemical profiles were also manually dereplicated using UV-absorption maxima and HRMS-MS/MS data against the Dictionary of Natural Products v 29.1 and Dictionary of Marine Natural Products 2019 (Taylor and Francis Group, Abingdon, United Kingdom), MarinLite (University of Canterbury, Christchurch, New Zealand) and SciFinder (CAS) databases as described by El-Elimat et al. (2013), for fungal and bacterial small molecules. For candidate search, exact mass accuracy was set to 5 ppm (Sumner et al., 2007). Heatmap visualization of metabolomic data table was generated in MetaboAnalyst (Pang et al., 2020).
Results
Microbial taxonomic composition
Overall, 40 prokaryote phyla were detected from the 16S rDNA gene libraries (Fig. S1A), and a total of 6,063 different ASVs were found, with a total proportion of 11.8% of unclassified reads (Table S3). Bacteroidetes was the most abundant (high read count) phylum, representing around 23% of all sequences in the samples, followed by Proteobacteria with ≈19% and Cyanobacteria with nearly 18%. Spirochaetes and Chloroflexi represented ≈8% and ≈4%, respectively. Nearly 2% were Firmicutes, Patesibacteria and Halanaerobiaeota (Fig. S1A).
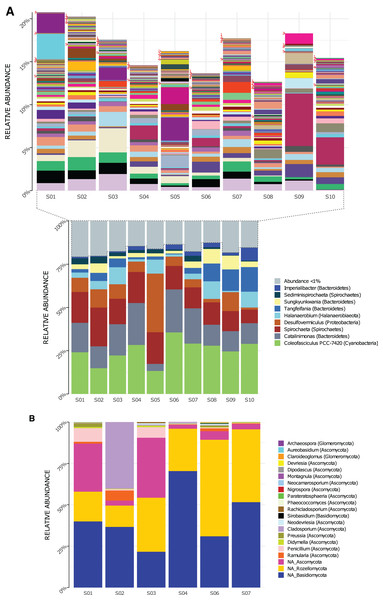
Genera composition is shown in Fig. 2. A total of 219 genera of Bacteria were detected in the 10 sampled sites (S1–S10) at a 1.5-m scale. From those, only nine genera had high read counts (representing ≈80% of the total proportion, Fig. 2A bottom): Coleofasciculus (formerly Microcoleus), Spirochaeta, Catalinimonas, Desulfovermiculus, Halanaerobium, Tangfeifania, Sungkyunkwania, Sediminispirochaeta and Imperialibacter. The remaining 210 genera can be classified as rare biosphere (<1% relative abundance each), with ≈20% of the total proportion (Fig. 2A, top), having a heterogeneous distribution between sites (percentage of relative abundance and percentage of total proportion are used as synonyms).
Figure 2: Genera relative abundance in Archaean Domes, Cuatro Ciénegas Basin sample.
(A) Bacteria (bottom: shows the most common genera, more than 1% of total proportion each; top: shows genera less than 1% of total proportion each; for labels of 210 corresponding genera, check Table S10) (B) Fungi (using Merge data, only fungal ASVs. NA is for ASVs that were not assigned to any genera, but phylum only).As for 16S rDNA ASVs, 18 of the 6,063 were abundant (>1% relative abundance each), representing 49% of the relative abundance of reads (Table S5). This 18 ASVs were distributed within the previously mentioned highly abundant (high read count) genera and phyla (except Firmicutes and Patesibacteria).
Conversely, 6,045 ASVs were rare taxa (<1% relative abundance each) which represents 51% of total reads: 4,124 of them were non-classified, and the rest (1,921) were distributed within the whole 40 bacterial phyla and almost all (217) genera (Table S5). The two exceptions of genera with no rare ASVs were Tangfeifania with two highly proportionated ASVs, and Coleofasciculus with only one high-read count ASV. Moreover, 28 ASVs of Archaea were obtained using the 16S primers, comprising two phyla, Woesearchaeota and Euryarchaeota (Fig. S1A). We detected four genera within Halobacteria class: Halovivax, Halorubrum, Halorussus and Haloasta.
For the ITS region libraries, we successfully obtained reads for six of the sampled sites (S1 to S4, S6 and S7). For the Forward-Only ITS data, we detected 923 different ASVs (Table S4). Many of the classified reads belong to Alveolata protists (77%, Fig. S1B), followed by Fungi (11%) and Viridiplantae (8%); Metazoa, Protista, Amoebozoa, Rhodoplantae, Chromista and Stramenophila comprised the remaining 4%. Unassigned taxa were 50% of all reads (Table S3). The majority of ASVs within Forward ITS data could not be accurately classified at lower taxonomic categories, particularly for three common ASVs (ASV0 (unclassified), ASV1 (Ciliophora) and ASV2 (Ciliophora)), which comprise nearly 70% of the proportion of ITS reads for all sites.
Using Merge ITS ASVs data (Table S1) for a better taxonomic assignment, Fungal ASVs comprising 18 genera were detected, with phyla Ascomycota, Basidiomycota and Rozellomycota present in all samples (Fig. 2B). Non-fungal Eukarya Merge ITS sequences classification (hits <90%) are shown in Table S2. Fabrea salina (Alveolata), and Vannella simplex (Amebozoa) were two species that constituted nearly 0.5% of the total proportion of Merge ITS ASVs data, among other classified but low- read count eukaryotic ASVs.
Alpha and beta diversity
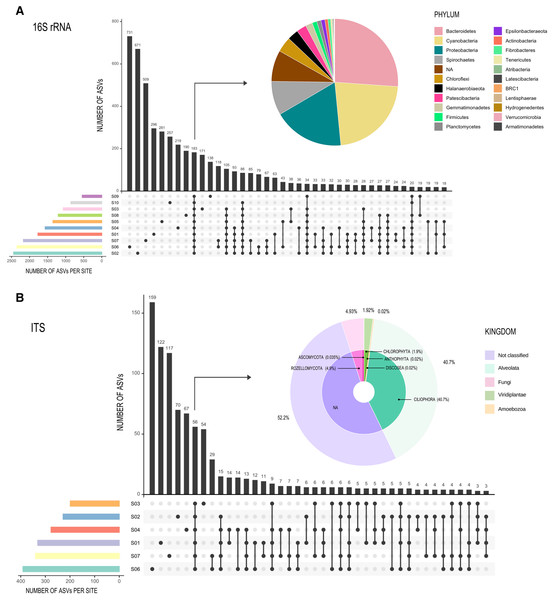
Rarefaction curves (Figs. S2A and S2B) showed similar results for both the Forward-Only ITS region libraries (S1, S2, S3, S4, S6, S7), and the 16S rDNA gene libraries, as all sites reached an asymptote, suggesting an adequate sampling effort. Only 183 ASVs (3% of the total ASV read count) were shared between all sites for 16S rDNA gene libraries (Fig. 3A), forming a very small core, but comprised 72.5% of all reads (composed of twenty-one phyla), while most of the ASVs were unique to a specific site or shared between two or three sites. For ITS libraries, the 56 shared ASVs (6%) comprised 95% of all reads (Fig. 3B).
Figure 3: Graphic representation of shared ASVs within Archaean Domes sampling sites.
Number of shared ASVs is represented by black bars, showing the exact number on the top. Dots below bars denote sites where those ASVs are shared, and lines joining dots indicate the shared sites. Colored bars at bottom left of the main figure are for ASVs number within each site. Pie charts show taxonomy of the few shared ASVs (corresponding bars are pointed with arrows) within all sites. All of these shared ASVs had a high proportion within the whole ASVs (72.5% of 16S rDNA data and 95% of Forward-Only ITS data). (A) 16S Archaea and Bacteria, rDNA gene data; (B) Fungi and other Eukarya, Forward-Only ITS data.The 16S rDNA ASVs alpha diversity (Table 1) ranged from 2,399 observed ASVs in S2 (Shannon = 7.4, highest Shannon index value), to 544 observed ASVs in S9 (Shannon = 6.1). In the case of the ITS region libraries (Forward-Only ITS data), the highest ASV number for Eukarya was S6 with 383 ASVs and a Shannon diversity of 2.5; S3 had the lowest observed ASVs with 199, but this site had the highest Shannon value of three (Table 1).
| α diversity | Phylogenetic diversity | ||||
|---|---|---|---|---|---|
| Sample | Observed | Shannon | Faith PD | Faith PD rare | Faith PD abundant |
| ITS data | |||||
| 1 | 322 | 1.827 | 86.309 | 42.596 | 18.161 |
| 2 | 226 | 2.713 | 60.622 | 40.846 | 14.516 |
| 3 | 199 | 3.062 | 47.289 | 30.370 | 14.774 |
| 4 | 270 | 1.783 | 83.008 | 51.513 | 16.287 |
| 6 | 383 | 2.501 | 131.435 | 75.352 | 28.794 |
| 7 | 337 | 2.008 | 114.983 | 60.352 | 18.865 |
| 16S rDNA data | |||||
| 1 | 1,761 | 6.863 | 544.704 | 290.491 | 44.159 |
| 2 | 2,399 | 7.402 | 789.807 | 368.430 | 84.442 |
| 3 | 1,068 | 6.256 | 211.745 | 156.204 | 20.064 |
| 4 | 1,564 | 6.499 | 500.787 | 259.828 | 40.627 |
| 5 | 1,344 | 6.882 | 220.536 | 172.299 | 21.557 |
| 6 | 2,345 | 6.301 | 851.168 | 302.103 | 131.337 |
| 7 | 2,156 | 6.838 | 759.536 | 328.238 | 97.486 |
| 8 | 1,195 | 6.487 | 205.987 | 139.839 | 22.798 |
| 9 | 544 | 6.126 | 160.368 | 94.201 | 18.577 |
| 10 | 828 | 5.115 | 283.017 | 148.655 | 36.514 |
Note:
Alpha diversity indices for 16S rDNA data and Forward-Only ITS region data from Archaean Domes CCB microbial mats.
Pairwise dissimilarities between samples for both the 16S rDNA gene and ITS region data ranged from 0.2 to 0.7 (16S) and 0.2 to 0.6 (ITS) for the Bray-Curtis distance values and 0.5 to 0.8 (16S) and 0.3 to 0.7 (ITS) according to the Jaccard-1 values (Figs. S3A and S3B). For both distance values, S10 is different from the rest of the 16S rDNA gene libraries, and S1, S2, S4, S6, S7 were associated in the dendogram clusters. In the ITS libraries, there are two main clusters (S1, S2 and S4; S3, S6 and S7). A significant geographic pattern in the Mantel test (p = 0.0402, r = 0.3224) was found for Jaccard-1 of the 16S library, however we did not find significant values based on geography for ITS libraries for either Jaccard-1 or Bray-Curtis (Table S6).
Phylogenetic diversity
For both libraries, Faith Phylogenetic Diversity indicated that rare (low read count) ASVs on each site, were more diverse than abundant (high read count) ASVs (Table 1).
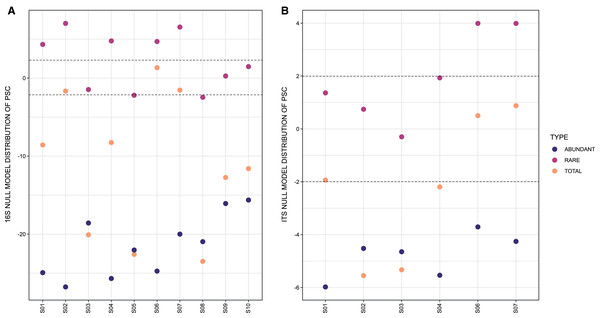
In respect to the null model distribution of Phylogenetic Species Clustering (nmdPSC), the 16S and ITS communities analyzed per site had in some sites a phylogenetic clustering distribution, and in others sites a null model distribution compared with chance alone (Figs. 4A and 4B, dots between lines or below −2).
Figure 4: Graph representing null model distribution of Phylogenetic Species Clustering (nmdPSC).
(A) 16S Archaea and Bacteria, rDNA gene data; (B) Forward-Only ITS data. Dotted lines are showing nmdPSC significant values: >+2 is consistent with a sample that had more phylogenetic overdispersion than expected by chance alone, and nmdPSC value <−2 indicates more phylogenetic clustering than expected by chance alone; values in the range +2 and −2 had a null model distribution. nmdPSC values are reported in Table S7.When analyzing rare and abundant subcommunities using an equivalent ASV number per site, nmdPSC indicated that abundant ASVs were phylogenetically clustered within all sites and for both libraries, while rare ASVs were subcommunities with a null model distribution in some sites, or phylogenetically dispersed in other sites, for bacterial and fungal libraries (Figs. 4A and 4B, Table S7).
Metabolomic profile
Based on the LC-MS results of S1–S10, a wide range of metabolites were visualized on a heatmap analysis chart (Figs. S4A and S4B) and a GNPS molecular networking analysis (Fig. 5 and Table S8).
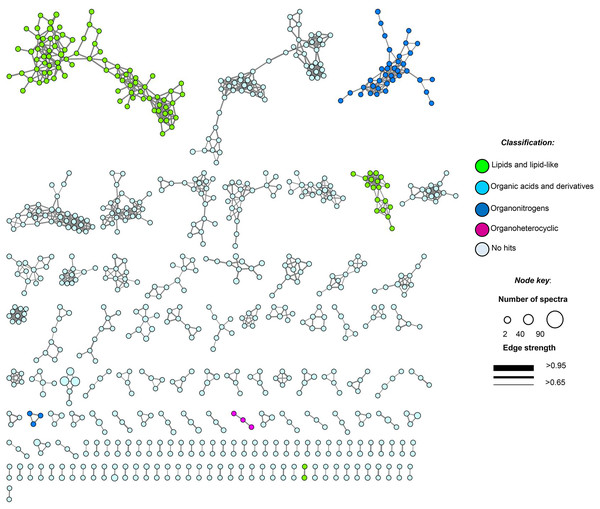
Figure 5: Molecular network of soil samples (S1–S10) grouped metabolite features into 139 chemical families.
Each node in the network represents one metabolite feature. Nodes connected to each other are structurally-related (cosine MS2 similarity score ≥0.7). Matching compounds are not shown for clarity (included in Table S8).Untargeted metabolome analysis of microbial communities in soil or environmental samples is difficult, due to the complexity and heterogeneity of this matrix and the scarcity of the secondary metabolites extracted. In the heatmap, the 1,341 molecular features at unique retention times (Figs. S4A and S4B) shared several common regions across all samples (molecular features), allowing us to group them into three main clusters (cluster 1, samples 3–5 and 10; cluster 2, samples 1, 2 and 6; and cluster 3, samples 7–9, Fig. S4B). We found highly heterogeneous metabolic profiles in all samples, and no significant associations were observed between metabolite features and ITS or 16SrDNA community composition matrices (Table S6).
Moreover, feature-based GNPS molecular networking analysis grouped the metabolite features in 1,534 nodes distributed into 67 chemical families (sub-networks) of ≥3 members, 72 families of two metabolite features, and 765 singletons (Fig. 5). Chemical ontology analyses revealed the presence of superclasses of lipids and lipid-like compounds, organic acids, and organonitrogen and organoheterocyclic compounds, all commonly found in soil samples (Fig. 5). Detected compounds in the GNPS and manual metabolome analysis of the main ions observed in LC-MS/MS data focused on microbial metabolites, allowing tentative identification of several secondary metabolites that passed our quality criteria (≤5 ppm mass error; Table S8). Finally, some plant-derived secondary metabolites and small molecules reflecting human activity were also observed (Table S8).
Discussion
Microbial composition at a small scale
Our study describes microbial diversity on a 1.5-m scale in a rare elastic microbial mat located at an extreme site. Altogether, we found 40 prokaryotic phyla, including 219 genera of Bacteria and 4 of Archaea, as well as 18 Fungal genera (Fig. 2 and Fig. S1).
Bacteria
The bacterial phyla (Fig. S1A) detected in our study site are common components of hypersaline microbial mats (Bolhuis, Cretoiu & Stal, 2014; Visscher et al., 2010): Bacteroidetes, Proteobacteria, Cyanobacteria, Chloroflexi, and Firmicutes, among other abundant (high read count) phyla, which are similar at this broad taxonomic level to other sites in CCB (Bonilla-Rosso et al., 2012; Breitbart et al., 2009; De Anda et al., 2018; Nitti et al., 2012). As expected from a microbial mat, a primary producer was present and abundant in all of our samples: Coleofasciculus (Fig. 2), a filamentous nitrogen-fixing cyanobacteria genus well-adapted to saline conditions (De Wit et al., 2013; Ramos et al., 2017) that has been reported in Guerrero Negro microbial mats (Des Marais, 2010; Wong, Ahmed-Cox & Burns, 2016). We also found the abundant taxon Catalinimonas, which is a halophilic Bacteroidetes marine genus (Choi et al., 2013) that has not been previously reported in microbial mats. Another common taxon in our samples is the Spirochaeta genus (Bolhuis, Cretoiu & Stal, 2014; Margulis et al., 2006), an anaerobic chemo-organotrophic taxon abundant in hypersaline marine mats (Ben Hania et al., 2015). Noteworthy is that the Spirochaetes phylum has not been detected previously in any CCB mats (Bonilla-Rosso et al., 2012; De Anda et al., 2018; Peimbert et al., 2012). We also observed Desulfovermiculus, a sulfate-reducing Deltaproteobacteria, and Halanaerobium, a thiosulfate-reducing Firmicute that is halophilic and can form spores (Liang et al., 2016), both genera are common in marine microbial mats (Bolhuis, Cretoiu & Stal, 2014), reinforcing previous evidence from CCB studies that this site can harbor taxa that are phylogenetically similar to marine lineages (see Rebollar et al., 2012; Souza et al., 2006, 2018).
Archaea
Archaea data presented here should be taken cautiously, since the primers for 16S rDNA used are universal but were not designed specifically to amplify archaea and would likely recover only 1% or less of archaeal diversity. However, we decided to only describe the groups detected, avoiding any interpretations regarding quantity where there could be a strong bias. We detected two main phyla (Fig. S1A); these were Woesearchaeota, a phylum that potentially has a syntrophic interaction with methanogens (Liu et al., 2018; Ortiz-Alvarez & Casamayor, 2016), and Euryarchaeota, within which we found four halophilic genera: Halovivax, Halorubrum, Halorussus and Haloasta, all of them previously reported in saline habitats and sediments (McGonigle et al., 2019; Zaitseva et al., 2018).
In a recent metagenomic study of the Archaean Domes (Medina-Chávez et al., 2019), some archaeal taxa were detected in all sampled sites, forming a “core taxa”. In those metagenomes, Archaea represented nearly 5% of the total reads. The metagenomic data (Medina-Chávez et al., 2019) and the 16S rDNA gene amplicons were sampled from the same pond at different times and locations, and both contained these lineages common to hypersaline habitats.
Fungi
Fungal members represent a diverse and common component of hypersaline water columns and mats (Cantrell et al., 2013) and have been suggested to play a role in the degradation of EPS (exopolymeric substances) (Cantrell & Duval-Pérez, 2013). Our results suggest that unidentified uncultured members of the Ascomycota and Rozellomycota comprise the core mycobiota of the analyzed mats, as they occurred across all the samples (Fig. 3B). In addition, it is feasible that these abundant heterotrophs occupy a niche at the micro-scale of microbial mats, where they can degrade the abundant EPS produced in the CCB microbial mats (Breitbart et al., 2009; De Anda et al., 2018; Peimbert et al., 2012). In particular, halotolerant taxa such as members of the genera Aspergillus, Penicillium, and Nigrospora have been repeatedly reported in microbial mats at other locations (Cantrell et al., 2013; Cantrell & Duval-Pérez, 2013), evidencing their adaptation to extreme conditions. Thus, it appears that, similar to other microbial mats, fungal members could occupy an EPS-degrader role at the micro-scale of the Archaean Domes (Cantrell et al., 2011).
Other eukaryotes
In our sample, there are three abundant (high read count) ASVs of Alveolata protists that represent 70% of the ITS sequences (Fig. S1B, Forward-Only ITS data) in all sequenced sites (S1–S4, S6, S7). Although the ITS primer pair used in this study was originally designed for fungal amplification, it has been reported that there are potential matches with non-fungal eukaryotic sequences as well (Toju et al., 2012). Nevertheless, these results should be interpreted in a descriptive sense, considering that several eukaryotic taxa are not being detected due to primer bias.
Two of the three ASVs are Ciliophora (i.e., ciliates), which is the most diverse phylum of protists, with 3,500 described species, most of which are cosmopolites (Gao et al., 2016). It will therefore be interesting to analyze more molecular markers in the future to distinguish the protist lineages, as well as to microscopically study the protists to reach a closer taxonomic identification. Other Protozoa ASVs were observed at low numbers and belong to the Amoebozoa phylum, and the ancestral algae from the kingdom Chromista were also observed at a low proportion (Fig. S1B).
Although research on halophilic protozoan is still young, it has made great progress in recent years (Harding & Simpson, 2018). Protozoa in general are grazing organisms, as is the case with Fabrea salina, one of the few classified low proportion ITS ASVs in our study (Merge data, Table S2). This ciliate is present in microbial mats and feeds on Cyanotece sp., a Cyanobacteria associated with algae mats (Carrasco & Perissinotto, 2012). Indeed, in hypersaline environments, most ciliates are known to be important components of the pelagic microbial food loop (Liu et al., 2016). Amoebae have also been observed as grazers (Hauer, Rogerson & Anderson, 2001; Hauer & Rogerson, 2005; Post et al., 1983) on cyanobacteria in surface mud samples from a salt pond in Eilat, within the Salton sea in Southern California (Hauer & Rogerson, 2005). Even though the diversity of protists is low, their prevalence, along with the large amount of viruses found in metagenomic samples (28% of the metagenomes from Medina-Chávez et al., 2019), strongly suggests that predator-prey interactions (e.g., protist-bacteria, Saleem et al., 2013) could be very important for the dynamics of the system (Carreira et al., 2020).
Alpha and beta diversity
The Shannon Index accounts for both species evenness and abundance; hence, it might not reflect the total richness if most of the found taxa are part of the rare biosphere. For example, Site three for the Forward-Only ITS data had the highest Shannon value, despite having the lowest ASV number (Table 1), due to its higher evenness distribution of taxa. Meanwhile, the rarefaction and observed diversity reflects the richness of the site (Figs. S2A and S2B; Table 1).
Beta diversity analysis showed values that represent a large ASV turnover between sites despite the small scale (Fig. S3), illustrated by the heterogenous composition observed within bacterial genera (Fig. 2A top). Accordingly, the different domains analyzed (i.e., the bacterial 16S rDNA data or the Eukarya Forward only ITS data) had a distinctive association pattern. This turnover pattern should be taken cautiously, as ASVs turnover might a methodological artifact: beta diversity analysis uses data on relative abundances, based on compositional data; this means that the presence of abundant ASVs might “push” the rare ASVs below the detection threshold. Therefore, this pattern should be corroborated in a future time-series using metagenomic data, and additionally, a transcriptome approach would explore if the rare ASVs are an active part of this community. A statistically marginal but significant geographic association was found only for the bacterial 16S rDNA data (p = 0.0402, r = 0.3224); this suggests a geographic pattern that also should be explored with a more extensive sampling.
Bacterial community comparison with other CCB layered microbial communities
We compared our data with four previously published metagenomes of layered microbial communities from the CCB: one microbialite from a large spring-fed river Rio Mesquites (Nitti et al., 2012), one stromatolite from a blue pool in Pozas Azules (Breitbart et al., 2009) and two microbial mats, one from a small and shallow pond called Lagunita in Churince (De Anda et al., 2018), and the other from a green pool in Pozas Azules (Bonilla-Rosso et al., 2012).
At the phylum level, we found seven taxa present in all these five microbial communities: Actinobacteria, Bacteroidetes, Chloroflexi, Cyanobacteria, Firmicutes, Planctomycetes and Proteobacteria. All of them shared among all sites on Archaean Domes mats and had a high read count (Fig. 3A).
Additionally, Bonilla-Rosso et al. (2012) reported 28 orders that were shared within the microbial mat from a green pool (Bonilla-Rosso et al., 2012), the stromatolite (Breitbart et al., 2009) and a coastal hypersaline non-thermophilic microbial mat from Guerrero Negro (Kunin et al., 2008). We found 18 of those 28 orders in the Archaean Domes community (Table S9).
We also explored lower taxonomic levels but did not find a consistent pattern.
The common pattern at higher taxonomic ranks reflects, as Bonilla-Rosso et al. (2012) mentioned, that layered microbial communities assemble in biogeochemical gradients related to defined ecological niches (e.g., photosynthesis, sulfate reduction, heterotrophy) according to their functional traits, rather than their species (Burke et al., 2011, Escalas et al., 2019).
Phylogenetic diversity
The null model distribution of Phylogenetic Species Clustering (nmdPSC, Fig. 4) of whole bacterial and eukaryotic communities showed negative or null values, indicating a clustered pattern compared with the null model. Phylogenetic clustering is predicted to be evident in environments with poor nutrient availability (Mondav et al., 2017), such as the microbial mat conditions. Since little is known about patterns and processes of rare and abundant bacterial and eukaryotic taxa in microbial mats, we also analyzed these two microbial subcommunities.
Abundant (high read count) taxa
The nmdPSC pattern of clustering for the abundant ASVs (Fig. 4) could be similar to some soil bacterial communities that typically show a coexistence of phylogenetically close relatives (Bryant et al., 2009; Horner-Devine & Bohannan, 2006), which may be the product of abiotic filtering but also of competitive exclusion (Goberna et al., 2014). Competitive exclusion seems to be a likely explanation for the phylogenetic clustering observed for abundant bacterial and eukaryotic ASVs, as proposed by Goberna et al. (2014), since the presence of compounds with antimicrobial properties (check Metabolomic profile sub-section below) suggests high levels of competition among abundant microbes.
Rare (low read count) taxa
The rare biosphere, when analyzing equivalent numbers of ASVs within abundant taxa, was more phylogenetically diverse (Faith Pylogenetic Diversity index, Table 1), as well as more phylogenetically disperse (nmdPSC, Fig. 4) than the abundant taxa within these samples for both the 16S rDNA and ITS libraries, suggesting that environmental filtering is not occurring for the rare biosphere in this site.
The two analyzed subcommunities (abundant and rare) showed different phylogenetic dispersion patterns. The clustering pattern for abundant taxa --and also for the whole community--, suggests that the associated assembly processes are abiotic filtering or competitive exclusion (Wang et al., 2013; Mondav et al., 2017). In contrast, communities that show phylogenetic dispersion, such as the rare taxa of the Archaean Domes, could be more affected by stochastic processes such as dispersal and drift (Wang et al., 2013; Mondav et al., 2017). Thus, both processes, deterministic and stochastic could be occurring in the Archean Domes community.
These rare and abundant patterns described suggest that rare taxa could be a large reservoir of genetic diversity, having diverse metabolic functions within this microbial mat (Jia, Dini-Andreote & Falcão Salles, 2018) and perhaps presenting collective adaptations to the extreme conditions. This is in correspondence with the unique metabolome profile showed for each site, despite the homogeneous abundant taxa. These ideas should be taken cautiously, since rare taxa may not be an active part of the community, as stated previously.
Metabolomic profile
The dynamic and complex metabolomic profiles (Figs. S4A and S4B, Fig. 5 and Table S8) of the studied microbial mat was consistent with the complex microbial community observed in the metagenomic analysis (Medina-Chávez et al., 2019).
Even though the expression of regulatory genes responsible for secondary metabolites production depends on several factors (e.g., nitrogen or phosphate starvation, stressors like heat, pH and damage to the cell wall, among others) (Baral, Akhgari & Metsä-Ketelä, 2018), along with the lack of soil metabolomics studies and the solubility of the compounds for LCMS analysis, we were still able to tentatively annotate several classes of compounds with interesting biological properties (Fig. 5 and Table S8). Many of these compounds were found to overlap between most of the samples: the antibiotics 8,9-dihydroindanomycin (Li, Roege & Kelly, 2009), neoindanomycin (Rommel, Li & Kelly, 2011) described from Streptomyces species; the cytotoxic daryamide C (Asolkar et al., 2006), gibbestatin B (Kinashi & Sakaguchi, 1984) and furaquinocin H (Ishibashi et al., 1991), from Streptomyces strains; the germination inhibitor colletofragarone A1, from fungi Colletotrichum fragariae and C. capsiciae (Inoue et al., 1996); periconiasin G (Zaghouani et al., 2016) from S. nitrosporeus and Periconia sp. F-31 with free radical scavenger and weak anti-HIV activities; and phomamide and tomaymycin I from Phoma lingam (Ferezou et al., 1980) and Nocardia sp. (Tozuka, Takasugi & Takaya, 1983) with no biological activity described. Other metabolites of interest were found in specific samples, for example the antifungal agents griseofulvin and dechlorogriseofulvin, produced by microorganisms of the genera Penicillium, Aspergillus, Xylaria, Coriolus, Palicourea, Memnoniella, Khauskia, Carpenteles, Arthrinium, Nigrospora, Stachybotrys, and Streptomyces (Atta-ur-Rahmanro, 2005; Kimura et al., 1992; Knowles et al., 2019; Park, Cho & Simpson, 2006) were identified only in S7; the antimitotic agent nostodione A from the blue-green alga Nostoc commune (Kobayashi et al., 1994) in samples S2 and S6–S8; and the mycotoxins citreohybridone B (Kosemura, 2003), setosusin (Fujimoto et al., 1996), roridin E (Böhner et al., 1965) and ganoboninone A (Ma et al., 2015) in samples S8–S10. Interestingly, several observed ions (Fig. 5) do not match with any data reported and could represent potentially new secondary metabolites. Finally, plant- and animal-derived metabolites and compounds resulting from human activity (contaminants), were also annotated (Table S8).
The association of compounds between fungi and osmotrophs, as well as to other Eukarya, reflect the importance of studying the potential role of microorganisms besides bacteria within microbial mats (Carreira et al., 2020). It is important to mention that metabolomics studies of samples containing diverse communities are scarce; thus, this work significantly expands our understanding of soil metabolites, especially from elastic hypersaline microbial mats.
Conclusions and Perspectives
We found a very low number of taxa that comprised a considerable proportion of the mat and were shared across all sampling points, whereas the rare biosphere was more phylogenetically diverse (FPD index) and phylogenetically disperse (using a null model distribution of Phylogenetic Species Clustering) than the abundant (high read count) taxa for both analyzed libraries. We also found a distinctive metabolome profile for each sample and were able to tentatively annotate several classes of compounds with relevant biological properties.
In view of these results, we are interested to investigate if the metabolomic data is associated with local adaptive processes that could be following an eco-evolutionary dynamic in these fluctuating communities (i.e., taxa are constantly and actively modifying their own ecological niche and that of others (niche construction) (Laland, Matthews & Feldman, 2016; Odling-Smee, Laland & Feldman, 2003; Van Der Hooft et al., 2020)). We also want to explore the high phylogenetic diversity and phylogenetic dispersion shown by the rare biosphere and use transcriptomic data to analyze if the rare biosphere is an active part of this community. We hypothesized that there is a diverse “seed bank” in the aquifer of CCB, a deep biosphere fed by magmatic energy (Wolaver et al., 2013) that could be constantly “seeding” the Archaean Domes community, along with deep water and particular minerals.
These ideas could be tested in a deep and spatial-temporal sampling approaches.
Supplemental Information
Taxa relative proportion.
(A) Phylum composition within 16S rDNA gene data.
Taxa relative proportion.
(B) Kingdom composition (based on UNITE database eukaryotic taxonomy).
UPGMA dendrogram showing composition similarities among sampling sites.
Branch length represents Bray-Curtis dissimilarity coefficient, based on 16S rDNA ASVs.
UPGMA dendrogram showing composition similarities among sampling sites.
Branch length represents Jaccard-1 dissimilarity coefficient, based on 16S rDNA ASVs.
UPGMA dendrogram showing composition similarities among sampling sites.
Branch length represents Bray-Curtis dissimilarity coefficient, based on Forward-Only ITS ASVs.
UPGMA dendrogram showing composition similarities among sampling sites.
Branch length represents Jaccard-1 dissimilarity coefficient, based on Forward-Only ITS ASVs.
Metabolite heatmap analysis.
Relative proportion of metabolite features (MS ions in positive mode) in the Archaean Domes mats samples. On the scale bar, brick red color indicates increased metabolite levels, and blue color represents decreased levels. (A) non-clustered samples and sites, (B) clustered samples and sites.
List of ASVs Blast hits with <90%, of non-fungal Merge ITS data.
Phyla and kingdom community composition tables.
16S rDNA gene phyla and ITS region kingdom taxa community composition ASVs tables, and percentage of classified and unclassified ASVs.
Forward-Only ITS-ASV community composition and taxonomy table.
Mantel test negative results of metabolites distance matrix vs beta diversity distance matrix.
Identified microbial metabolites.
Tentatively identified microbial metabolites in samples S1–S10 by GNPS and detailed metabolomic analysis.
List of 18 orders shared between Archaean Domes and other layered microbial communities.
Labels of Figure 2A (up).
Low read count genera (less than 1% of total proportion of taxa) labels from Figure 2A (up). Five repeated sequences of colors are used, distinguished here by red lines, and numerated red arrows in figure.