Roles of H2S and NO in regulating the antioxidant system of Vibrio alginolyticus under norfloxacin stress
- Published
- Accepted
- Received
- Academic Editor
- Piotr Dąbrowski
- Subject Areas
- Agricultural Science, Marine Biology, Microbiology
- Keywords
- Cystathionine-β-synthase (CBS), Nitric oxide synthetase (NOS), Antioxidant system, H2S, NO, Norfloxacin, Vibrio alginolyticus
- Copyright
- © 2021 Chen et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Roles of H2S and NO in regulating the antioxidant system of Vibrio alginolyticus under norfloxacin stress. PeerJ 9:e12255 https://doi.org/10.7717/peerj.12255
Abstract
Antioxidant system is of great importance for organisms to regulate the level of excessive reactive oxygen species (ROS) under the environmental stresses including antibiotics stress. Effects of norfloxacin (NOR) on cystathionine-β-synthase (CBS), nitric oxide synthase (NOS) and antioxidant enzymes were investigated, and interaction between NO and H2S and their regulation on the antioxidant system of Vibrio alginolyticus under NOR were determined as well in the present study. After treated with 2 µg/mL NOR (1/2 MIC), CBS content, H2S and NO contents decreased while H2O2 accumulation and the antioxidant-related genes mRNA level increased. Additionally, the endogenous H2S content in V. alginolyticus was increased by the exogenous NO, while H2O2 accumulation and the relative expression level of SOD (Superoxide dismutase gene) decreased under exogenous NO or H2S. And the content of endogenous NO and NOS in V. alginolyticus increased under the exogenous H2S as well. Taken together, these results showed that anti-oxidative ability in V. alginolyticus was respectively enhanced by the gas molecules of H2S and NO under NOR-induced stress, and there may be a crosstalk regulative mechanism between H2S and NO. These results lay a foundation for the research of regulation network of H2S and NO, and provide a hint to synthesize anti-vibrio drugs in the future.
Introduction
Under the environmental stresses including antibiotics stress, the excessive reactive oxygen species (ROS) produced by bacteria have deleterious effects on their growth and reproduction. Antioxidant system, comprised of antioxidant enzymes and nonenzymatic antioxidants, is of great significance for bacteria to resist and reduce ROS under the adverse condition (Ding et al., 2012). Superoxide dismutase (SOD), glutathione reductase (GR) and catalase (CAT) are the significant members of the antioxidant enzymes. Glutathione (GSH) is an important nonenzymatic antioxidant, and it can be regenerated through reduction of GSSG (oxidized glutathione) catalyzed by NADPH-dependent glutathione reductase (GR) (Hicks et al., 2007). Hydrogen sulphide (H2S) has been proved to be a gasotransmitter (Nandi, Ravindran & Kurian, 2018; Arif et al., 2020), and plays a vital role in regulating the antioxidant system by activating SOD, a crucial antioxidase, to reduce ROS and balance the oxidation-reduction level in Escherichia coli (MG1655) (Shatalin et al., 2011). Another ubiquitous gasotransmitter, nitric oxide (NO), which is proved to induce the tolerance of biological cells under high temperature stress by increasing H2S content (Li et al., 2013), also acts as the mediator of antioxidant system to protect bacteria from the antibiotics stress (Gusarov et al., 2009). Although the mechanisms and the detailed network of crosstalk between NO and H2S remain unclear, some studies have demonstrated that the two gases can act synergistically to keep the cellular redox homeostasis (Shatalin et al., 2011; Li et al., 2013; Iqbal et al., 2021).
Vibrio alginolyticus is a gram-negative bacterium, and a marine pathogenic species which can cause diseases of many marine animals with heavy economic loss in aquaculture industry (Ardiç & Ozyurt, 2004; Liu et al., 2004; Zhou et al., 2013). In order to prevent the vibriosis, antibiotics is considered as the most effective and economic method, and has already been used widely, even though it can cause new environmental problems. The bactericidal mechanism varies with the different antibiotics (Walsh, 2000), but most of them would increase ROS level in the bacterial cells and induce the cellular death (Davies et al., 2009; Dwyer, Kohanski & Collins, 2009; Mols & Abee, 2011). Therefore, it is necessary and reasonable that V. alginolyticus uses its antioxidant system to eliminate the excessive ROS to protect itself from the oxidative damage induced by antibiotics. However, whether H2S and NO can act synergistically in regulating its antioxidant system in V. alginolyticus is still unknown.
In this paper, in order to discover and obtain fundamental information about the role of H2S and NO on the antioxidant system of V. alginolyticus treated with NOR, the content of hydrogen sulfide synthase (cystathionine-β-synthase, CBS) and nitric oxide synthase (NOS), H2S and NO and hydrogen peroxide (H2O2), and the relative expression levels of antioxidant-related genes such as superoxide dismutase (SOD), glutathione reductase (GR) and catalase (CAT), were measured under different gas donors and scavengers treatment, which is expected to provide a hint to synthesize drugs to resist Vibrio spp. in the future.
Materials and Methods
Bacteria strain, culture media and harvest
V. alginolyticus HY9901 strain used in this work was isolated and preserved in our laboratory, Guangdong Provincial Key Laboratory of Pathogenic Biology and Epidemiology for Aquatic Economic Animals in China (Cai et al., 2007). Bacteria were cultured in Tryptone Soy Broth medium (TSB with an adjusted pH of 7) with 15 g/L tryptone, 5 g/L soy peptone and 5 g/L NaCl. Bacteria cells were collected by centrifugation. Subsequently, the pellets were resuspended and washed with phosphate buffer saline (PBS) for 2–3 times for the following biochemical measurements after sonication. While RNA was isolated from the collected cells (in 1 mL) without washing after centrifugation.
Primers and reagents
Genes encoding antioxidant-related enzymes of SOD, GR and CAT in V. alginolyticus were cloned and sequenced in our previous work, and qPCR primers were designed according to the sequenced results (Table 1), referenced by 16S rDNA gene. Primers were synthesized by Sangon Biological Engineering Technology & Services Co., Ltd. (China). The kits of RNA extraction and reverse transcription were from Beijing TransGen Biotech Co., Ltd. (China). Sodium hydrosulfide (NaSH, H2S donor), hypotaurine (HT, H2S scavenger), sodium nitroprusside (SNP, NO donor) and 2-phenyl-4, 4, 5, and 5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO, NO scavenger) came from Sigma-Aldrich Co. LLC (USA), while NOR came from Guangzhou Technology Company Limited (China). ELISA kits for microorganism CBS and NOS, H2S and NO were provided by Shanghai Jianglai Industrial Ltd (China). Hydrogen Peroxide Assay Kit and Total Protein Quantification Assay Kit were from Nanjing Jiancheng Bioengineering Institute (China).
| Gene | Sequences (5′- 3′) | Amplicon size (bp) | Reference |
|---|---|---|---|
| SOD (s) | TTATGGCGTTGTTTTTAC | 162 | This study |
| SOD (a) | TGCTTCCCTGTGTTGTTA | ||
| CAT (s) | AAAAAGATTGGCAAGGGA | 172 | This study |
| CAT (a) | GCGAATGGCACAGATACA | ||
| GR (s) | GGTGGTCGTCCTACTATTCC | 196 | This study |
| GR (a) | TACGCAGTGGTGACTCTTTAC | ||
| 16S rDNA (s) | AAAGCACTTTCAGTCGTGAGGAA | 156 | Rui et al. (2008) |
| 16S rDNA (a) | TGCGCTTTACGCCCAGTAAT |
Preparation for stock solutions
NaSH, SNP, HT and PTIO were respectively weighed, and then dissolved and diluted with cold double-distilled water to yield stock solutions in a final concentration of 100.0 mM. Except for NaSH and SNP are prepared before using, all the other liquors were preserved in −20 °C for one week, after filtering through 0.22 µm microfiltration membrane.
0.15 g of NOR was dissolved with 10 mL 12 mol/L cold HCl to yield a stock solution with a final concentration 15,000 µg/mL and preserved in −80 °C for one week.
Determination of the minimum inhibitory concentration (MIC) of NOR against V. alginolyticus
V. alginolyticus was respectively cultured in total volume of 0.5 mL at 28 °C for 48 h with NOR from 0.125 to 256 µg/mL (at final concentration) using two-fold serial dilution method. Briefly, the stock solution of NOR was added into 10 mL V. alginolyticus culture in logarithmic period with OD600 of 0.1, to yield 256 µg/mL NOR treatment, and subsequently 0.5 mL of V. alginolyticus culture with 256 µg/mL NOR was transferred into 0.5 mL of V. alginolyticus culture in logarithmic period without NOR, to yield 128 µg/mL NOR treatment. Similarly, repeat the above dilution till NOR concentration reach 0.125 µg/mL. As a control, a same volume of HCl was added into 10 mL V. alginolyticus culture, and diluted with the same method as described above. Growth of V. alginolyticus in different concentration groups was recorded to determine MIC value of NOR against the bacterium. Every treatment including control were triplicated (n = 3).
Sample treatment with NOR
V. alginolyticus was transferred to 50 mL fresh TSB medium and cultured at 28 °C for 10 h till OD600 value with 0.5, and subsequently treated with 0 (control) and 1/2 MIC concentration NOR, respectively, and continued to culture for 2 h. Then harvested cells and measured CBS and NOS, H2S and NO contents, and H2O2 accumulation as well as the relative expression levels of the antioxidant-related genes. Every treatment was triplicated (n = 3).
Measurement of the total protein content
Total protein was measured according to the bicinchoninic acid method (with standard sample) (Smith et al., 1985) with Total Protein Quantification Assay Kit (Nanjing Jiancheng Bioengineering Institute, China). In briefly, the principle of assessment total protein concentration is that Cu+ reduced from Cu2+ by proteins in the alkaline condition can react with the bicinchoninic acid (BCA) reagent to form the purple complex which can be spectrophotometrically read at 562 nm, and to quantify the protein by comparing with the standard curve. The absorbance is proportional to the protein concentration, so concentration can be obtained following the formula: total protein = (ODsample- ODblank)/(ODstandard- ODblank) × standard sample (524 µg/mL) × sample dilution times.
Interaction of H2S and NO
To determine the effect of NO on CBS content and H2S content, V. alginolyticus was treated with a final concentration of the following resolution respectively, (1) control; (2) 1.0 mM SNP; (3) 0.2 mM PTIO under NOR for 2 h. Every treatment was repeated thricely. Similar to the above, in order to determine the effect of H2S on NOS content and NO content, V. alginolyticus was treated with a final concentration of the following resolution respectively, (1) control; (2) 1.0 mM NaSH; (3) 0.2 mM HT for 2 h. Every treatment was triplicated (n = 3).
For studying the effect of NO and H2S on the antioxidant-related enzymes, V. alginolyticus was treated for 2 h with a final concentration of the following resolution respectively: (1) control; (2) 1.0 mM SNP; (3) 0.2 mM PTIO; (4) 1.0 mM NaSH; (5) 0.2 mM HT. Every treatment was triplicated (n = 3). H2O2 accumulation level and relative expression level of the antioxidant-related genes were subsequently determined.
Measurement of CBS and NOS contents
CBS and NOS contents were measured with ELISA method of the quantitative sandwich immunoassay technique (Sandwich ELISA) (Stynen et al., 1995). The principles and methods are briefly described that the purified antibody against CBS or NOS was pre-coated in microtiter plate wells in advance, subsequently the sample containing CBS or NOS was added into the microtiter plate, to form antigen-antibody complex through 30 min incubation with closure plate membrane at 37 °C. After washing with wash buffer and discarding residue liquid by swing completely, 50 µL combined antibody of CBS or NOS with horseradish peroxidase (HRP)-conjugate reagent were introduced into plate wells, forming the antibody-antigen-antibody (HRP enzyme labeled) complex after 30 min incubation at 37 °C. Subsequently, microtiter plate was washed with wash buffer and swing completely, and 50 µL carbamide peroxide [CO(NH2)2⋅H2O2)] and 50 µL substrate solution of 3, 3, 5, 5-tetramethylbenzidine (TMB) were added to plate wells. TMB substrate became blue after HRP enzyme catalyzed in 30 min at 37 °C, and this chromogenic reaction was terminated by the addition of 50 µL 2M sulphuric acid solution for the spectrophotometric measurement at 450 nm. The standard curve was set up on the same plate simultaneously, which was used for calculating CBS or NOS contents, and the result was further calibrated by the corresponding total protein concentration. The final results were showed with U/g protein.
Measurement of H2S and NO contents
H2S contents were measured with the sandwich ELISA method (Zheng et al., 2016). Briefly, H2S was caught by the pre-coated antibody in microtiter plate wells in advance, then sample containing H2S were introduced to microtiter plate wells in triplicate, forming antigen-antibody complex after incubation at 37 °C. The following steps refered to the above sandwich ELISA method of CBS and NOS. The result was further calibrated by the corresponding total protein concentration to get the final results (µmol/g protein).
Similar to the sandwich ELISA method of H2S measurement, NO was detected by the ELISA kit according to the manufacturer’s instructions (Abd El Dayem et al., 2019), and subsequently further calibrated by the corresponding total protein concentration, to yield the final results (µmol/g protein).
Measurement of H2O2
H2O2 was measured with Hydrogen Peroxide Assay Kit according to the manufacturer’s recommendations. The measurement principle was that this kind of ROS can react with molybdic acid to form peroxomolybdic acid complex which can be read at 450 nm. The accumulation of H2O2 in the samples were subsequently recorded by comparing OD value of the samples with the standard.
Measurement of the relative expression level of antioxidant-related genes
qPCR was used to measure the gene expression level of SOD, GR and CAT. Total RNA was extracted using RNA extraction kit, and subsequently converted to cDNA using the reverse transcription kits. All RNA samples were adjusted to a same concentration prior to the reverse transcription with RNase free water. cDNA was used to qPCR with a program: 1 denaturation cycle at 94 °C for 5 min, 40 amplification cycles at 60 °C for 20 s, and 72 °C for 45 s with Bio-Rad iQ5 Real Time PCR System (USA). 16S rDNA gene of V. alginolyticus was used as a reference gene in qPCR. All samples were triplicated.
Statistical analysis
Differences between the control and treatments were determined by the statistical t-Test method and F test with GraphPad Prism 5. Results were considered statistically significant if P < 0.05 and presented as means with standard error (SEM).
Results
Minimum inhibitory concentration (MIC) of NOR
NOR minimum inhibitory concentration (MIC) against V.alginolyticus was 4 µg/mL. Hence subinhibitory concentration as 1/2 MIC concentration (2 µg/mL) of NOR was used to treat V. alginolyticus to assess the interaction of H2S and NO under NOR stress.
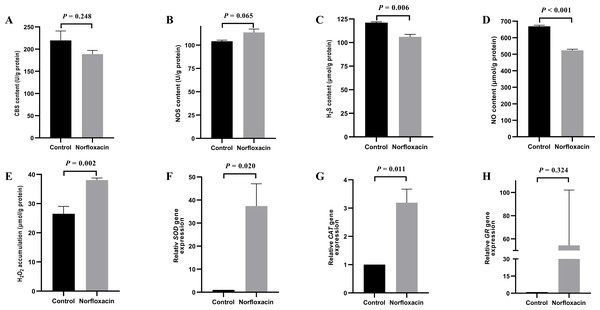
Figure 1: Effect of NOR on Vibrio alginolyticus.
(A) Effect of NOR on CBS content. (B) Effect of NOR-induced stress on the NOS content. (C) Effect of NOR-induced stress on the H2S content. (D) Effect of NOR-induced stress on NO content. (E) Effect of NOR-induced stress on H2O2 content. (F) Effect of NOR-induced stress on the relative expression of SOD. (G) Effect of NOR-induced stress on the relative expression of CAT. (H) Effect of NOR-induced stress on the relative expression of GR. The differences between the control and treatments were determined by unpaired t test of the statistical t-Test (df = 4), and F test was used to compare the variances. “P” stands for P value (two-tailed).Effect of NOR treatment on V. alginolyticus
Under 1/2 MIC NOR stress, CBS content decreased without a significant statistical difference (P = 0.248), and H2S content was lower than that in the control group (P = 0.006) (Figs. 1A and 1C). It is interesting that, NOR could evidently down-regulate NO content (P < 0.001) (Fig. 1D) though it had no significant influence on NOS content in cells (P = 0.065) (Fig. 1B). Besides, H2O2 accumulation (P = 0.002) and the relative expression level of SOD (P = 0.020) and CAT (P = 0.011) increased under NOR stress compared to the control (Figs. 1E, 1F and 1G), and the relative expression level of GR also increased in cells treated with NOR (P = 0.324) (Fig. 1H).
Effect of NO on CBS and H2S content in V. alginolyticus
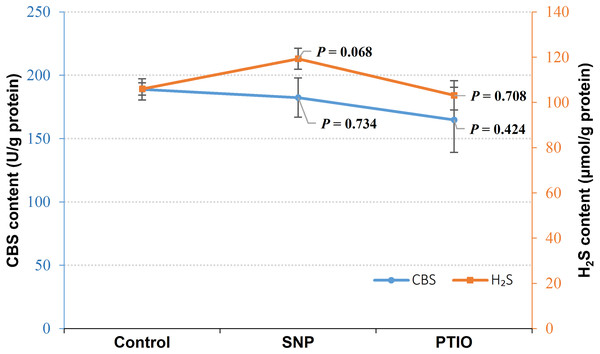
Under NOR stress, the endogenous H2S level increased in the treatment groups with exogenous NO by SNP treatment (P = 0.068), whereas CBS content did not change evidently in the groups treated with SNP compared to the control (Fig. 2). Both of CBS (P = 0.424) and H2S (P = 0.708) content decreased in the group treated with NO scavenger of treatment (PTIO), though P-value failed to achieve a statistical significance (Fig. 2).
Figure 2: CBS content and H2S content in Vibrio alginolyticus after treated with NO donor or scavenger under NOR-induced stress.
The differences between the control and treatments were determined by unpaired t test of the statistical t-Test (df = 4), and F test was used to compare the variances. “P” stands for P value (two-tailed).Effect of H2S on NOS and NO content in V. alginolyticus
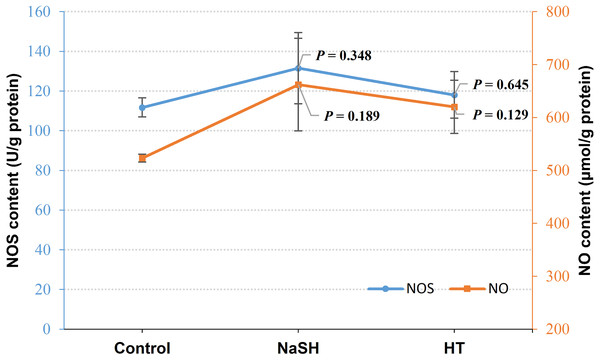
Exogenous H2S (NaSH treatment) improved NOS content in V. alginolyticus (P = 0.348, Fig. 3) and NOS content did not change significantly when treated with H2S scavenger (HT). Interestingly, NO content was up-regulated by both the exogenous H2S (P = 0.189) and H2S scavenger (P = 0.129) treatment compared to the control (Fig. 3).
Figure 3: NOS content and NO content in Vibrio alginolyticus after treated with H2S donor or scavenger under NOR-induced stress.
The differences between the control and treatments were determined by unpaired t test of the statistical t-Test (df = 4), and F test was used to compare the variances. “P” stands for P value (two-tailed).H2O2 accumulation and relative expression level of antioxidant-related genes under donors or scavengers
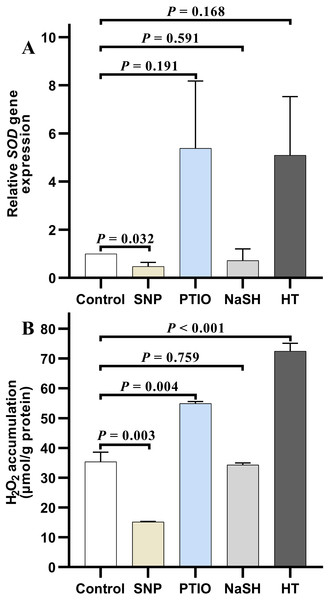
Under NOR stress, the relative expression level of SOD in V. alginolyticus treated with SNP decreased significantly (P = 0.032) compared to the control, but it increased slightly after treated with PTIO (P = 0.191) (Fig. 4A). Also, the relative expression level of SOD in the treatment group with NaSH was lower than that in the control, but it increased quickly when bacteria were treated with HT (P = 0.168) (Fig. 4A). It is noteworthy that, the relative expressions level of CAT decreased significantly in the treatment groups with different donors or scavengers (Data not shown).
Figure 4: H2O2 accumulation and SOD relative expression level in Vibrio alginolyticus after treated with H2S and NO and their donors or scavengers under NOR-induced stress.
(A) relative expression level of SOD. (B) H2O2 accumulation. The differences between the control and treatment were determined by unpaired t test of the statistical t-Test (df = 4), and F test was used to compare the variances. “P” stands for P value (two-tailed).Similar to the change of relative expression level of SOD, H2O2 accumulation in bacterial cells decreased significantly in the treatment group with SNP (P = 0.003), and also decreased in the treatment group with NaSH (P = 0.759), whereas it rose up when treated with PTIO (P = 0.004) or HT (P < 0.001) with significant difference (Fig. 4B) compared to the control group.
Discussion
Low dosage of ROS can act as a cellular signal in regulating the activation of protein kinases (Tobiume et al., 2001) and substance synthesis (Steinite, Gailite & Ievinsh, 2004) in a normal cell. Hence, low levels of ROS in cells are necessary and have a positive role on themselves. However, over-production of ROS induced by the environmental stress can influence on the balance of oxidant/reduction in cells, and will lead to serious destruction or dysfunction of cells (Haigis & Yankner, 2010; Shukla, Mishra & Pant, 2011). Many studies showed that H2S and NO play a great role in regulating the antioxidant system without an identical mechanism (Chen et al., 2013; Li, 2013). Interestingly, H2S has been proved to regulate the antioxidant system by enhancing the activities of the antioxidative enzymes (Shatalin et al., 2011; Chen et al., 2013), while NO was found to has a negative effect on the antioxidative enzymes (Vital et al., 2008), which suggest that it may act as a direct scavenger of ROS to decrease excessive O and H2O2 (Patel et al., 1999; O’Donnell & Freeman, 2001). Though the effects of H2S and NO on the antioxidant system and the cross-talk between H2S and NO have already been studied in some species (Li, 2013), it still remains unknown in V. alginolyticus.
In this work, it was initially found that H2S and NO contents as well as CBS content decreased significantly, while H2O2 accumulation and the relative expression of anti-oxidation related genes increased significantly in V. alginolyticus under NOR stress (Fig. 1). These showed that NOR could induce significantly H2O2 accumulation and the expression of anti-oxidation related genes in V. alginolyticus. Increased expression of antioxidant-related genes should be induced directly by accumulating H2O2 as a kind of ROS and a small molecular messenger (Ding et al., 2012). H2S content was positively related to the content of CBS. But we don’t know whether the antibiotics of NOR affect the biosynthesis of NO, or the decrease in NO content were due to its consumption in alleviating the excessive ROS.
Under NOR stress, H2S content increased after treated with SNP (NO donor) while it decreased after treated with PTIO (NO scavenger) (Fig. 2), and NO content increased when treated with NaSH (H2S donor) and HT (H2S scavenger) (Fig. 3), indicating that there is a regulation and compensation mechanism between H2S and NO to response to NOR-induced stress, and H2S may be the upstream signal molecule in regulating the bio-synthesis of NO. However, the results presented that CBS content also decreased in the treatment of exogenous NO scavenger but no evident change after being treated with the exogenous NO treatment (Fig. 2), while NOS content was up-regulated by exogenous H2S but did not change significantly when H2S was scavenged (Fig. 3). These suggested that H2S and NO have a different effect on each other in regulating their synthases, but still demands more scientific details by the further studies to disclose it.
Many studies focused on the synergistical regulation between the two gases (H2S and NO) in smooth muscle, vasodilation in animal, and cobalt toxicity and heat tolerance in plant (Hosoki, Matsuki & Kimura, 1997; Liew et al., 2007; Ozfidan-Konakci et al., 2020; Hassan, Maulood & Salihi, 2021), whereas the research on the synergistic effect of the two gas molecules in regulating the antioxidant system of V. alginolyticus was hardly reported. In fact, the results mentioned above showed that there is significant correlation between the antioxidant system and two gas molecules of H2S and NO under the antibiotics stress. It showed that under NOR stress, the endogenous H2S and NO contents increased after treated respectively with exogenous NO and H2S (Figs. 2 and 3), while H2O2 accumulation and the relative expression level of SOD were down-regulated in V. alginolyticus (Fig. 4). That is to say, H2O2 induced by NOR was alleviated by the up-regulation of the exogenous NO and H2S on the anti-oxidative ability, while its accumulation was facilitated by the NO and H2S scavenger in the treatment with PTIO and HT, respectively (Fig. 4B). Decreased H2O2 down-regulated the gene expression level of anti-oxidative enzyme SOD. These also suggested that both NO and H2S, directly or indirectly, take part in eliminating ROS induced by NOR in V. alginolyticus, and also showed that H2S and NO may work synergistically to regulate the antioxidant system in this bacterium under NOR stress. But interestingly, it had been found that NO content induced by abscisic acid (ABA) in plants decreased under the exogenous H2S (Lisjak et al., 2011). And NO content increased whereas H2S decreased in rats under chronic restraint stress (Moustafa, 2021), suggesting that there also exists antagonistic effect between these two gases in cells (Decréau & Collman, 2015). Therefore, whether H2S and NO act synergistically on the antioxidant system is inconsistent in all the organisms and should be species-specific.
Finally, the medicines that can disturb the metabolic pathway of H2S and NO are potential to enhance the bactericidal effect, hence it is worthwhile to develop and synthesize these anti-vibrio drugs in the future, even though it needs more endeavors and cross studies in this fields. We believe that it was, and it will be a research hotspot in the future.
Conclusion
Anti-oxidative ability in V. alginolyticus was respectively enhanced via the regulation of H2S and NO under NOR-induced stress, and there may be a crosstalk mechanism between H2S and NO to regulate the antioxidant system of V. alginolyticus treated with NOR, which lay a foundation for the research of regulation network of H2S and NO, and for study on the anti-drug mechanism. It also provides a novel target for the synthesis of anti-vibrio drugs.