Semidry acid hydrolysis of cellulose sustained by autoclaving for production of reducing sugars for bacterial biohydrogen generation from various cellulose feedstock
- Published
- Accepted
- Received
- Academic Editor
- Ela Eroglu
- Subject Areas
- Biotechnology, Microbiology
- Keywords
- Cellulose, Dark fermentation, Escherichia coli, Hydrogen gas, Polysaccharides, Semidry acid hydrolysis
- Copyright
- © 2021 Morsy et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Semidry acid hydrolysis of cellulose sustained by autoclaving for production of reducing sugars for bacterial biohydrogen generation from various cellulose feedstock. PeerJ 9:e11244 https://doi.org/10.7717/peerj.11244
Abstract
Cellulosic biowastes are one of the cheapest and most abundant renewable organic materials on earth that can be, subsequent to hydrolysis, utilized as an organic carbon source for several fermentation biotechnologies. This study was devoted to explore a semidry acid hydrolysis of cellulose for decreasing the cost and ionic strength of the hydrolysate. For semidry acid hydrolysis, cellulose was just wetted with HCl (0 to 7 M) and subjected to autoclaving. The optimum molar concentration of HCl and period of autoclaving for semidry acid hydrolysis of cellulose were 6 M and 50 min respectively. Subsequent to the semidry acid hydrolysis with a minimum volume of 6 M HCl sustained by autoclaving, the hydrolysate was diluted with distilled water and neutralized with NaOH (0.5 M). The reducing sugars produced from the semidry acid hydrolysis of cellulose was further used for dark fermentation biohydrogen production by Escherichia coli as a representative of most hydrogen producing eubacteria which cannot utilize non-hydrolyzed cellulose. An isolated E. coli TFYM was used where this bacterium was morphologically and biochemically characterized and further identified by phylogenetic 16S rRNA encoding gene sequence analysis. The reducing sugars produced by semidry acid hydrolysis could be efficiently utilized by E. coli producing 0.4 mol H2 mol−1 hexose with a maximum rate of hydrogen gas production of 23.3 ml H2 h−1 L−1 and an estimated hydrogen yield of 20.5 (L H2 kg−1 dry biomass). The cheap cellulosic biowastes of wheat bran, sawdust and sugarcane bagasse could be hydrolyzed by semidry acid hydrolysis where the estimated hydrogen yield per kg of its dry biomass were 36, 18 and 32 (L H2 kg−1 dry biomass) respectively indicating a good feasibility of hydrogen production from reducing sugars prepared by semidry acid hydrolysis of these cellulosic biowastes. Semidry acid hydrolysis could also be effectively used for hydrolyzing non-cellulosic polysaccharides of dry cyanobacterial biomass. The described semidry acid hydrolysis of cellulosic biowastes in this study might be applicable not only for bacterial biohydrogen production but also for various hydrolyzed cellulose-based fermentation biotechnologies.
Introduction
Cellulosic biowastes are of the most abundant renewable biomass available for hydrolysis for a cost-effective fermentation bioindustries. One of these fermentation biotechnologies is the production of hydrogen gas by bacteria. Hydrogen gas is one of the applicable sources of future renewable energy that is potentially promising to replace the present worldwide used fossil fuels (Yokoi et al., 2001; Nath & Das, 2004; Kapdan & Kargi, 2006; Chong et al., 2009; Abd-Alla, Morsy & El-Enany, 2011; Budiman & Wu, 2018; Noblecourt et al., 2018; Morsy et al., 2019). The hydrogen gas production by eubacteria is a cost-effective way where cheap organic wastes can be utilized in such future biological industry (Yu et al., 2002; Zhang, Brunsb & Logan, 2006; Pattra et al., 2008; Lo et al., 2009; Cheng & Chang, 2011; Noparat, Prasertsan & Sompong, 2011; Hay et al., 2016; Morsy, 2017; Morsy, Elbahloul & Elbadry, 2019). Cellulose feedstock come at the top of interest to be utilized as an organic form source of carbon for producing H2 by eubacteria, however most hydrogen producing eubacteria cannot utilize cellulosic biowastes. Hydrolysis of cellulose is thus fundamental for an efficient utilization of cellulosic biowastes in the production of hydrogen by many eubacteria. The efficiency of acid hydrolysis of polysaccharides in general depends on its polymeric complexity where cellulose is one of the difficult types of polysaccharides to hydrolyze. Due to abundant cellulose feedstock all over the world (Sukumaran, Singhania & Pandey, 2005), its hydrolysis is of the hot topics for the production of reducing sugars for various biofuels fermentation biotechnological industries. In the past, cellulosic feedstocks were used in heating but now with the modernization of developing countries, it is no longer used and replaced by biogas. Thus, farmers in developing countries sometimes burn most of the crop plants straw in-situ of their farms to get rid of it. This leads to a high-risk pollution and increases the CO2 in the atmosphere; a trouble that shares to some extent, besides CO2-liberating industries and extensive use of fossil fuels, in increasing the percent of CO2 gas in the atmosphere enhancing global warming. Many works of literature seek useful utilization of cellulose feedstocks in producing hydrogen gas biologically. Acid and enzymatic hydrolysis of the polysaccharide cellulose is usually used for producing reducing sugars (Camacho et al., 1996; Iranmahboob, Nadim & Monemi, 2002; Sun & Cheng, 2002; Xiang et al., 2003; Taherdazeh & Karimi, 2007; El-Zawawy et al., 2011; Heinonen et al., 2012; Ni et al., 2013; Pulidindi, Kimchi & Gedanken, 2014; Vo et al., 2014; Yoon, Han & Shin, 2014). The acid hydrolysis is more applicable in high mass of cellulose at industrial scale. Reducing the amount of acid used in the hydrolysis is useful in two aspects; first it reduces the cost by reducing the amount of acid per-se and the amount of base used in subsequent neutralization step; second it will reduce the final ionic strength of the fermentation medium that might adversely affect the fermentation process. In this study a semidry acid hydrolysis of cellulose was conducted and its efficiency in producing reducing sugars was investigated. Applicability of acid hydrolysis of lignocelluloses and other polymeric carbohydrates is extensively reported in many fields of biotechnologies (Cuzens & Miller, 1997; Baruah et al., 2018; Bhatia et al., 2021). Hydrogen gas production by E. coli strain TFYM from hydrolyzed pure cellulose and various cellulose feedstock was investigated as an example of the many biotechnological applications that can be served by semidry acid hydrolysis of cellulose in a cost-effective way.
Materials and Methods
Semidry acid hydrolysis of cellulose
Cellulose [Cellulose powder RM126, HiMedia Laboratories Pvt. Ltd. India], retaining no humidity confirmed by drying at 70 °C for 20 h and re-weighting and stored in dry conditions, was used for semidry acid hydrolysis where it was just wetted by HCl in a ratio of 1:1 [1g cellulose: 1 ml HCl], in loosely screw capped glass tubes, followed by autoclaving at 121 °C using LabTech vertical autoclave (Model LAC-51005, Daihan LabTech Co., LTD, Namyangju-City, Kyonggi-Do, Korea).
Optimization of HCl molar concentration and period of autoclaving for semidry acid hydrolysis of cellulose
Optimization experiments of semidry acid hydrolysis was conducted in loosely screw capped glass tubes retaining 1 g cellulose and 1 ml of 0 (H2O) to 7 M HCl (255.2 g/L). For optimization of HCl molar concentration and period of autoclaving for semidry acid hydrolysis of cellulose, optimum molarity of HCl for hydrolysis was determined first. The optimization of HCl molar concentration for semidry acid hydrolysis of cellulose was conducted using various molarities of HCl (0 to 7 M) at a constant period of autoclaving for 30 min. Subsequently, the optimization of the autoclaving period for semidry acid hydrolysis of cellulose was investigated at constant optimum molarity of HCl using various autoclaving periods (0 to 70 min). The determined optimum molarity was 6 M HCl for semidry acid hydrolysis of cellulose and thus, determination of the optimum period of autoclaving was conducted at constant 6M HCl. The period (0.0) of autoclaving is after acid treatment (addition of 6M HCl to cellulose) before autoclaving. Control samples with no acid treatment (acid replaced by distilled water) were subjected to the various periods of autoclaving used.
Hydrolysate neutralization and preparation
Subsequent to autoclaving, the hydrolysate was diluted with distilled H2O and filtered through Whatman No. 1 filter paper (Cat. No. 1001-090, Whatman International Ltd, Maidstone England). The hydrolysate filtrate was neutralized by 0.5 M NaOH solution and the reducing sugars content was measured by Nelson reagent (Nelson, 1944).
Semidry acid hydrolysis of various cellulosic biowastes
Semidry acid hydrolysis was applied on various cellulose feedstocks including wheat bran, sawdust and sugarcane bagasse. Wheat bran was purchased from the market. Sawdust was obtained from a carpenter shop. Sugarcane bagasse was obtained from Egypt and ground to small pieces. All feedstocks were washed with distilled water, filtered through cloth filter and dried at 70 °C up to constant weight, stored in dry conditions and subsequently used for semidry acid hydrolysis. Cellulose and various cellulose feedstocks (wheat bran, sawdust and sugarcane bagasse) were subjected to semidry acid hydrolysis in loosely screw capped glass bottles (250 ml SCHOTT Duran-group, Germany) retaining 10 g dry biomass and 10 ml of optimum HCl molar concentration (6M HCl) in a ratio of 1:1 [1g biomass: 1 ml HCl] and subjected to optimum autoclaving period (50 min.). The hydrolysates were diluted, filtered and neutralized as described above. The released reducing sugars content in the filtrates was measured by Nelson reagent (Nelson, 1944) where the efficiency of the semidry acid hydrolysis represented in the percent hydrolysis of the cellulosic biowastes was calculated as follows: The neutralized filtrate retaining the released reducing sugars from semidry acid hydrolysis of cellulose and various cellulosic feedstocks were used subsequently for dark fermentation hydrogen gas production by E. coli TFYM.
Isolation and identification of E. coli TFYM
Isolation of E. coli and its phenotypic characterization was conducted by standard protocols. Isolation of the bacterium E. coli TFYM was conducted using lactose broth following the techniques [MPN (Most Probable Number)] (MacFaddin, 1985) from wastewater sample at Saudi Arabia. Confirmation of the positive tubes was performed using Eosin Methylene Blue (EMB) agar and it was further characterized as E. coli bacterium on MacConkey medium. Other characterizations of the bacterium were conducted following Bergey’s Manual (Brenner et al., 2005).
Molecular biological identification and phylogenetic analysis of E. coli TFYM 16S rRNA gene sequence
The 16S rRNA gene amplification by PCR
The bacterial cells genomic DNA was extracted by Promega Wizard Genomic DNA Purification Kits (Promega, USA) according to the kit manufacturer instructions. Subsequently, the 16S rRNA encoding gene amplification was conducted to a near-full length by PCR using the genomic DNA template and the universal forward 27F primer with a sequence of (5′-AGAGTTTGATC[A/C]TGGCTCAG-3′) and reverse 1492R universal primer with a sequence of (5′-G[C/T]TACCTTGTTACGACTT-3′) (Lane, 1991). The amplification by PCR was performed in a reaction mixture (25 µl) composed of 2.5 µl of 10 × Taq buffer (100 mM Tris–HCl, pH 8), 100 µM dNTPs, 1.25 mM MgCl2, 1.2 µM forward and reverse universal primers, 0.5U of the Taq DNA polymerase, in addition to the genomic DNA of about 5 ng as a template. The PCR amplification was performed using Model 2720, USA Applied Biosystem Thermal Cycler following a program for PCR of 5 min at 95 °C (initial denaturation), 35 cycles of [1 min at 94 °C (denaturation), 1 min at 56 °C (annealing), and 1 min at 72 °C (extension)] followed by 10 min at 72 °C as a final extension. The PCR amplification product was subsequently analyzed by standard protocol of agarose-electrophoresis using 1% agarose-gel retaining 5 µg/mL of ethidium bromide. A 1 kb Plus DNA ladder size marker (Invitrogen, USA) was used.
Sequencing, accession number and phylogenetic analysis of 16S rRNA encoding gene nucleotides sequence
The amplification product of the PCR was purified by PCR Purification Kit [Invitrogen PureLink (Invitrogen, USA)]. Subsequently the purified PCR product was photometrically quantified. Using same forward and reverse primers, the PCR purified product was cycle sequenced in both directions using automated florescent dye terminator sequencing method (Sanger, Nicklen & Coulson, 1977) at Macrogen Korea sequencing facility, (Seoul, Korea). The 16S rRNA encoding gene sequence reads were assembled and compared with its nearest matches found by searching in the nucleotide-nucleotide BLAST of the GenBank website search tools of the NCBI website. The alignments of the base sequences of the 16S rRNA encoding gene were conducted using the website of Clustal W1.83 XP (Thompson et al., 1997). The 16S rRNA gene sequence derived phylogenetic tree was constructed through the use of neighbor-joining method (Saitou & Nei, 1987) using MEGAX software (Kumar et al., 2018). Bacillus cereus strain ATCC14579 (NR_074540.1) was used as outgroup. The obtained base sequence in this study of E. coli TFYM 16S rRNA encoding gene has been deposited as near full length sequence of this gene in the GenBank website of nucleotide sequence database under accession number MK332445.1.
Growth of E. coli on reducing sugars prepared by semidry acid hydrolysis of cellulose as indicator of its utilization of as a carbon source
The utilization of semidry acid hydrolyzed cellulose by E. coli for growth was investigated using BM (Basal Mineral) medium supplemented with 5 g/L reducing sugars of semidry acid hydrolyzed cellulose. BM medium was composed of the followings (per liter): K2HPO4, 4.4 g; (NH4)2SO4, 1.3 g; NaH2PO4, 3.5 g; MgSO4.6H2O, 0.9 g and 1 ml of trace elements solution. The trace elements solution was composed of the followings (per 100 ml): FeSO4 .7H2O, 0.37 g; CaCl2.2H2O, 4.8 g; MnCl2, 0.1 g; CoCl2.6H2O, 0.04 g; Na2MoO4.2H2O, 0.02 g. The aerobic growth of E. coli on reducing sugars of semidry acid hydrolysis of cellulose on basal medium (BM) was followed spectrophotometrically by absorbance at wavelength 600 nm quantified using UV/Vis spectrophotometer (6320D Jenway). A calibration of the growth followed by OD at 600 nm versus dry cell weight (DCW) was conducted in 100 ml cultures of E. coli on reducing sugars of semidry acid hydrolysis of cellulose in Basal Mineral (BM) medium.
Hydrogen gas production by E. coli TFYM dark fermentation
Prior to fermentation, E. coli TFYM was grown in LB medium [10 g/l tryptone, 10 g/l NaCl, 5 g/l yeast extract] at 35 °C. E. coli TFYM batch dark fermentation experiments were conducted for H2 formation from reducing sugars prepared as described above by semidry acid hydrolysis of cellulose and cellulosic feedstock biowastes (wheat bran sugarcane bagasse and sawdust). A one-liter glass fermentation bottle was used with a working volume of 970 ml. A volume 870 ml of neutralized hydrolysate was put in the fermentation bottle and supplemented with E. coli TFYM (OD600 equal 0.25) 100 ml inoculum. The fermentation bottle was closed with a rubber bung and subsequently sparged with nitrogen gas for 20 min to accelerate installing anaerobic conditions. The fermentation bottle was kept in dark with continuous stirring at 35 °C. The evolved H2 gas was collected using a CO2-free H2 gas collection system (Morsy, 2015) relying on passing the evolved gas on NaOH solution for absorbing CO2 and subsequently collecting the CO2-free H2 gas by water displacement. The collected H2gas compared to pure H2 gas was measured using a Clark-type platinum-coated electrode computerized system [purchased from (Hansatech Instruments, Inc.) for Taibah University, Saudi Arabia] according to manufacturer instructions. The rate of H2production was estimated as previously described (Morsy, Elbahloul & Elbadry, 2019) at each point of measurements of the produced cumulative H2 gas as follows: Where (Vx − Vp) is the difference in volume of collected cumulative H2 gas at measurement time point (x) and the previous measurement time point (p). (Tx − Tp) is difference in time between the two cumulative H2gas measurement points.
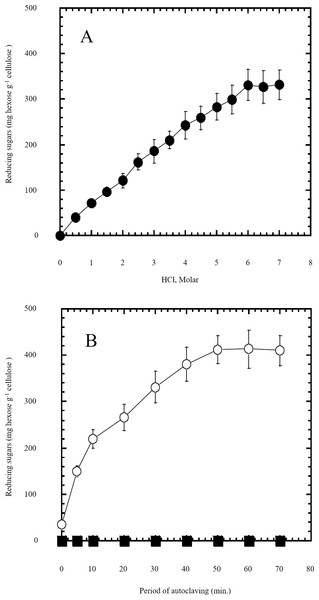
Figure 1: Semidry acid hydrolysis of cellulose.
(A) shows the optimization of HCl molar concentration in semidry acid hydrolysis of cellulose sustained by autoclaving for 30 min. Semidry acid hydrolysis of cellulose using various molarities of HCl for a constant period of autoclaving for 30 min. (B) shows the autoclaving period optimization for semidry acid hydrolysis of cellulose at 6M HCl. The determined optimum molarity of 6 M HCl for semidry acid hydrolysis of cellulose was used (open circles) for determining the optimum period of autoclaving. Control samples (closed squares) with no acid treatment (replaced by water) were subjected to various periods of autoclaving used. The mean values of three replicates and standard errors are shown.Results
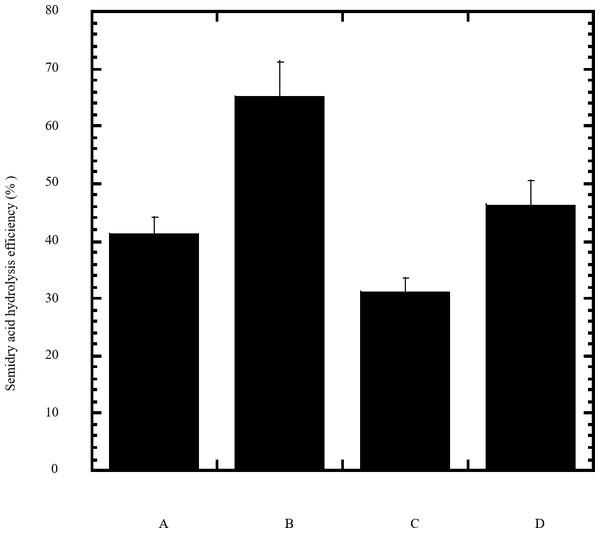
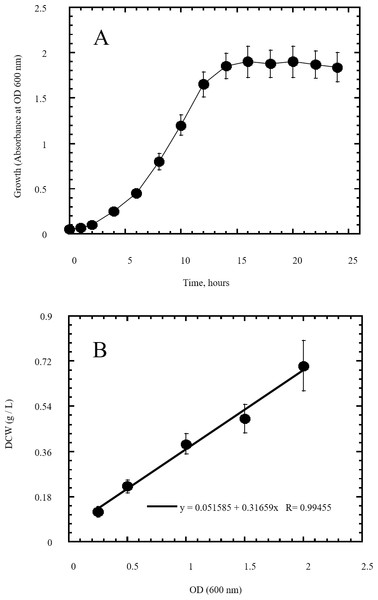
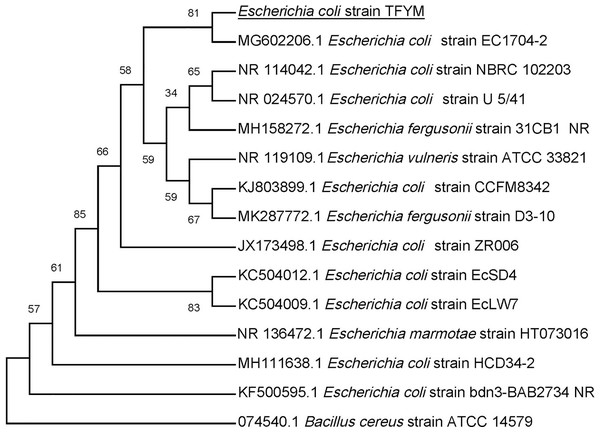
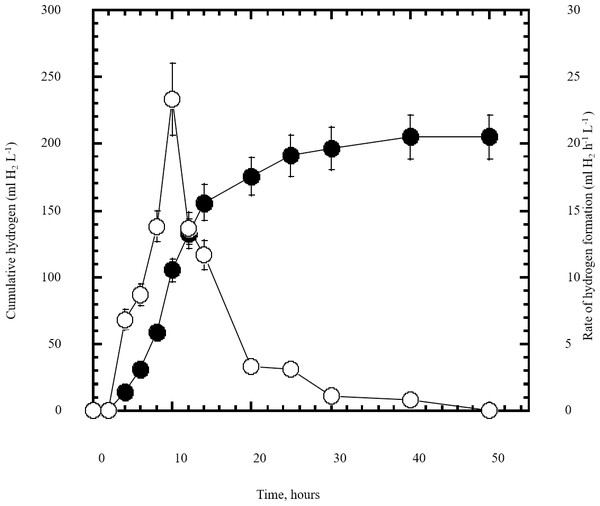
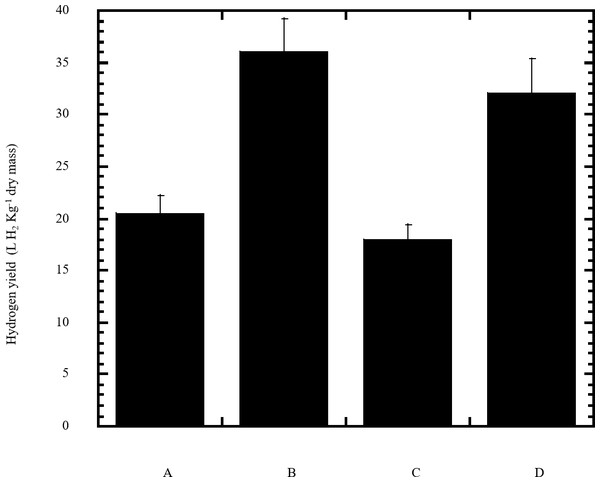
In this study a semidry acid hydrolysis of cellulose and cellulosic feedstock biowastes was conducted for producing reducing sugars that can be used in many fermentation biotechnologies including production of hydrogen gas by bacterial dark fermentation. The semidry acid hydrolysis of cellulose was performed in a ratio of 1:1 [1 g biomass:1 ml of HCl where the cellulose was just wetted by the acid and subjected to autoclaving. The optimum HCl molar concentration for semidry acid hydrolysis of cellulose was 6 M determined first at autoclaving period of 30 min (Fig. 1A). The optimum autoclaving period was subsequently determined as 50 min (Fig. 1B) at optimum 6M HCl. These optimum 6M HCl and 50 min autoclaving conditions was efficient (Fig. 2) in hydrolyzing various cheap cellulosic feedstock biowastes (wheat bran, sawdust and sugarcane bagasse). The percent hydrolysis of wheat bran [65% ± 10.58 (Standard Deviation)] and sugarcane bagasse (46.3% ± 7.18) was more than pure cellulose (41.2% ± 5.31) possibly due to hydrolyzing cellulose and other less complicated more hydrolysable polysaccharides such as hemicellulose and others that is known to be present in natural plant residues. The semidry acid hydrolysis was also applied on non-cellulosic biomass of the potent extracellular polysaccharides producing cyanobacterium Nostoc commune where it produced 0.51 ± 0.12 g reducing sugars/ g dry mass. The ability of E. coli to utilize the reducing sugars produced by semidry acid hydrolysis of cellulose was conducted on Basal Mineral (BM) medium, so that the medium contains only minerals and a soli organic carbon source (the reducing sugars of semidry acid hydrolyzed cellulose). The results showed good ability of E. coli for utilization of the reducing sugars prepared by semidry acid hydrolysis of cellulose as investigated in a basal mineral medium where the bacterium could grow efficiently (Fig. 3) using the hydrolysate reducing sugars as an organic carbon source. The aerobic growth of E. coli on reducing sugars of semidry acid hydrolysis of cellulose in Basal Mineral (BM) medium followed by OD at 600 nm (Fig. 3A) was calibrated (Fig. 3B) versus dry cell weight (DCW) where a calibration value of OD600 nm of 1 equals 0.368 g/L. E. coli like most other hydrogen producing bacteria cannot utilize cellulose as a carbon source and its utilization of reducing sugars prepared by semidry acid hydrolysis is a representative for efficiency of using such hydrolysate in dark fermentation. The bacterium used in this study was E. coli TFYM as a representative of the hydrogen producers to investigate the suitability of the hydrolysate prepared by semidry acid hydrolysis. This isolated bacterium was identified morphologically and biochemically (Table 1) following standard protocols. The identification was also confirmed by phylogenetic analysis of the 16S rRNA encoding gene sequence (Fig. 4). As the bacterium could utilize the reducing sugars in the hydrolysate prepared by semidry acid hydrolysis of cellulose, it was used for investigating the suitability of the hydrolysate for dark fermentation biohydrogen gas production. For the batch dark fermentation hydrogen production, the semidry acid hydrolysis of 10 g cellulose or various cellulose feedstock of wheat bran, sawdust and sugarcane bagasse was conducted and the hydrolysate retaining the reducing sugars was filtrated and neutralized to be used as an organic source of carbon for hydrogen formation by E. coli TFYM dark fermentation. The rate of hydrogen gas production gradually increased and was maximum (23.3 ml H2 h−1 L−1) at 10 h after the start of fermentation followed by a decline at the late stationary phase of cumulative hydrogen production (Fig. 5). The reducing sugars produced by semidry acid hydrolysis (4.12 g/ 10 g cellulose) were efficiently used for hydrogen production by E. coli producing 0.4 mol H2 mol−1 hexose which is comparable to previously reported yield by E. coli from expensive pure sugars and sugar wastes (Table 2). The estimated hydrogen yield by E. coli (Fig. 6) from the reducing sugars prepared by the semidry acid hydrolysis of the cheap cellulosic biowastes of wheat bran, sawdust and sugarcane bagasse was 36, 18 and 32 (L H2 kg−1 dry biomass) respectively. These results indicate a good feasibility of hydrogen production from reducing sugars prepared by semidry acid hydrolysis of such cheap cellulosic biowastes.
Figure 2: Efficiency of semidry acid hydrolysis of cellulose.
The efficiency of semidry acid hydrolysis of various cellulosic feedstock are represented in percent hydrolysis of pure cellulose (column A), wheat bran (column B), sawdust (column C), sugarcane bagasse (column D). The mean values of three replicates and standard errors are shown.| Characteristics | Escherichia coli | Strain TFYM |
|---|---|---|
| Morphological features | ||
| Colonies on EMB agar | Green Metallic sheen | Green Metallic sheen |
| Gram staining of cell wall | -ve | -ve |
| 3% KOH | Viscous and thread like slime | Viscous and thread like slime |
| Cell shape | Unicellular short rods | Unicellular short rods |
| Bacterial cell motility | Motile | Motile |
| Spore | -ve | -ve |
| Biochemical tests | ||
| Characteristic Growth on MacConkey | +ve | +ve |
| Catalase test | +ve | +ve |
| Oxidase test | -ve | -ve |
| Methyl Red (MR) | +ve | +ve |
| Indole | +ve | +ve |
| Voges-Proskauer (VP) | -ve | -ve |
| Citrate | -ve | -ve |
| Gas | +ve | +ve |
| Presumptive test | +ve | +ve |
| Urease | -ve | -ve |
| Gelatin liquefaction | -ve | -ve |
Figure 3: Growth of Escherichia coli as indicator of its utilization of reducing sugars prepared by semidry acid hydrolysis of cellulose as a carbon source.
The growth of Escherichia coli was conducted on basal mineral medium supplemented with hydrolysate of cellulose and followed photometrically at 600 nm (A) to explore the ability of Escherichia coli for utilizing the reducing sugars produced from semidry acid hydrolysis of cellulose. (B) shows the calibration of OD (600 nm) versus dry cell weight (DCW) of Escherichia coli aerobic growth on reducing sugars of semidry acid hydrolyzed cellulose in Basal Mineral (BM) medium. The mean values of three replicates and standard errors are shown.Figure 4: Phylogenetic tree of isolated Escherichia coli TFYM indicating the relationship of this strain with its nearest bacterial strains neighbors from NCBI.
The evolutionary relationships of Escherichia sp. TFYM to other species of Escherichia sp were deduced using the Neighbor-Joining method to represent the taxa analyzed evolutionary history (Felsenstein, 1985). The evolutionary comparisons considered the variations in the composition bias among sequences (Tamura & Kumar, 2002). Bacillus cereus strain ATCC14759 was used as outgroup for comparison. The evolutionary analysis were performed using MEGA X.Figure 5: Dark fermentation cumulative hydrogen gas production by Escherichia coli TFYM from reducing sugars prepared by semidry acid hydrolysis of cellulose.
The cumulative hydrogen gas production (closed circles) by Escherichia coli TFYM dark fermentation was followed. The rate of hydrogen gas production (open circles) was estimated along the fermentation period. The mean values and standard errors of three independent fermentation experiments are shown.Figure 6: Estimated hydrogen gas yield of Escherichia coli TFYM from reducing sugars prepared by semidry acid hydrolysis of various cellulosic feedstock.
The hydrogen yield was estimated for dark fermentation by Escherichia coli TFYM from reducing sugars of semidry acid hydrolyzed cellulose (column A), wheat bran (column B), sawdust (column C) and sugarcane bagasse (column D). The mean values and standard errors of three independent fermentation experiments are shown.| Microorganism(s) | substrate | Yield [mol of H2/ molof hexose] | Ref. |
|---|---|---|---|
| Escherichia coli HD701 | Acid hydrolyzed potato starch residue stream | 0.45 | Morsy (2014) |
| Escherichia coli W3110 | Glucose | 0.54 | Fan, Yuan & Chatterjee (2009) |
| Escherichia coli ZF3a | Glucose | 0.96 | Fan, Yuan & Chatterjee (2009) |
| Escherichia coli S3 | Glucose | 0.84 | Junyapoon, Buala & Phunpruch (2011) |
| Escherichia coli SH3b | Glucose | 1.48 | Kim et al. (2009) |
| Escherichia colic | Glucose | 0.17 | Manish, Venkatesh & Banerjee (2007) |
| Escherichia coli Δ ldhd | Glucose | 0.23 | Manish, Venkatesh & Banerjee (2007) |
| Escherichia coli HD701 | Acid hydrolyzed molasses | 0.46 | Morsy (2011) |
| Escherichia coli DJT135e | Glucose | 1.51 | Ghosh & Hallenbeck (2009a) |
| Escherichia coli DJT135 | Fructose | 1.27 | Ghosh & Hallenbeck (2009a) |
| Escherichia coli DJT135 | Galactose | 0.69 | Ghosh & Hallenbeck (2009a) |
| Escherichia coli DJT135 | Glucose | 1.69 | Ghosh & Hallenbeck (2009b) |
| Escherichia coli BW25113f | Glucose | 1.35 | Maeda, Sanchez-Torres & Wood (2007) |
| Escherichia coli SR15g | Glucose | 1.82 | Yoshida et al. (2006) |
| Escherichia coli WDHLh | Galactose | 1.12 | Rosales-Colunga, Razo-Flores & Rodriguez (2012) |
| Escherichia coli WDHL | Lactose + galactose | 1.02 | Rosales-Colunga, Razo-Flores & Rodriguez (2012) |
| Escherichia coli WDHL | Glucose + galactose | 1.02 | Rosales-Colunga, Razo-Flores & Rodriguez (2012) |
| Escherichia coli WDHL | Glucose | 0.3 | Rosales-Colunga, Razo-Flores & Rodriguez (2012) |
| Escherichia coli strain TFYM | Semidry acid hydrolyzed pure cellulose | 0.4 | This study |
Notes:
Discussion
Cellulosic biomass wastes are cheap and abundant. Thus, upon hydrolysis, these polymeric renewable organic materials can be used in many fermentation biotechnological industries. This study describes a semidry acid hydrolysis of cellulosic feedstock biowastes where the biomass was just wetted with 6M HCl and subjected to autoclaving for 50 min where a considerable amount of reducing sugars were produced where the percent hydrolysis of pure cellulose was 41.2%. Assessment of semidry acid hydrolysis of cellulose was conducted using pure cellulose in first part of the study where 412 ± 53.1 mg reducing sugars/g of pure cellulose were produced. Subsequently application of semidry acid hydrolysis on crude cellulosic biowastes of wheat bran, sawdust and sugarcane bagasse biomasses was conducted. The cellulose, hemicellulose, lignin and other organic materials composition of these crude biowastes were previously reported (Table 3) where cellulose is basic structural components of these biowastes. In comparison to pure cellulose, semidry acid hydrolysis showed higher percent of hydrolysis in case of wheat bran (65%) and sugarcane bagasse (46.3%) mostly due to presence of complicated more hydrolysable polysaccharides associated with cellulose in these natural plant residues such as hemicellulose which is present along with cellulose in wheat bran (Merali et al., 2015), sugarcane bagasse (Sanjuán et al., 2001) and almost all terrestrial plant cell walls (Scheller & Ulvskov, 2010). The ground tissue in sugarcane stalks retains abundant parenchyma cells whose cell wall is primary one which is composed mainly of cellulose and hemicellulose. Subsequent to crushing sugarcane stalks for extracting its juice by crusher machine in the initial steps of sugar industry, the sugarcane bagasse obtained is rich in residues of the primary cell walls of parenchyma tissue retaining cellulose and hemicellulose. Thus, these natural waste biomasses, including sugarcane bagasse and also wheat bran retaining both cellulose and the less complicated hemicellulose, are highly susceptible for semidry acid hydrolysis. This indicates that semidry acid hydrolysis is efficient for hydrolyzing cellulose and other less complicated polymeric carbohydrates in the cellulosic feedstock wastes. The semidry acid hydrolysis described in this study was thus highly efficient for hydrolysis of non-cellulosic cyanobacterial biomass. The potent extracellular polysaccharides producing (Hill, Peat & Potts, 1994; Hill et al., 1997; Helm et al., 2000; Tamaru et al., 2005; Morsy et al., 2008) cyanobacterium Nostoc commune biomass was efficiently hydrolyzed (51%) by semidry acid hydrolysis indicating that semidry acid hydrolysis was highly effective for hydrolyzing also this less complicated non-cellulosic renewable biomass. Subjecting cellulosic biomaterials to high concentrated H2SO4 in a two-step hydrolysis was described elsewhere (Iranmahboob, Nadim & Monemi, 2002; Chang et al., 2018). A two step acid hydrolysis was described to hydrolyze wood chips where the first step included treatment with high concentrated 80% H2SO4 at room temperature with a mass ratio of 500 g H2SO4/200 g dry mass of wood chips followed by addition of distilled boiling water to reach 26 wt% H2SO4 with boiling for 30 min and stirring followed by filtration where the filtrate containing cellulose was heated in a second step for extra 2 h resulting in 78–82% overall hydrolysis efficiency of cellulose theoretical values in wood (Iranmahboob, Nadim & Monemi, 2002). The Two-Step cellulose hydrolysis was also described elsewhere where in the first step cellulose was treated with high concentrated 72 wt% H2SO4 at 30 °C over 2 h in a ratio of H2SO4/dry mass of cellulose of 36 followed by a partial second step neutralization through using 20 wt% NaOH at 2.3–2.5 molar ratio for H+/OH− with subjecting to more hydrolysis 10 min autoclaving at a temperature of 121 °C (Chang et al., 2018). Cotton cellulose was found to completely dissolve at room temperature in high concentrated sulfuric acid above 55% (by volume) and a reduced sugar yields from the initial cotton cellulose concentrations of 30–70 g/L were varied from 64.3 to 73.9% (g R-sugar/g cotton cellulose) at a temperature of 40 (Chu et al., 2011). The percent hydrolysis of sawdust was lower than cellulose possibly due to presence of highly complicated lignin in wood and lower cellulose content. Around 20% to 30% of sawdust content is lignin (Sınağ et al., 2009) which is highly complicated to be hydrolyzed. However, the use of sawdust for obtaining reducing sugars through semidry hydrolysis would depend in the source of the sawdust where the cellulose content would depend on the type of wood. The resulted reducing sugars were utilizable by E. coli as a representative of the fermenting bacteria indicating that the reducing sugars prepared by semidry acid hydrolysis are fermentable and can be utilized not only for dark fermentation hydrogen production but also for other fermentation-based biotechnologies. In this study, the organic carbon sources (the reducing sugars of semidry acid hydrolyzed cellulose and various cellulosic feedstocks) used for dark fermentation hydrogen production, are already subjected to autoclaving during semidry acid hydrolysis and hence it contains no native microbiota. Thus, the effect of native microbiota described elsewhere (Dauptain et al., 2020) is not applicable in the present study where fermentation was conducted by the supplied E. coli inoculum. Confirmative control experiments with no E. coli inoculated to the fermentor, did not produce hydrogen gas indicating no native microbiota effect is there where E. coli is the soli hydrogen producing bacterium in the fermentor through its utilization of the reducing sugars of semidry acid hydrolyzed cellulose and various cellulosic feedstocks. E. coli produce only hydrogen and CO2 gas (Penfold, Forster & Macaskie, 2003) where CO2 is absorbed by NaOH in the collection system and the collected hydrogen gas was fully pure compared to reference samples of pure hydrogen in measurements. The use of the abundant cellulose feedstock for hydrogen production would be cost effective through the semidry acid hydrolysis. The feasibility of such industry would be of importance in future upon exhaustion of fossil fuels (Muradov & Veziroglu, 2008). In fact, more and more research on the valorization of agricultural residual into biofuel has attracted great attention, mainly due to the positive effects from both economic and environmental aspects and long-term energy sustainability with greenhouse gas mitigation (Ho, Ong & Wu, 2019). The attempt to use cellulose feedstock would also be of importance in many agricultural countries. Besides, the re-activation of this biological hydrogen production industry would encourage farmers in developing countries to make use of crop plants straw and avoiding the harsh burning of such straw and cellulosic agricultural wastes. The data shown in this study either for growth of E. coli on the reducing sugars obtained from semidry acid hydrolysis or its utilization for hydrogen production did not show any inhibition or toxicity against the bacterium by the hydrolysate contents. As it requires minimum amount of acid and hence minimum amount of base for subsequent neutralization step, the semidry acid hydrolysis of cellulosic biowastes would be cost effective for bacterial hydrogen production biotechnology. The semidry acid hydrolysis of the cheap and abundant cellulosic wastes feedstock might possibly be applicable not only for bacterial H2 production but also for other cellulose dependent biotechnologies. The described semidry acid hydrolysis reduces the amount of high molarity HCl required for hydrolysis to minimum. Thus, the amount of NaOH required for the tedious neutralization step required for various fermentation biotechnologies comes to minimum. Further future studies for modification of semidry acid hydrolysis such as combination with other hydrolysis protocols would be of interest for the best making use of the abundant cellulosic biowastes in various fermentation biotechnologies.
| Biomass | Cellulose | Hemicellulose | Lignin | References |
|---|---|---|---|---|
| Sawdust | 40–50% | 25–35% | 20–30% | Sınağ et al. (2009) |
| Wheat bran | 31.4 ± 1.6% | 20.3 ± 1.0% | 22.3 ± 0.3% | Cantero et al. (2015) |
| Sugarcane bagasse | 32–45% | 20–32% | 17–32% | Alokikaa et al. (2021) |