In vitro anti-Leishmania activity of 8-hydroxyquinoline and its synergistic effect with amphotericin B deoxycholate against Leishmania martiniquensis
- Published
- Accepted
- Received
- Academic Editor
- Bernardo Franco
- Subject Areas
- Biochemistry, Cell Biology, Parasitology
- Keywords
- Leishmaniasis, Leishmania martiniquensis, Mundinia, 8-Hydroxyquinoline, Synergistic effect, Drug combination, Amphotericin B deoxycholate
- Copyright
- © 2022 Chanmol et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. In vitro anti-Leishmania activity of 8-hydroxyquinoline and its synergistic effect with amphotericin B deoxycholate against Leishmania martiniquensis. PeerJ 10:e12813 https://doi.org/10.7717/peerj.12813
Abstract
Leishmania (Mundinia) martiniquensis is responsible for visceral leishmaniasis in patients with no known underlying immunodeficiency, and visceral or disseminated cutaneous leishmaniasis in HIV-infected patients. The available anti-Leishmania drugs for treatment have limitations such as high toxicity and variable efficacy. To improve the therapeutic index of anti-Leishmania drugs, the search for a new drug or a new natural compound in combination therapy instead of using monotherapy to reduce drug side effect and have high efficacy is required. In this study, anti-Leishmania activity of 8-hydroxyquinoline (8HQN) and its synergistic effect with amphotericin B (AmB) against L. martiniquensis were evaluated in vitro for the first time. These results showed that 8HQN presented anti-Leishmania activity against L. martiniquensis with IC50 1.60 ± 0.28 and 1.56 ± 0.02 µg/mL for promastigotes and intracellular amastigotes, respectively. The selectivity index (SI) value of 8HQN was 79.84 for promastigotes and 82.40 for intracellular amastigotes, which highlight promising results for the use of 8HQN in the treatment of L. martiniquensis-infected host cells. Interestingly, four combinations of 8HQN and AmB provided synergistic effects for intracellular amastigotes and showed no toxic effects to host cells. These results provided information of using a combination therapy in treating this Leishmania species leads to further development of therapy and can be considered as an alternative treatment for leishmaniasis.
Introduction
Leishmaniasis is an infectious disease caused by different species of the protozoan parasite of the genus Leishmania, which invades a host to grow inside macrophages (Laranjeira-Silva, Hamza & Pérez-Victoria, 2020). Leishmania parasites are transmitted to humans and mammals through the bite of the sandfly vector that carries the infective metacyclic promastigotes (Giraud et al., 2019). Depending on the species of parasite, patients infected with Leishmania spp. may present a broad spectrum of clinical manifestations, from cutaneous (CL) to visceral leishmaniasis (VL) and be a carrier of parasites for long period with or without symptoms (Ghorbani & Farhoudi, 2017).
Both CL and VL disease are endemic in 56 and 55 countries, respectively. An estimated 50,000 to 90,000 new cases of VL and 600,000 to 1 million new cases of CL occur worldwide annually (WHO, 2021).
Leishmaniasis has been reported as emerging disease in several countries. In Southeast Asia, most of cases are infected with Leishmania martiniquensis, responsible for VL in a patient with no known underlying immunodeficiency, and VL or disseminated cutaneous leishmaniasis (DCL) in a HIV patient (Chusri et al., 2012; Pothirat et al., 2014). L. martiniquensis is of particular interest because it is in a new subgenus L. (Mundinia) (Espinosa et al., 2018) and few studies on drug treatment are available.
The treatment of leishmaniasis mainly relies on chemotherapy. Antimonials, miltefosine, paromomycin and amphotericin B deoxycholate (AmB) are antileishmanial drugs that have been used historically to treat a disease. However, there have been reports of antimony resistance in several regions, especially in the Indian subcontinent (Dumetz et al., 2018). Thus, miltefosine, paromomycin and AmB are increasingly used (Ponte-Sucre et al., 2017).
Most cases of L. martiniquensis infections were treated with AmB, since it is an available drug in many countries (Leelayoova et al., 2017). Recently, Phumee et al. (2020) using the colorimetric prestoblue resazurin-based assay, demonstrated that IC50 of amphotericin B was twice increased for L. martiniquensis (Phumee et al., 2020). Some fluorinated rhodacyanine analogues have shown better values than AmB (IC50 = 40–85 nM) against the promastigote and axenic amastigote stages (Lasing et al., 2020).
AmB is an antifungal agent, that provides reliable and broad-spectrum therapeutic alternative and also for use in the treatment of leishmaniasis. The drug causes the formation of an aqueous pore in cell membrane of the parasite, with an excellent leishmanicidal activity. However, the use of this drug is accompanied by dose-limited toxicities, highly toxic side effects, infusion-related reactions, and nephrotoxicity (Sundar & Chatterjee, 2006; Hamill, 2013). Therefore, to improve the therapeutic index of AmB, the search for a new drug or a new natural compound in combination with AmB therapy instead of using AmB in monotherapy may reduce drug side effects and have high efficacy.
8-Hydroxyquinoline (8HQN) is a plant-derived or synthetic quinoline. The compound has been used in agriculture, textile, wood and paper industries as a preservative, since it has the metal property of being a fungicidal activity (Short et al., 2006). Previous studies found that 8HQN showed great interest in a broad range of biological activities, such as antioxidant (Chobot et al., 2018), anticancer (Xie, Cai & Peng, 2018), antibacterial (Odingo et al., 2019), antifungal (Pippi et al., 2017), antiviral (Lai, Sridhar Prasad & Padmanabhan, 2013) and antischistosomal (Allam, Eweas & Abuelsaad, 2013) activities. It has been shown to have anti-Leishmania activity against L. tropica, L. major and L. infantum (Dardari et al., 2004). The compound was also effective against the intracellular stage of L. braziliensis, L. amazonensis and L. infantum with low toxicity to primary cells (Costa Duarte et al., 2016). The 8HQN has not been tested on L. martiniquensis. Thus, the aims of this study were to determine the anti-Leishmania activity of 8HQN, followed by the investigation of the synergistic effect between 8HQN and AmB against L. martiniquensis. To the best of knowledge, the results provided information of using a combination therapy in treating L. martiniquensis infection that may be useful for future treatment.
Materials and methods
Compound and drug
8HQN was commercially purchased (Sigma-Aldrich, Saint Louis, MO, USA). A stock solution of 8HQN (7,500 µg/mL) was prepared in 50% (v/v) ethanol (C2H5OH) and stored at −20 °C. AmB (250 µg/mL) was purchased (Gibco, Grand Island, NY, USA). Various concentrations of 8HQN and AmB were prepared in the fresh medium and used immediately.
Parasites
Leishmania martiniquensis (MHOM/2013/LSCM3) was obtained from the Department of Parasitology, Faculty of Medicine, Chulalongkorn University. Promastigotes were grown in Schneider’s insect medium (SIM), pH 6.8 (Sigma-Aldrich, Saint Louis, MO, USA), containing 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) and 25 µg/mL gentamicin sulfate (Sigma-Aldrich, Saint Louis, MO, USA) and incubated at 26 °C. Promastigotes were maintained in a logarithmic phase of growth by sub-passage every 3–4 days, starting the cultures with 106 parasites/mL.
THP-1 cells
The human monocytic cell line (THP-1) was used as a mammalian host cell for cytotoxicity assay, intracellular amastigote assay and drug combination assay. THP-1 cells were offered by Dr. Sirida Yangshim, Department of Microbiology, Faculty of Medicine, Chiang Mai University. THP-1 cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% FBS, 25 µg/mL gentamicin sulfate at 37 °C, 5%CO2. The sub-passaged was performed every 2–3 days, starting cultures with 5 × 104 cells/mL. THP-1 cells were treated with 10 ng/mL of phobalmyristate acetate (PMA) (Sigma-Aldrich, Saint Louis, MO, USA) to induce macrophages differentiation before starting the experiment.
Promastigote assay
To determine the anti-Leishmania activity of 8HQN against L. martiniquensis promastigotes, the logarithmic phase promastigotes were cultivated in the culture medium with the presence of various concentrations of 8HQN. AmB was used as a control. Parasite viability was assessed using alamarBlue cell viability assay (Thermo Fisher Scientific, Waltham, MA, USA) as described by Intakhan et al. (2020). Briefly, 8HQN (0.39–25 µg/mL) and AmB (0.004–0.625 µg/mL) were prepared in SIM supplemented with 10% FBS and 25 µg/mL gentamicin sulfate. A total of 50 µL of different dilutions of 8HQN and AmB were plated in a 96-well culture plate (Nunc, Roskilde, Denmark) and then added with 50 µL of 2 × 106 logarithmic phase promastigotes of L. martiniquensis. Parasites were incubated with compound and drug at 26 °C for 48 h. To determine parasite proliferation using alamarBlue cell viability assay, 10 µL of alamarBlue reagent (Thermo Fisher Scientific, Waltham, MA, USA) was added into each well of a 96-well culture plate. The culture was further incubated for 24 h at 26 °C. Then, the absorbance was measured at wavelength of 570 and 600 nm using an Eon™ microplate spectrophotometer (BioTek, Winooski, VT, USA). Wells with medium alone and medium containing 10 µL of alamarBlue reagent were included as controls. Dose response curves obtained from % growth inhibition were generated using GraphPad Prism software (GraphPad Prism Software Inc., San Diego, CA, USA) The IC50 value (concentration of the compound or drug that inhibits 50% of the parasite viability after 72 h of incubation) was determined and expressed as mean ± SD. Results are from three independent experiments.
Cytotoxicity assay
The cytotoxic activity of 8HQN was evaluated in THP-1 cells. Cells were treated with PMA to induce differentiation towards macrophages. Briefly, THP-1 cells were prepared in RPMI-1640 medium (10% FBS and 25 µg/mL gentamicin sulfate) containing 10 ng/mL of PMA. 100 µL of cells were placed into each well of 96-well culture plates (2.5 × 104 cells/well). PMA-treated cells were incubated at 37 °C, 5%CO2 for 24 h to allow cell adhesion (complete differentiation). Then, cultures were washed and replaced with culture medium containing different dilutions of 8HQN (0.49–500 µg/mL), AmB (0.0049–10 µg/mL) and incubated for another 72 h at 37 °C, 5%CO2. To determine cell viability after exposure to compound and drug using alamarBlue cell viability assay, 10 µL of alamarBlue reagent was added into each well of a 96-well culture plate. Then, the incubation was continued for 4 h at 37 °C, 5%CO2 before reading the absorbance at wavelengths of 570 and 600 nm using an Eon™ microplate spectrophotometer. Wells with medium alone and medium containing 10 µL of alamarBlue reagent were included as controls. Dose response curves obtained from % survival of THP-1 cell were generated using GraphPad Prism software. The CC50 value (concentration of compound or drug that induces 50% of cell death after an incubation period of 72 h) was determined and expressed as mean ± SD. Results are from three independent experiments.
Preparation of Leishmania-infected macrophages
Promastigotes were sub-passaged into RPMI-1640 medium supplemented with 20%FBS, 25 µg/mL gentamicin sulfate, pH 5.5 to stimulate metacyclogenesis (Zakai, Chance & Bates, 1998). Promastigotes were incubated at 26 °C for 5 days to yield metacyclic promastigotes. The culture with the presence of 70% of metacyclic promastigotes (body length < 12.5 µm, body width 1.5 µm and flagellum > body length) according to the criteria of Chanmol et al. (2019) was used for host cell infection. THP-1 cells were treated with 10 ng/mL of PMA for 24 h to differentiate into host cell macrophages in 96-well culture plates. Then, macrophages were infected with promastigotes (obtained from the culture with 70% metacyclic promastigotes) at a parasite/macrophage ratio of 10:1 to reach an optimal level of infection and incubated at 37 °C, 5%CO2.
Intracellular amastigote assay
To determine the anti-Leishmania activity of 8HQN against L. martiniquensis amastigotes, intracellular amastigotes in THP-1 derived macrophages were treated with various concentrations of 8HQN. AmB was used as a control. The viability of amastigotes was assessed through the parasite rescue and transformation assay, a method described by Jain et al. (2012). Briefly, THP-1 cells were prepared in RPMI-1640 medium containing 10 ng/mL of PMA and dispended into 96-well culture plates. After 24 h of cell differentiation, THP-1 derived-macrophages were infected with promastigotes at a parasite/macrophage ratio of 10:1. Then, infected macrophages were treated with 8HQN (0.156–20 µg/mL) and AmB (0.004–0.5 µg/mL) prepared in RPMI-1640 medium supplemented with 2% FBS and 25 µg/mL gentamicin sulfate and incubated at 37 °C, 5%CO2. After exposure to the compound and drug for 48 h, survived amastigotes in macrophages were assessed by means of parasite rescue and formation assay. Leishmania-infected macrophages were washed with serum-free RPMI-1640 medium. Then, 0.05% (w/v) of sodium dodecyl sulfate (SDS) prepared in RPMI-1640 medium (20 µL/well) was added and incubated for 30 s to lyse the macrophage cell membrane and release intracellular amastigotes. Then, SIM supplemented with 20% FBS, 25 µg/mL gentamicin sulfate (180 µL/well) was added into each well to neutralize the SDS. The culture was incubated at 26 °C for 96 h to allow the amastigotes to grow, transform into promastigotes and start proliferation. Then, parasite proliferation was assessed using alamarBlue cell viability assay. The culture was added with alamarBlue reagent (20 µL/well) and incubated at 26 °C for another 24 h. Then, the absorbance was measured at wavelengths of 570 and 600 nm using an Eon™ microplate spectrophotometer. Wells with non-infected macrophage treated with 0.05% SDS (30 s) and medium containing 20 µL of alamarBlue reagent were included as controls. Dose-response curves were generated using GraphPad Prism software. The IC50 value was determined and expressed as mean ± SD. Results are from three independent experiments.
Drug combination assay and analysis of interaction
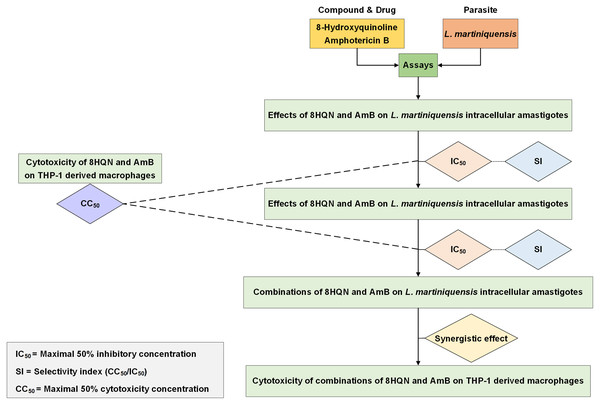
Drug interaction was investigated by using the method as previously described by Intakhan et al. (2020). Double concentration of IC50 of 8HQN and AmB were prepared and serially diluted twice in 2%FBS RPMI-1640 medium. Different dilutions of 8HQN and AmB were combined using checker board assay (Zhao, Kelnar & Bader, 2014). The matrix of 8HQN/AmB provided 12 different combinations. Leishmania-infected macrophages were prepared in 96-well culture plates as describe above and treated with all combinations at 37 °C, 5%CO2 for 48 h. After exposure to combinations, macrophages surviving amastigotes were assessed using parasite rescue and transformation assay as described above. Promastigote proliferation in the culture at 96 h of culture was determined using alamarBlue cell viability assay. Each well was added with 20 µL of alamarBlue reagent and incubated for 24 h. Controls were wells with non-infected macrophages treated with 0.05% SDS (30 s) and medium containing 10 µL of alamarBlue reagent. Then, the absorbance was measured at wavelengths of 570 and 600 nm using an Eon™ microplate spectrophotometer (BioTek, Winooski, VT, USA). %Growth inhibition was used in the analysis of drug interaction. The interaction of 8HQN and AmB was analyzed using the Chou-Talalay combination index method by using CompuSyn software (Chou, 2008). Then, combinations were classified as synergism, additive or antagonism. Overall study is shown in Fig. 1.
Figure 1: Overall study for 8HQN and AmB treatment in L. martiniquensis.
The diagram shows processes for determining activity of 8HQN and AmB against promastigotes, intracellular amastigotes and THP-1 cells and processes for investigation of drug interaction and cytotoxicity of combinations.Statistical analysis
All data were recorded, edited and entered using GraphPad Prism software (GraphPad Prism Software, Inc., San Diego, CA, USA). Mean and standard deviation was calculated from three independent experiments. The statistical differences of %growth inhibition between drug combination and 8HQN alone was evaluated using one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison tests using GraphPad Prism software (GraphPad Prism Software, Inc., San Diego, CA, USA). The statistical differences of cytotoxicity of drug combinations and drug or compound alone were evaluated using ANOVA, followed by Bonferroni’s multiple comparison tests. Differences were considered significant when p values were < 0.05.
Results
Effect of 8HQN against L. martiniquensis promastigote and intracellular amastigote
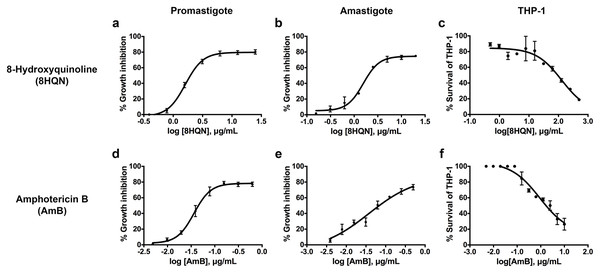
8HQN has effect on both the promastigote and intracellular amastigote stages of L. martiniquensis. After 8HQN or AmB treatment, parasite growth was determined by the alamarBlue assay. The percentage of growth inhibition was plotted as a function of compound or drug concentration by fitting the values to a non-linear regression analysis. The dose response curves of 8HQN or AmB for promastigotes and amastigotes were generated by GraphPad Prism as shown in Fig. 2. These dose-response curves agree with the law of mass action and inhibition followed a sigmoidal curve. The IC50 value of 8HQN for promastigotes was 1.61 ± 0.28 µg/mL (Table 1). Intracellular amastigotes were more sensitive to 8HQN than promastigotes with IC50 value of 1.56 ± 0.02 µg/mL. The drug AmB showed IC50 value of 0.04 ± 0.02 µg/mL and 0.04 ± 0.01 µg/mL for promastigotes and amastigotes, respectively.
Figure 2: Dose response curves of 8HQN and AmB on L. martiniquensis and THP-1 cells.
Parasites and THP-1 cells were treated with 8HQN and AmB. Percentages of parasite growth inhibition and THP-1 cell viability were determined using alamarBlue assay. Non-linear regression trendlines obtained by a serial compound or drug dilution were generated by using GraphPad Prism software. (A–C) Dose response curves of 8HQN for promastigotes, amastigotes and THP-1 cells, respectively. (D–F) Dose response curves of AmB for promastigotes, amastigotes and THP-1 cells, respectively.Cytotoxicity and selectivity index of 8HQN
The cytotoxicity of 8HQN was evaluated in THP-1 cells. The CC50 value of 8HQN was 128.55 ± 0.92 µg/mL, showing that it was less toxic to THP-1 cells than the drug, as shown in Table 1. The CC50 value of AmB was 0.95 ± 0.32 µg/mL. To compare the selectivity index (SI) of 8HQN and AmB, the SI value was calculated from the CC50 value divided by IC50 value. The SI values of 8HQN obtained from promastigotes and intracellular amastigotes were 79.84 and 82.40, respectively. The SI value of AmB was 23.75 for both promastigotes and intracellular amastigotes. These results showed that 8HQN was more effective in inhibiting parasite growth and safer to host cells than the drug.
Synergistic effects of 8HQN in combination with AmB on L. martiniquensis amastigote in THP-1 cells
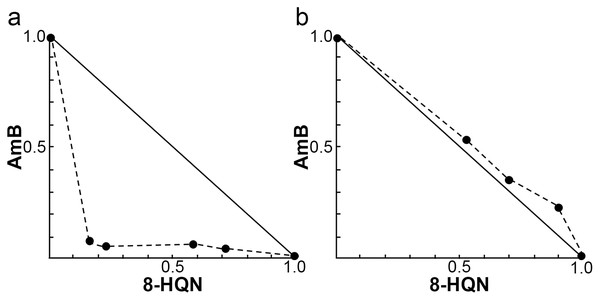
The investigation of the synergistic effect of 8HQN in combination with AmB was performed using the Chou-Talalay combination index method. The IC50 values of 8HQN and AmB were 1.47 ± 0.02 µg/mL and 0.09 ± 0.02 µg/mL, respectively, which were similar to the previous IC50 results. The different concentrations of the combination were obtained from the checkerboard method. After treatment with each combination, the percentage of parasite growth inhibition was calculated from the percentage of treated parasites compared to untreated parasites (control) and then determined the CI value to classify the type of interaction, such as very strong synergism, strong synergism, synergism, moderate synergism, slight synergism, nearly additive, slight antagonism, moderate antagonism, antagonism, strong antagonism, or very strong antagonism. Results showed that the interaction between 8HQN and AmB was found from strong antagonism, nearly additive, to strong synergism as shown in Table 2. Two combinations of 8HQN/AmB (1.6:0.05 and 1.6:0.025 µg/mL) were classified as strong synergism. Combination of 1.6 µg/mL of 8HQN plus 0.0125 or 0.00625 µg/mL of AmB were classified as synergism and moderate synergism, respectively. Result showed nearly additive obtained from two combinations of 8HQN/AmB (0.8:0.05 and 0.8:0.025 µg/mL). The synergistic and additive effects of the combinations are presented as shown in the isobologram (Fig. 3). The result showed that four data points were below the line of additivity, indicating the synergy of 8HQN and AmB as shown in Fig. 3A. Some combinations indicated additive and antagonistic effects, showing data points near or above the line of additivity, as shown in Fig. 3B. A combination of 8HQN/AmB (1.6:0.00625 µg/mL) had the highest dose reduction index (DRI) values in the reduction of AmB used with DRI value of 22.7 (Table 2).
Figure 3: Representative normalized isobolograms of interaction between 8HQN and AmB against intracellular amastigote stage of L. martiniquensis.
(A) 1.6 µg/mL 8HQN plus 0.05, 0.025, 0.0125 or 0.00625 µg/mL AmB. (B) 0.8 µg/mL 8HQN plus 0.05 or 0.025 µg/mL AmB. Data points (dots) located on, below, or above the line indicate additivity, synergy or antagonism, respectively.| Drug combination non-constant ratio (μg/mL) | %Growth Inhibition | Combination Index (CI) | Interpretation | Dose reduction index (DRI) | ||
|---|---|---|---|---|---|---|
| 8HQN | AmB | 8HQN | AmB | |||
| 0 | 0 | 0 | ||||
| 0.4 | 0 | 18.23 ± 5.41 | ||||
| 0.8 | 0 | 15.03 ± 2.62 | ||||
| 1.6 | 0 | 49.67 ± 4.16 | ||||
| 0 | 0.00625 | 16.43 ± 2.23 | ||||
| 0 | 0.0125 | 24.57 ± 1.37 | ||||
| 0 | 0.025 | 26.44 ± 1.80 | ||||
| 0 | 0.05 | 43.66 ± 1.34 | ||||
| 0.4 | 0.00625 | 1.00 ± 1.03*** | 4.10 | Strong antagonism | 0.60 | 0.16 |
| 0.4 | 0.0125 | 22.00 ± 3.58 | 1.80 | Antagonism | 1.84 | 3.80 |
| 0.4 | 0.025 | 20.00 ± 1.73 | 1.14 | Slight antagonism | 1.73 | 1.78 |
| 0.4 | 0.05 | 25.00 ± 0.96 | 1.45 | Antagonism | 2.02 | 1.04 |
| 0.8 | 0.00625 | 16.00 ± 3.98 | 1.51 | Antagonism | 0.74 | 6.15 |
| 0.8 | 0.0125 | 28.00 ± 4.53* | 1.13 | Slight antagonism | 1.10 | 4.51 |
| 0.8 | 0.025 | 38.30 ± 1.85*** | 1.05 | Nearly additive | 1.42 | 2.90 |
| 0.8 | 0.05 | 51.00 ± 5.41**** | 1.05 | Nearly additive | 1.87 | 1.91 |
| 1.6 | 0.00625 | 68.50 ± 3.35** | 0.76 | Moderate synergism | 1.40 | 22.70 |
| 1.6 | 0.0125 | 76.00 ± 6.55*** | 0.65 | Synergism | 1.72 | 13.88 |
| 1.6 | 0.025 | 94.50 ± 5.04**** | 0.29 | Strong synergism | 4.31 | 17.19 |
| 1.6 | 0.05 | 97.00 ± 3.60**** | 0.24 | Strong synergism | 6.07 | 12.07 |
Note:
8HQN and AmB were combined at a non-constant ratio. Dose dependent effects were analyzed by CompuSyn software. CI value of each combination was classified as very strong synergism (CI < 0.1), strong synergism (CI = 0.1–0.3), synergism (CI = 0.3–0.7), moderate synergism (CI = 0.7–0.85), slight synergism (CI = 0.85–0.90), nearly additive (CI = 0.90–1.10), slight antagonism (CI = 1.10–1.20), moderate antagonism (CI = 1.20–1.45), antagonism (CI = 1.45–3.30), strong antagonism (CI = 3.30–10), very strong antagonism (CI > 10). DRI is the fold dose of dose reduction allowed in a combination for a given degree of effect as compared with the dose of each 8HQN or AmB alone. Statistical differences between the effects of AmB alone and in combination with 8HQN are indicated as follows: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001.
Cytotoxicity of 8HQN/AmB combinations to THP-1 derived macrophages
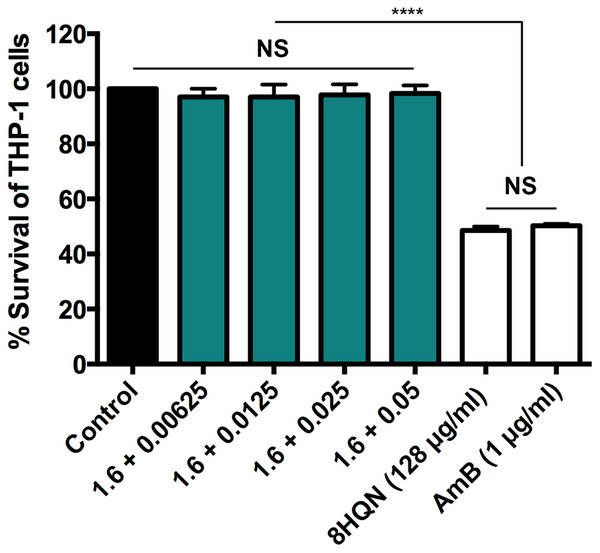
Four combinations (1.6 µg/mL 8HQN plus 0.05, 0.025, 0.0125 or 0.00625 µg/mL AmB) that provided synergistic effects (Table 2) were tested for their cytotoxicity to THP-1 derived macrophages. 8HQN and AmB, which were prepared at the concentration of CC50, were used as controls. The concentration of combinations providing synergistic effects against parasites showed no toxic effects for host cells, whereas both 8HQN and AmB at the CC50 concentration showed about 50% of the host cell viability, as shown in Fig. 4.
Figure 4: Cytotoxic activity of 8HQN in combination with AmB on THP-1 cells.
The x axis represents different concentration (µg/mL) of 8HQN plus AmB. Solid bar, untreated control; Green bars, THP-1 cells treated with 1.6 µg/mL 8HQN plus 0.05, 0.025, 0.0125 or 0.00625 µg/mL AmB; white bars, THP-1 cells treated with 8HQN (128 µg/mL) or AmB (1 µg/mL). Statistical differences between the effects of 8HQN or AmB at CC50 values and their combinations (8HQN plus AmB) or untreated control are indicated as follows: NS, non-significance; ****, p ≤ 0.0001.Discussion
L. martiniquensis is a member of the subgenus Leishmania (Mundinia) and is one of the etiological agents of disseminated cutaneous and visceral leishmaniasis in humans (Chusri et al., 2012; Leelayoova et al., 2017; Espinosa et al., 2018; Burza, Croft & Boelaert, 2018; Valero & Uriarte, 2020). AmB is an available drug used for chemotherapy of L. martiniquensis infection (Leelayoova et al., 2017). It has proven to be effective against L. martiniquensis in vitro (Intakhan et al., 2020). However, the use of this drug is associated with highly toxic side effects (Grela et al., 2018). Thus, to improve the treatment and reduce the side effects of using monotherapy, the search for a new drug or a new natural compound in combination therapy that is less toxic and highly effective has been required.
In this study, the anti-Leishmania activity of 8HQN against L. martiniquensis was determined both in promastigote and intracellular amastigote stages of parasites, followed by the investigation of drug interaction between 8HQN and AmB. Previously, 8HQN has been shown to inhibit the multiplication of L. tropica, L. major and L. infantum at micromolar concentrations (Dardari et al., 2004). In the present study, L. martiniquensis was treated with 8HQN for the first time. 8HQN was able to inhibit the multiplication of promastigotes of L. martiniquensis in a microconcentration range. This confirms the efficacy of 8HQN, although this Leishmania species is in a distinct subgenus, Leishmania (Mundinia). The compound was also able to reach and inhibit the growth of intracellular amastigotes of L. martiniquensis within THP-1 derived macrophages, showing the ability to cross the host cell membrane and treat Leishmania-infected host cells. Interestingly, 8HQN had low toxicity to THP-1 derived macrophages, similar to a previous study in murine macrophages (Costa Duarte et al., 2016). Upon drug discovery, the compound with anti-Leishmania activity is expected to have less toxicity to host cells, showing an SI value higher than 10 (Don & Ioset, 2014).
Our results showed that 8HQN had SI values of 79.84 and 82.40 for promastigote and intracellular amastigote of L. martiniquensis, respectively. These SI values were higher than 10 and higher than the SI value of AmB, which highlight promising results for the use of 8HQN in the treatment of Leishmania-infected host cells. The mode of action of 8HQN is different, depending on the organism in which the compound acts. In Schizosaccharomyces pombe and Clostridium difficile, 8HQN acts as a chelating agent, which was able to inhibit RNA synthesis (Fraser & Creanor, 1974; Novakova et al., 2014). In intracellular microbes such as Cryptococcus neoformans and Mycobacterium tuberculosis, 8HQN activated the Cu-independent antibacterial host responses to kill intracellular pathogens (Festa et al., 2014; Shah et al., 2016). In the present study, a decrease in the number of intracellular amastigotes of L. martiniquensis in macrophages after treatment with 8HQN was similar to the results of a previous study in L. braziliensis, L. amazonensis and L. infantum (Costa Duarte et al., 2016). The compound failed to stimulate the production of nitric oxide by macrophages, showing that 8HQN did not activate the host response to kill parasites. However, some anti-Leishmania compounds showed the ability to kill parasites by up-regulating the level of ROS, causing oxidative stress (Mesquita et al., 2013). Costa Duarte et al. (2016) proved that 8HQN was unable to induce oxidative stress, but acted directly on parasite mitochondria, affecting the parasite mitochondrial membrane potential, causing parasite death. In addition, 8HQN did not affect the plasma membrane permeability of parasites, which is in contrast to the mechanism of AmB that acts on parasite plasma membrane by binding to ergosterols to form pores, resulting in parasite cell lysis (Mishra, Saxena & Singh, 2007). Furthermore, 8HQN alone and in a form of compound-containing polymeric micelle system have been proved to be effective against L. amazonensis-chronically infected BALB/c mice, suggesting the use of this compound in vivo (Costa Duarte et al., 2016; Lage et al., 2016).
In an attempt to reduce the side effects of AmB in monotherapy, 8HQN was combined with the drug and tested in intracellular amastigotes of L. martiniquensis to investigate its efficacy. Our results showed that 8HQN/AmB combinations provided synergistic effects without cytotoxicity to host cells. The combination between 8HQN 1.6 µg/mL and AmB 0.00625 µg/mL provided the highest DRI, showing approximately a 22-fold reduction in AmB used in the combination.
To date, there are no reports of synergistic effects of 8HQN in combination with AmB on any other species of Leishmania. Based on our finding, the synergistic effect of 8HQN with AmB on intracellular amastigotes of L. martiniquensis, can be explained by the ability of AmB to form pores in the parasite membrane, as well as potential direct effects of 8HQN on mitochondrial function.
Drug combinations were recommended to reduce the treatment duration and toxicity and delay the emergence of resistance (van Griensven et al., 2010). However, Leishmania parasites could develop resistance to drug combinations, leading to the potential risk of resistance to the different drugs used in combinations (García-Hernández et al., 2012). Thus, synergistic effects of 8HQN/AmB combinations found in this study can also be considered as an alternative combination therapy for leishmaniasis treatment.
Conclusions
In conclusion, 8HQN and its combination with AmB were tested on L. martiniquensis in vitro for the first time. 8HQN was effective against L. martiniquensis and worked synergistically with AmB to reduce AmB used in the combination against parasites. These findings suggest that 8HQN can be considered as an alternative treatment for L. martiniquensis infection. The use of combinations may also bring significant advantages and better therapeutic effects than AmB alone.