Effect of Elaeagnus umbellata (Thunb.) fruit extract on H2O2-induced oxidative and inflammatory responses in normal fibroblast cells
- Published
- Accepted
- Received
- Academic Editor
- Anne Fernandez
- Subject Areas
- Biotechnology, Cell Biology, Food Science and Technology, Molecular Biology, Plant Science
- Keywords
- Elaeagnus umbellata, Autumn olive, Plant extract, Anti-oxidative, Anti-inflammatory, Fibroblast, Hydrogen peroxide
- Copyright
- © 2021 Zglińska et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Effect of Elaeagnus umbellata (Thunb.) fruit extract on H2O2-induced oxidative and inflammatory responses in normal fibroblast cells. PeerJ 9:e10760 https://doi.org/10.7717/peerj.10760
Abstract
Background
Elaeagnus umbellata is a plant commonly used in traditional Asian medicine for its many health benefits and strong antioxidative activity. Its therapeutic potential is believed to be connected to its effect on fibroblasts. This study aimed to investigate E. umbellata methanol-acetone extract’s (EUE) defense against hydrogen peroxide (H2O2)-induced fibroblast damage.
Methods
Because the main biologically active compounds of E. umbellata are water-insoluble, we evaluated the effects of methanol-acetone fruit extracts using liquid chromatography (for ascorbic acid and beta-carotene) and spectrophotometry (for lycopene and total phenolics). The extract’s antioxidative activity was measured using DPPH radical inhibition, and EUE’s effect on human fibroblasts was also evaluated. We assessed the metabolic activity and apoptosis of HFFF-2 fibroblasts exposed to EUE and/or H2O2using the XTT test and flow cytometry, respectively. Superoxide dismutase activity and reactive oxygen species (ROS) production were evaluated using colorimetric and fluorometric assays, respectively. We measured pro-inflammatory cytokine (MIF, fractalkine, MCP-4, BLC, GCP-2, NAP-2, Eotaxin-2, and Eotaxin-3) expression in HFFF-2 cells using immunocytochemistry.
Result
The extract increased HFFF-2 cell proliferation and reduced cell death caused by H2O2-induced stress. H2O2-treated fibroblasts had greater ROS production than cells treated with both H2O2 and EUE. Additionally, the group treated with H2O2 alone showed higher pro-inflammatory cytokine (MIF, MCP-4, NAP-2, Eotaxin-2, and Eotaxin-3) expression.
Conclusion
EUE protected human fibroblasts from H2O2-induced oxidative stress and reduced the fibroblast-mediated inflammatory response triggered by ROS.
Introduction
Fibroblasts are the most common type of cells in mammalian connective tissue. In addition to their role as a structural ingredient, fibroblasts contribute to various immune and inflammatory responses. Human chronic inflammatory diseases can develop into a number of debilitating fibrotic disorders, proving that permanent activation of the immune system can lead to strong disturbance in fibroblast function (Williams, 1998; Mescher, 2017). Fibroblasts respond to the actions (attendance) of many types of cytokines, and these mediators trigger fibroblasts and leucocytes to act together during complex healing processes such as wound healing. Fibroblasts can modify the chemical composition of their secretions in response to other cell mediators (Mescher, 2017). Additionally, fibroblasts are frequently exposed to inflammatory agents related to infections and UV or ionizing external radiation exposure. It is well known that any type of physical injury in multicellular animals immediately produces a cascade of enzymatic and cellular reactions within the destructed tissues (Watanabe et al., 2004). Therefore, compounds that can protect fibroblasts against oxidative stress and inflammation are extremely important.
The Elaeagnus genus includes 70 to 80 species, the most studied of which being Elaeagnus angustifolia L. (Russian olive). Another oft-mentioned variety is Elaeagnus umbellata, frequently referred to as the autumn olive, silverberry, or spreading oleaster. It can be found in Asia, North America, or its place of origin, Southeast Asia. The Elaeagnus sp. berry is an excellent source of antioxidative compounds, including carotenoids, phenolic, flavonoids, and vitamins, specifically vitamins A, C, E, and D (Uddin & Rauf, 2012; Khattak, 2012; Hamidpour et al., 2016; Ozen et al., 2017). The carotenoids beta-carotene and lycopene have proven antioxidative and anti-cancer effects. The carotenoids and α-tocopherol work with reactive oxygen species (ROS), especially peroxyl radicals, and singlet oxygen. Beta-carotene and lycopene act as antioxidants during lipid phases by neutralizing singlet oxygen or ROS (Sies & Stahl, 1995). Vitamin E mediates cell signaling and proliferation, as well as gene expression regulation. α-tocopherol, the most prevalent form of this vitamin in human tissue, exhibits antioxidant and anti-inflammatory properties (Patel, 2015).
Lycopene is the main compound in tomatoes, and is found in six to 16 times greater quantities in autumn olive berries (Fordham et al., 2001; Patel, 2015). Elaeagnus fruits are also a good source of high-quality oils. Fat extracted from E. augustiflora seeds mostly consists of unsaturated fatty acids, including linolenic acid, with well-tested strong anti-inflammatory properties (Hamidpour et al., 2016).
Due to the complex nature of E. umbellata’s antioxidative mechanism, a combination of methods should be applied in order to correctly determine its complete properties. The reduction of ROS secretion by cells subjected to oxidative stress also confirms the effects of these antioxidant properties. Under certain physiological conditions, ROS are present in the cell at very low levels and only execute signalling functions. The increase of ROS intracellular levels results from oxidative stress (Schieber & Chandel, 2014). ROS secretions can be measured in vitro in living cells using a test in which ROS react with a fluorogenic sensor localized in the cytoplasm, resulting in a fluorometric product amount that is proportional to the amount of ROS present in the studied sample (Cusimano et al., 2017).
Hydrogen peroxide (H2O2) is mainly generated from superoxide anions produced by mitochondria and NADPH oxidases. Physiological levels are involved in normal cell metabolism, whereas increased levels of superoxide or hydroxyl radicals can invoke oxidative stress. Accordingly, H2O2 has been shown to be involved in the formation of numerous pathologies. More specifically, H2O2 can exert its toxic effects through the ferrous iron-dependent formation of highly reactive hydroxyl radicals, which in turn can alter the structure of lipids, proteins, and DNA (Halliwell & Gutteridge, 1992). Oxidative stress can trigger the activation of cytokines or chemokines, as well as the aggregation of metabolites resulting from oxidation. Many oxidative stress promoters recruit inflammatory cells either directly or indirectly, therefore increasing ROS production and creating an increased inflammatory state. Oxidative stress may be critical during the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF- κB), based on the observations that the cytokine-induced activation of NF- κB could be prevented by antioxidant or metal chelator treatment (Nagata, 2005). The effects of H2O2 on epidermis cells was found to vary depending on the dose amount. Song et al. (2012) showed that concentrations of H2O2 over 100 µM significantly increased the expression of tumour necrosis factor α (TNF-α) mRNA in human middle ear epithelial cells, suggesting that this dose was appropriate for studying anti-inflammatory effects.
Inflammation, controlled by chemotactic cytokines known as chemokines, manifests itself in many diseases such as arthritis, meningitis, respiratory system disorders, and neurodegeneration. These disease’s chemokines can trigger leukocyte accumulation and activation in tissues. By controlling the movement of inflammatory cells, previous studies have proposed that different chemokines and their receptors could provide direction for future therapeutic interventions (Luster, 1998; Nagata, 2005).
Chemokines have been found to play an important role in connective tissue diseases by recruiting eosinophils to the inflammation site and releasing ROS, causing tissue damage and propagating the inflammatory response. One therapeutic strategy to reduce excessive inflammatory responses is to inhibit chemokine activity or block their receptors (Waśniowska, 2004). Both inflammation and connective tissue injuries have also been associated with collagen degradation, a process where matrix metalloproteinase proteins (MMPs) are activated. Endothelial cells and monocytes secrete MMPs in response to various inflammatory stimuli and oxidative stress (Galis et al., 1998; Calabriso et al., 2016).
Additionally, there is evidence of the involvement of MMP-2 and MPP-9 during neurodegeneration. Under particular neuro-inflammatory conditions, MMPs have been shown to change the permeability of the blood-brain barrier, resulting in white matter damage. Therefore, MMPs may be related to amyloid formation in Alzheimer’s disease, as well as the death of dopaminergic neurons in Parkinson’s disease (Rosenberg, 2009). The number of neurons in the brain has been found to increase in the presence of hepatocyte growth factor (HGF), which has also been shown to regulate the migration and mobility of newly-created cells (Respondek & Buszman, 2015).
When searching for natural products that are able to moderate oxidative stress and inflammation, traditional medicine provides several options. The E. umbellata berry has been used in traditional Asian medicine as an anti-inflammatory and anti-nociceptive agent (Uddin & Rauf, 2012; Ahmad et al., 2009; Hamidpour et al., 2016; Ozen et al., 2017). Its antidiabetic (Nazir et al., 2018; Spínola et al., 2019) and anti-proliferative (Wang, Bowman & Ding, 2007; Ozen et al., 2017) potential have been also demonstrated. However, scientific data on the molecular mechanisms of Elaeagnus sp. extracts’ antioxidative and anti-inflammatory effects remain limited. To our knowledge, there have been no studies on the effects of autumn olive fruit extract on normal human fibroblast cells.
In this study, we investigated the potential antioxidative and anti-inflammatory effects of E. umbellata fruit extract during ROS production and inflammation-associated cytokine expression in H2O2-induced human fibroblast cells.
Materials & Methods
Samples
Fruits of Elaeagnus umbellata were collected from a farm located in the Łódź Voivodeship in central Poland. Fruits were harvested after ripening. The species classification was confirmed by Piotr Banaszczak (Rogów Arboretum, Warsaw University of Life Sciences, Poland). The fruits were then lyophilized.
A field permit was not required as the fruit was bought at an ornamental plant farm.
Extract preparation
First, samples were incubated with methanol and acetone (1:50:10) in an ultrasound bath for 30 min. Then, extracts were filtered and the process of extraction was repeated. Extracts were combined and methanol was evaporated under vacuum in a rotary evaporator (40 °C). Next, they were kept in −20 °C until analysis. All extraction procedures were performed in darkness.
Extract characteristics
High-performance liquid chromatography
Fat extraction and saponification of E. umbellata berry extracts was performed according to the AOAC procedure (1990) at 25 °C. Analysis of carotenoids, beta-carotene, D (group), E (α-tocopherol), K (phylloquinone) in crude fat was conducted using an Agilent 1,100 high performance liquid chromatography (HPLC) system (Agilent, Waldbronn, Germany). Separations were performed at 25 °C using a 4.6 × 150 mm and 5 µm Zorbax Eclipse XDB C8 column (Agilent). Solvent flow rate was maintained at 1.2 mL/min in a linear gradient of 90:10 (vol./vol.) methanol to water, with an injection volume of 10 µL. The temperature of the column oven was set at 30 °C. Detection with a UV–vis detector was carried out at 230 nm for α-tocopherol, and 265 nm for K, carotenoids, β-carotene, and vitamin D. The standard solution (1 mg/mL) was prepared separately by dissolving 10 mg of each individual vitamin in 10 mL of methanol and storing in darkness at 4 °C. N = 3
L-ascorbic acid levels was determined by HPLC using the method described by Odriozola-Serrano, Hernández-Jover & Martín-Belloso (2007). Briefly, 0.1 g of the extract was transferred into a volumetric flask and filled up to 10 mL of pure ethanol. A part of the mixture was filtered through a 0.45 µm PTFE syringe filter onto a chromatographic vial and 20 µL were analyzed using HPLC, an Onyx Monolithic C18 column (100 × 4.6 mm, 5 µm; Phenomenex, Torrance, CA, USA), and a UV-Vis detector (Shimadzu, Kyoto, Japan). The mobile phase was 0.1% m-phosphoric acid solution in ultrapure water. The analysis was carried out using isocratic conditions ( one mL/min) at a 25 °C column temperature, and detection was performed at 254 nm. The calibration curve was performed with L-ascorbic acid external standard solutions (N = 3).
Phenolic content
The total phenolic (TC) content was determined using the standard Folin–Ciocalteu method. The extract was was combined with 2% sodium carbonate in deionized water and vortexed the mixture well. Next, 50% Folin–Ciocalteau reagent was added to the mixture and vortexed. It was left to settle for 45 min and the absorbance was measured at 750 nm. TC contents were calculated with the use of calibration curve of quercetin. The results were presented as quercetin equivalent milligram per gram (mg/g) of E. umbellata extract dry matter (d.m.; N = 6).
Lycopene content
The spectrophotometric method was used to determine the amount of lycopene (Rao, Waseem & Agarwal, 1998). Lycopene was extracted from each sample using a mixture of butylated hydroxytoluene (BHT) in acetone, ethanol and hexane (1:1:2) and values were determined by measuring absorbance at λ = 503 nm in the hexane layer. The lycopene content was calculated using the lycopene extinction coefficient in hexane, at this wavelength (N = 3).
Antioxidant potential
α, α-diphenyl- β-picrylhydrazyl (DPPH) free radical scavenging
The anti-oxidant in vitro properties of extracts were measured using DPPH free radical scavenging test, as previously shown by Matusiewicz et al. (2009). Concentrations ranging from 100 to 1,000 µg/mL were used and the results were expressed as a percentage of DPPH inhibition. Total extract and α-tocopherol were diluted in methanol and L-ascorbic acid in deionized water before the analysis.
Regarding the scavenging activity of the DPPH? test, 0.2 mM DPPH? in methyl alcohol was mix with each extracts or standard antioxidant (L-ascorbic acid and α-tocopherol). After 30 min in darkness (25 °C), samples were centrifuged (1,600 g, 10 min) and then the absorbance was measured at 517 nm (N = 6).
Cell cultures and treatments
The HFFF-2 human foetal foreskin fibroblast cell line was obtained from the European Collection of Authenticated Cell Cultures (ECACC, 12 passage) and maintained in Dulbecco’s modified medium (DMEM, Gibco, Thermo Scientific, Waltham, MA, USA) supplemented with 10% foetal bovine serum (Sigma-Aldrich/Merck, Darmstadt, Germany), 1% penicillin, and streptomycin (Sigma-Aldrich/Merck, Darmstadt, Germany) at 37 °C in a humidified atmosphere of 5% CO2/95% air in a NuAire DH AutoFlow CO2 Air-Jacketed Incubator (Plymouth, MN, USA). Cells that reached about 70% confluence were used for the experiment.
The effect of extracts on cell viability
To measure cell viability, the XTT Kit (Sigma-Aldrich/Merck, Darmstadt, Germany), a colorimetric assay for the nonradioactive quantification of cellular proliferation, viability, and cytotoxicity, was used. The assay is based on the extracellular reduction of XTT by NADH produced in the mitochondria. XTT is only reduced to formazan by metabolically active cells. Cells were plated into 96-well plates at 17 000 cells/well and 100 µL DMEM was added to each well. After 24 h, 90 µL of fresh culture medium and 10 µL of extract at concentrations ranging from 100 to 500 µL/mL were added to each well.
The following groups were created:
I. Control without extract or H2O2
II. 100 µg/mL of extract without H2O2
III. 150 µg/mL of extract without H2O2
IV. 200 µg/mL of extract without H2O2
V. 250 µg/mL of extract without H2O2
VI. 500 µg/mL of extract without H2O2
VII. 100 µg/mL of extract with H2O2
VIII. 150 µg/mL of extract with H2O2
IX. 200 µg/mL of extract with H2O 2
X. 250 µg/mL of extract with H2O2
XI. 500 µg/mL of extract with H2O2
XII. H2O2 alone.
The extract was mixed with PBS and stirred until completely dissolution. Equal volumes of PBS were added to control cells. After 72 h, 100 µM H2O2 was added to induce oxidative stress.
Then, after another 24 h, the XTT test was performed according to the manufacturer’s protocol.
Optical density (OD) was measured at 450 nm using an enzyme-linked immunosorbent assay reader (Infinite M200, Tecan, NC, USA). Cell viability was expressed as the percentage of (ODtest −ODblank)/(ODcontrol −ODblank), where “ODtest” is the OD of cells incubated with the extract, “ODcontrol” is the OD of the control sample, and “ODblank” is the OD of the wells without cells (N = 6).
The effect of the extract and H2O2 on cell death type
Annexin V/PI Apoptosis Kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to estimate cell death pathways according to Szczepaniak et al. (2018). HFFF-2 cells were seeded in six-well plates at a density of 2 × 105 cells/well. After 24 h, the culture medium was changed to one containing 1% fetal bovine serum (FBS) and 100 µg/mL of 10% extract in PBS (two groups, six wells) or PBS alone (two groups, six wells). After 72 h, 100 µM of H2O2 was added to two groups (one with extract and another without extract). After 6 h of incubation, the cells were collected and centrifugated. Each cell pellet was then mixed with 100 µL of Annexin binding buffer. 5 µL of fluorescein isothiocyanate-labelled Annexin V and 1 µL of propidium iodide were pipetted into each vial. The samples were left for 15 min at 25 °C. Finally 400 µL of binding buffer and the samples on ice. A total of 10,000 events were recorded per sample and the apoptosis plots were generated using Flowing Software 2.5.1 (Turku Bioscience, Turku, Finland).
This protocol was repeated twice and in triplicate with H2O2 incubation times of 12 and 24 h, respectively.
ROS generation
To measure cell-generated ROS, the fluorometric intracellular ROS assay kit (MAK143; Sigma, St Louis, MO, USA)was used. The following groups were created:
I. Control without extract or H2O2
II. 100 µg/mL of extract with H2O2
III. 500 µg/mL of extract with H2O2
IV. H2O2 alone.
HFFF-2 cells were seeded at a density of 1,700 cells/mL on a 96-well plate with 100 µL of DMEM added to each well. The next day, the medium was removed and replaced with fresh medium containing 1% FBS and dilutions of E. umbellata extract at concentrations of 100 mg/L and 500 mg/L in PBS or appropriate volume of PBS. After 72 h, oxidative stress was induced by addition of 100 µM H2O2 to the groups receiving the extract and the group treated only with H2O2. H2O2 dose was chosen based on literature data (Szczepaniak et al., 2018). The controls were treated with PBS. Master Reaction Mix was added into the cell plates and fluorescence intensity was measured at λex520 nm/λem605 nm with a microplate reader.
ROS production was measured every 10 min for a total of six times.
Superoxide dismutase activity (SOD)
For the SOD activity assay, the following groups were created:
I. Control without extract or H2O2
II. 100 µg/mL of extract with H2O2 III. H2O2 alone.
Cells were seeded at 75 cm3 flask in complete medium with the FBS reduced to 1%. After 24 h, extract (100 µg/mL in PBS) or an equivalent volume of PBS was added to cells. After 72 h incubation, oxidative stress was induced by addition of 100 µM H2O2. Cell pellets from individual groups were pooled to obtain three samples: 100 µg/mL extract + H2O2, H2O2 alone, and control. Total protein was isolated and measured using the BCA method. The same amount of protein was used from each group. The isolated protein was analyzed using the SOD determination KIT (Sigma-Aldrich) according to the manufacturer’s instructions. This KIT allowed us to evaluate SOD activity by utilizing WST-1 [2-(4-Iodophenyl)- 3-(4-nitrophenyl)-5-(2,4-disulfophenyl)- 2Htetrazolium, monosodium salt], a highly water-soluble tetrazolium salt that produces a water-soluble formazan dye when reduced with a superoxide anion. The O2 reduction rate was linearly related to xanthine oxidase (XO) activity and was inhibited by SOD. The results were expressed as SOD activity (%), according to protocol.
Cytokine antibody microarrays
The expression of selected cytokines was measured using cytokine arrays, which are antibody-pair-based assays that are analogous to Western blots, but using a membrane as a substrate.
The following groups were created:
I. 100 µg/mL of extract with H2O2
II. H2O2 alone
III. Control –PBS only.
Cells at 75 cm3 flask in complete medium as described in section ‘Cell cultures and treatments’, except for the FBS concentration, which was reduced to 1%. After 24 h, extract (100 µg/mL in PBS) or an equivalent volume of PBS was added to cells. Following a 72 h incubation period, oxidative stress was induced by adding 100 µM H2O2. Cell pellets from individual groups were pooled to obtain 3 samples: 100 µg/mL extract + H2O2, H2O2 alone, and the control group. Total protein was isolated and measured using the BCA method. The isolated protein was analyzed using the human cytokine antibody array (Abcam), according to the manufacturer’s instructions. he same amount of protein was applied to each membrane. Chemiluminescence detection was performed using the ChemiDoc1 Imaging System and Quantity One Basic Software (Bio-Rad, Hercules, CA, USA). The captured images of each membrane were analysed using Fiji software (National Institute of Health, Bethesda, MD, USA). The results from each group were optimized according to the Abcam protocol (N = 4).
Statistical methodology
Statistical analyses were performed using the Statistica 13 program.
In order to determine antioxidant potential and viability statistical significance of differences between control and individual concentrations of the extract was examined using the ANOVA test. Equality of variances was confirmed using the Brown-Forsythe test. The differences between individual concentrations were checked using the Tukey’s post hoc test.
Results
Extract bioactive compounds
E. umbellata fruit is known to contain large amounts of phenolic compounds, carotenes, ascorbic acid, and fat-soluble vitamins. ethanol-acetone extracts from E. umbellata fruit were evaluated regarding total phenols [1749 (± 5.2) mg], lycopene [50 (± 0.2) mg], beta-carotene [73 (± 0.2) mg], and vitamins D [1.38 (± 0.1) mg], E (α-tocopherol) [1124 (± 3.2) mg], K [0.44 (± 0.08) mg], and C (L-ascorbic acid) [64 (± 1.8) mg] levels per 100 g of d.m.
E. umbellata extract antioxidant potential
α, α-diphenyl- β-picrylhydrazyl free radical scavenging
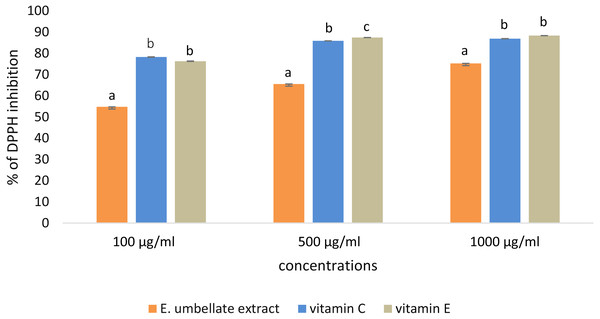
DPPH scavenging activity was dose-dependent in the tested concentrations (100–1,000 µg/mL) of E. umbellata fruit extract and the standard antioxidants. The results were expressed as the % of DPPH inhibition (Fig. 1).
Figure 1: Antioxidant potential of E. umbellata extract and standard antioxidants in 100 µg/ml, 500 µg/ml and 1,000 ug/ml concentration.
DPPH inhibition is expressed on the y axis in %. Different letters mean that the values for the bars differ statistically in the same concentration (p < 0.05) N = 6.L-ascorbic acid and α-tocopherol had similar antioxidant activity. The extract showed lower potential to reduce DPPH when compared to the standard antioxidants; the extract showed 54.78% potential in the 100 µg/ml dose versus 78.30% for vitamin C and 76.31% for vitamin E. However, this difference was less significant in a higher dose (1,000 µg/ml), reaching 75.25% potential for the extract and 86.24% and 88.37% for L-ascorbic acid and α-tocopherol, respectively.
Influence of extract and H2O2 on HFFF-2 cell viability
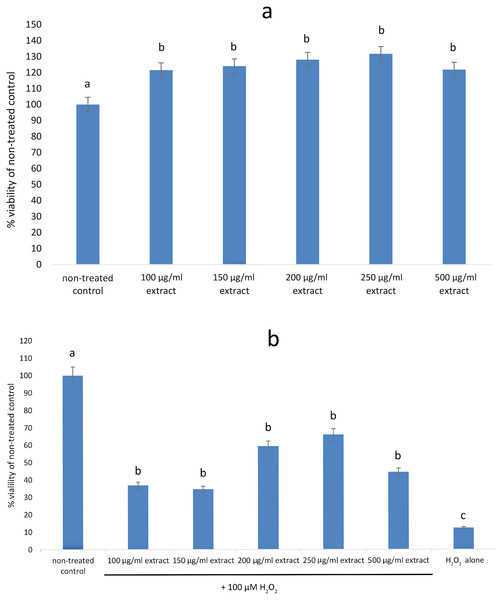
None of the tested concentrations (100–500 µg/mL) of the E. umbellata fruit extract showed any cytotoxic effects on HFFF-2 fibroblast cells. After 72 h of treatment, all of the extract concentrations increased cell viability when compared to the non-treated control. The observed effect was statistically significant (p < 0.05) across all tested doses (Fig. 2A). In contrast, addition of H2O2 decreased cell viability by 87.64% as compared to control. Pre incubation with extract for 48 h significantly protect cells from cell death. This effect was statistically significant across all tested doses (Fig. 2B). The differences in effects shown between the 100 µg/mL and higher doses were not statistically significant. Based on this result, we considered the 100 µg/mL dilution the most suitable for testing.
Figure 2: Viability of HFFF-2 cell treated with E. umbellata extract and/or H2O2.
(A) Cell treated by various concentration of extract for 72 h. (B) Cell treated by 100µM H2O2 after 48 h incubation with extract. Non-treated control and H2O2 only were incubated with PBS instead of extract. Results are expressed as % of non-treated control viability. Mean ± SD. Different letters mean that the values for the bars differ statistically (p < 0.05) N = 6.E. umbellata extract rescues fibroblasts from H2O2-induced cell death
Table 1 shows the ratio of necrosis to apoptosis across all dead cells. Apoptosis was predominant, but the data clearly show that the cells receiving only H2O2 had the highest rate of necrosis in the total mortality. Across different time intervals, the control or extract-only cells had the lowest necrosis rates, and the highest proportion of necrosis was found in the H2O2-only cells.
| Type of cell death after different time of H2O2 treatment (%) | Control | Extract only | H2O2 only | Extract + H2O2 | p-value |
|---|---|---|---|---|---|
| 6 h | |||||
| Necrosis | 0.60a | 0.77b | 5.20c | 2.46b | 0,000234 |
| Apoptosis | 3.38a | 2.80b | 7.04c | 6.65b | |
| Live | 96.07a | 96.62b | 87.55c | 91.34b | |
| 12 h | |||||
| Necrosis | 1.02a | 0.57a | 17.62b | 6.56c | 0,000292 |
| Apoptosis | 3.77a | 2.54a | 21.67b | 13.17c | |
| Live | 51.11a | 97.4a | 60.74b | 80.26c | |
| 24 h | |||||
| Necrosis | 0.38a | 0.47a | 38.47b | 11.64a | 0,000231 |
| Apoptosis | 2.05a | 2.12a | 62.06b | 43.55a | |
| Live | 97.57a | 97.42a | 17.05b | 44.81a | |
Notes:
Groups: control - untreated, extract only - treated with 100 µg / ml extract without H2O2, H2O2 only - treated with 100 µM hydrogen peroxide without the addition of extract, extract + H2O2 - treated with 100 µg/ml extract and 100 µM hydrogen peroxide. Mean ± SD followed by different letters (a,b,c) in the same verse were significantly different for p < 0, 05 (post hoc test).
The extract’s influence on ROS production in HFFF-2 cells
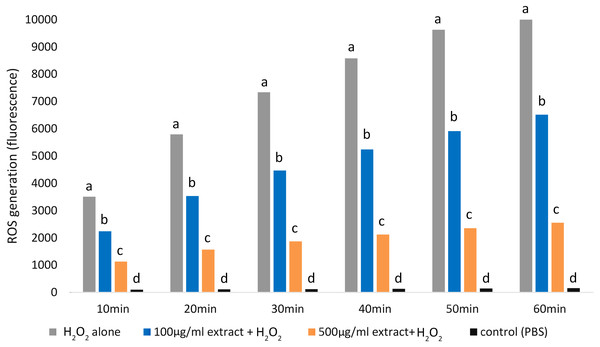
Adding both E. umbellata extract concentrations to cells 72 h before oxidative stress induction resulted in a statistically significant (p < 0.05) decrease in ROS production (Fig. 3).
Figure 3: Variations in ROS generation by fibroblasts relative to incubation time and concentrations of the extract of E. umbellata fruits.
Groups: 500 mg/L extract + H2O2, 100 mg/L extract + H2O2, and H2O2 alone were induced with 100 µM H2O2 after 72 h of pre-treatment with extract or PBS (H2O2 alone group). A negative control (not H2O2-induced) was also included. Different letters mean that the values for the bars differ statistically in the same time interval (p < 0.05) N = 6.Notably, the 500 mg/L extract concentration had a greater effect in inhibiting ROS production than the 100 mg/L concentration (p < 0.05).
E. umbellata extract reduced SOD H2O2-induced activity.
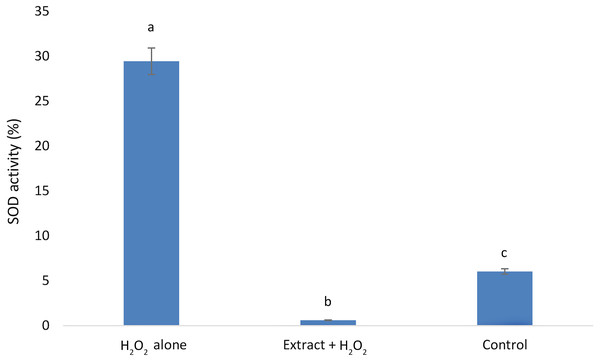
Our results showed that the H2O2alone group exhibited the highest SOD activity, followed by the control group. The lowest SOD activity was observed in the group that received E. umbellata extract 72 h before the addition of H2O2 (Fig. 4).
Figure 4: Superoxide dismutase activity in HFFF-2 fibroblast cells treated with E. umbellata extract and/or H2O2.
Groups: H2O2 alone –cells treated only with 100 µM H2O2. Extract + H2O2 –cells treated with 100 µg/mL extract of E. umbellata (day 2) and 100 µM H2O2 (day 5). Non-treat control–control without extract and/or H2O2. Values expressed as mean ± SD. Different letters mean that the values for the bars differ statistically (p < 0.05) N = 6.The E. umbellata extract changes the expression of selected growth factors in H2O2-induced HFFF-2 cells
The use of the extract 72 h before the induction of oxidative stress resulted in changes in chemokine expression in the cells that did not receive the extract and the non-treated control group (Table 2).
| Group | Extract + H2O2 | H2O2 alone | Control | p-value |
|---|---|---|---|---|
| BDNF | 308.63 ±11.1a | 135.83 ±1.1b | 0.00 ±0.0c | 0.000000 |
| FGF-7 | 63.64 ±0.4a | 31.97 ±2.8b | 32.75 ±5.7b | 0.000001 |
| HGF | 157.09 ±1.6a | 150.30 ±0.6b | 45.60 ±2.8c | 0.001910 |
| TIMP-1 | 1898.01 ±39.8a | 1371.93 ±61.2b | 711.88 ±37.9c | 0.000000 |
Notes:
H2O2 alone–cells not incubate with extract; control–cell treated only with PBS (no extract, no H2O2). N = 4; p-value–analysis of variance (ANOVA). Mean ± SD followed by different letters (a,b,c) in the same verse were significantly different for p < 0, 05 (post hoc test).
More specifically, H2O2-induced HFFF-2 cells treated with the extract showed lower expressions of macrophage migration inhibitory factor (MIF), fractalkine, monocyte chemoattractant protein 4 (MCP-4), B lymphocyte chemoattractant (BLC), granulocyte chemotactic protein 2 (GCP-2), neutrophil-activating peptide 2 (NAP-2), Eotaxin-2, and Eotaxin-3 than in cells treated only with H2O2. The non-treated control samples showed the lowest expression of chemokines, of which fractillin, BLC, GCP-2, and Eotaxin-3 were negligible. Cells treated with the extract and H2O2 had more than twice the expression of fibroblast growth factor 7 (FGF-7) and brain-derived neurotrophic factor (BDNF) after H2O2 administration than cells treated only with H2O2. Likewise, TIMP metallopeptidase inhibitor 1 (TIMP-1) and HGF expression was higher by over 40% and 5%, respectively (Table 3). The non-treated control samples had the lowest expression of these factors, and cells that were not exposed to the extract had undetectable BDNF levels (Table 3).
| Group | Extract + H2O2 | H2O2 alone | Control | p-value |
|---|---|---|---|---|
| BDNF | 308.63 ±11.1a | 135.83 ±1.1b | 0.00 ±0.0c | 0.000000 |
| FGF-7 | 63.64 ±0.4a | 31.97 ±2.8b | 32.75 ±5.7b | 0.000001 |
| HGF | 157.09 ±1.6a | 150.30 ±0.6b | 45.60 ±2.8c | 0.001910 |
| TIMP-1 | 1898.01 ±39.8a | 1371.93 ±61.2b | 711.88 ±37.9c | 0.000000 |
Notes:
H2O2 alone–cells not incubate with extract; control cell treated only with PBS (no extract, no H2O2) N = 4; p-value analysis of variance (ANOVA). Mean ± SD followed by different letters (a,b,c) in the same verse were significantly different for p < 0, 05 (post hoc test).
Discussion
This study showed that methanol-acetone extract from E. umbellata fruits protects fibroblasts against the effects of oxidative stress and inflammation triggered by the addition of H2O2.
Extract characteristics
HPLC determined that the extract’s main fat-soluble compound was α-tocopherol. This finding was in line with previous studies on the content of E. umbellata fruit extract. Ahmad, Sabir & Zubair (2006) found that autumn olive fruit possessed 0.9 mg/g d.m. of α-tocopherol. The extract in this study contained more than 10 times the concentration of this compound, possibly due to the fact that the extract is much more concentrated than the fruit by itself.
Similarly, the fruit had contained 27.8 mg/100 g d.m. of L-ascorbic acid, while the extract contained 65 mg/100 g d.m., which was in accordance with Khattak’s (2012) findings.
On the other hand, the relatively small amounts of lycopene and beta-carotene probably resulted from the choice of solvent and temperature used during extraction. Since the degree of lycopene extraction increases with temperature (50–60 °C is optimal), acetone, ethyl acetate, or boiling ethanol are the recommended solvents. However, this study’s temperature of extraction was 40 °C, and we used methanol and acetone as solvents in a 80:20 (vol/vol) ratio. Concurrently, beta-carotene has been found to more effectively dissolve in ethanol at lower temperatures (maximum extraction at 25 °C) (Calvo, Dado & Santa-María, 2007). Therefore, extraction conditions were selected to that could also accommodate temperature-sensitive phenols. Khattak (2012) found that E. umbellata fruit contained 23.3 mg/g of total phenols, a higher amount than found in our extract. However, their results were given as a gallic acid equivalent, while in our study, we used quercetin as a standard, so the results cannot be easily compared.
The extract’s antioxidant potential
The results of our study confirmed that E. umbellata fruit extract contained strong anti-oxidative properties. Ozen et al. (2017) found that 500 µg/mL of E. umbellata methanol, ethanol, and water extracts had more than 99.5% DPPH scavenging activity, whereas the extract described in this study had 65.55% DPPH scavenging potential. This difference could have been influenced by the use of acetone as a co-solvent for the extract, but also from subtle changes during the experiments (e.g., slight variations in incubation time or laboratory temperature).
The results showed that the extract was a weaker antioxidant than L-ascorbic acid and α-tocopherol, which are among the strongest known antioxidants. Although the extract contained both of these vitamins, it should be noted that our extraction protocol did not remove all of the fruit’s non-antioxidant compounds, as the procurement of pure bioactive compounds was outside the scope of the study.
Nutraceuticals and functional foods are becoming increasingly popular and contribute to the public’s growing fear of potential side effects of synthetic compounds. Consumers also have low confidence in supplements containing pure, isolated substances. Therefore, this study focused on evaluating the health benefits of an extract containing a mixture of bioactive compounds and other residues.
Influence of the extract and H2O2 on ROS production in HFFF-2 cells
In this study, we found that E. umbellata extract significantly reduced the H2O2-induced secretion of ROS in HFFF-2 fibroblast cells. As expected, administering H2O2 significantly increased ROS secretion in the cells. However, pre-treating the cells with the extract caused up to 4 times lower ROS secretion when compared to cells only treated with H2O2.
The E. umbellata extract’s ability to protect cells against oxidative stress might be a result of the presence of phenols, particularly polyphenols, which have the ability to reduce the oxidation rates of organic matter by transferring an H atom (from their OH groups) to chain-carrying ROO* radicals.
Antioxidants can also break radical chains, thereby preventing their propagation. One classic example of a chain-breaking antioxidant is α-tocopherol. When delivered to a cell, α-tocopherol has been shown to protect membranes against lipid peroxidation (Zhang et al., 2001). Concomitantly, phenols and other compounds that are present in the extract, such as vitamin A and carotenoids, have been reported to scavenge oxygen (Foti, 2007; Moise, Al-Babili & Wurtzel, 2014).
The second possible mechanism is related to antioxidant enzymes. SOD, which scavenges superoxide radicals to H2O2, is an important endogenous antioxidant enzyme that acts as the first line of defense against ROS, which are further processed by subsequent antioxidant enzymes. Jia et al. (2014) found that SOD activity was reduced in cells after exposure to 500 µM of H2O2 when compared to the control. This suggests that adding H2O2 caused oxidative damage to antioxidant enzymes. However, blackcurrant extract limited the decrease in SOD activity of the tested cells (Jia et al., 2014). In this study, 100 µM H2O2 were applied, which resulted in significantly lower cell mortality than that observed in the Jie et al. study. Accordingly, we showed that a 3 d incubation with extract of E. umbellata before the induction of oxidative stress resulted in reduced ROS secretion by HFFF-2 cells. A similar reduction was shown in the SOD activity in these cells, probably owing to the reduced association with oxygen radicals, which are substrates in a SOD catalysed reaction. The E. umbellata extract had such strong effects that a reduction in SOD activity was even observed relative to the non-stress induced control.
Administering H2O2 to the culture medium exposed cells to direct hydroxyl radical (OH) reactions resulting from H2O2 reacting with iron (II) ions (the Fenton reaction) or with iron and copper ions (the Haber-Weiss reaction). The strong OH oxidizing effect was directed against mitochondrial DNA. The reaction of these metal ions with AA is also important for this process, as L-ascorbic acid acts as a co-substrate of hydroxylases and oxygenases, which are enzymes involved in collagen biosynthesis. Overall, AA keeps the metal ions in a reduced state in the enzyme active site, which in turn enables optimal enzyme activity. L-ascorbic acid reduction of free transition metal ions generates oxygen radicals. Reduced metal ions, such as iron, react with H2O2to generate highly reactive OH or O2− ions. However, due to the limited availability of iron and copper ions in living organism tissue, it has not been confirmed that this reaction takes place in vivo. These metal ions are bound by proteins that are present in cells, such as ferritin, transferrin, and ceruloplasmin (Konopacka, 2004).
The E. umbellata extract provides some amounts of calcium and copper ions, but it also contains various antioxidants that may limit L-ascorbic acid’s pro-oxidative effects. Additionally, phenols that are present in the extract have the ability to form stable complexes with transition metal ions, such as Cu2+ or Fe2+, thus preventing Haber-Weiss and Fenton reactions (Foti, 2007).
Influence of the extract and H2O2 on HFFF-2 cell viability
Additionally, the extract’s effect on the viability of HFFF-2 cells was investigated. Our results showed a significant increase in HFFF-2 cell viability after incubation with E. umbellata extract at both tested concentrations, indicating the extract’s safety. This enhanced cell viability was probably related to increased FGF-7 expression. Moreover, the increased HGF expression in cells exposed to the extract further suggested that the extract can affect cell proliferation and differentiation. The E. umbellata extract limited cell viability due to the addition of H2O2, suggesting that it could protect fibroblast cells and even increase proliferation after oxidative stress-induced damage. However, more accurate studies are needed to confirm this result.
E. umbellata extract rescues fibroblasts from H2O2-induced cell death
Apoptosis is a non-inflammatory process resulting from the rapid identification and removal of dying cells before they secrete damage-associated molecular patterns (DAMP). In some instances, physiological inducers of apoptosis (i.e., TNF) can promote inflammatory cytokine expression in conjunction with apoptosis, resulting in an apoptotic process that appears pro-inflammatory. Conversely, necrosis is considered pro-inflammatory due to DAMP (Davidovich, Kearney & Martin, 2014).
We evaluated the cell death pathways that were activated by the extract. The percentage of necrosis decreased gradually across all groups except for the non-treated control group. Necrosis was most pronounced in cells treated with only the stress factor (H2O2), while cells exposed to both the extract and H2O2 showed significantly lower necrosis rates just 6 h after H2O2-induced stress. However, only 24 h after H2O2, administration, the group that received the extract and H2O2 showed similar necrotic activation as the group that received the extract without the stress factor. These results indicated that H2O2 causes rapid inflammation-related cellular changes, which HFFF-2 cells can partially neutralize. On the other hand, the extract reduced overall cell death and the incidence of necrosis at rates that were similar to the untreated control group, where no oxidative stress was induced.
The E. umbellata extract changes the expression of selected growth factors in H2O2-induced HFFF-2 cells.
During the same oxidative stress testing, we examined the expression of selected cytokines that were associated with inflammation. When compared to cells that were exposed to oxidative stress and did not receive the extract, the use of E. umbellata extract decreased expression of the following inflammatory mediators: MIF, fractalkine, MCP-4, BLC, GCP-2, NAP-2, and Eotaxin-2 and 3. As expected, chemokine expression was clearly lower in the control group, which was not treated with H2O2. Fibroblasts are known to produce inflammatory mediators in response to skin inflammation, similarly to pro-inflammatory cytokines during the progression phase of inflammation.
E. angustifolia has been shown to have anti-inflammatory properties when tested on animals. Total and endocarp extracts from this plant decreased formalin–induced paw oedema, similarly to sodium salicylate. Other researchers concluded that analgesia induced by E. angustifolia fruit extract did not depend on the opioid system, but flavonoids and terpenoids were the main candidates for the observed analgesic and anti-inflammatory effects (Ahmadiani et al., 2000). Another study showed a decrease in formalin-induced inflammation in mice following treatment with E. angustifolia aqueous extract. Moreover, this extract was found to inhibit type 1 and type 2 cyclooxygenase enzymes (Farahbakhsh, Arbabian & Emami, 2011). Liao et al. (2012) found that Elaeagnus oldhamii maxim methanol extracts increased SOD and glutathione peroxidase (GPx) activity in liver tissue and decreased malondialdehyde (MDA), nitric oxide (NO), interleukin (IL) 1 β, 6 (IL-6), TNF-α, and cyclooxygenase-2 (COX-2) levels in paw oedema tissue after λ-carrageenan-induced inflammatory reactions. Nikniaz et al. (2014) observed the anti-inflammatory properties of E. angustifolia fruit powder in women with knee osteoarthritis. They observed a significant decrease in serum pro-inflammatory cytokine (TNF- α, MMP-1) levels and a rise in serum anti-inflammatory cytokine (IL-10) levels after 8 weeks of supplementation. No significant changes were observed in the IL-1 β and MMP-13 serum levels (Nikniaz et al., 2014). These in vivo results were consistent with our in vitro results regarding E. umbellata extract, although in this study, we studied a larger and more diverse number of cytokines.
The mechanisms that affect pro-inflammatory cytokine expression in vitro. Maas, Deters & Hensel (2011) measured the anti-inflammatory activities of Eupatorium perfoliatum L. extracts by significantly downregulating the expression of cytokines, including colony stimulating factor 3 (CSF-3), IL-1 α, IL-1 β, and the majority of chemokines, such as C-C motif chemokine ligand 2 (CCL2), CCL22 (MCP-1) and C-X-C motif chemokine ligand 10 (CXCL10, IP-10) in LPS-stimulated murine macrophage cells.
Chemokines play an important role in connective tissue diseases by mediating the recruitment of eosinophils to the inflammation site and ROS release, leading to tissue damage and inflammatory response propagation (Elsner et al., 1997). One therapeutic strategy used to reduce excessive inflammatory responses is to inhibit chemokine activity or block their receptors (Waśniowska, 2004). In this study, we observed a decrease in chemokines that were eosinophil chemo-attractants (eotaxins and MCP-4) in oxidative stress-subjected fibroblasts treated with E. umbellata extract. Elsner et al. (1997) proposed that using a therapeutical approach that blocks these cytokines would prevent the invasion and destruction caused by eosinophils in certain conditions, such as allergic asthma and connective tissue diseases.
In this study, E. umbellata extract decreased the expression of four cytokines that induce MMP expression: NAP-2, CPG-2, MIF/CXCR2, and Fractalkine/CX3CL1. As previously mentioned, MMPs are associated with collagen degradation during oxidative stress. Lee, Seo & Lee (2019) found that Skullcapflavone II (a flavonoid derived from the Scutellaria baicalensis root) decreased MMP-1 expression and improved the integrity of type I collagen in foreskin fibroblasts. In recent years, there has been a greater use of natural substances which, instead of reducing MMP expression, increase the expression of their inhibitors (tissue inhibitors of metalloproteinases, TIMPs). The imbalance between MMPs and TIMPs has been characteristic for inflammatory diseases, cancer, and neurodegenerative diseases (Rosenberg, 2009; Calabriso et al., 2016). In this study, E. umbellata extract increased TIMP-1 expression by 43% compared to the non-treated control. A similar result was obtained when Spatholobus suberectus stem extract was applied to UVB-induced human keratinocyte cells (Kwon et al., 2019), polyphenol-rich red grape skin extract on vascular inflammation cell models (Calabriso et al., 2016), and kuding tea polyphenols on UVB-induced skin aging in hairless mice (Yi et al., 2019).
Proteins from the MPP family are also known to contribute to neurodegenerative diseases by destroying white matter and neurons (Rosenberg, 2009). In addition to increasing MPP inhibitor secretion, E. umbellata extract also increased BDNF expression in fibroblast cells under oxidative stress. BDNF is a neurotrophic factor known to prevent axonal and neuronal damage (Stadelmann et al., 2002) and promote the formation of new neurons. HGF has shown a similar effect on cell proliferation in the brain, and also has been reported to regulate the migration and mobility of newly-created cells (Respondek & Buszman, 2015). Insignificant increase in HGF and a marked increase in FGF-7 expression were also observed in our study, suggesting that E. umbellata extracts could potentially be used in the treatment of disorders with neuro-inflammation, such as Alzheimer’s or Parkinson’s diseases. However, further research is required on a different model to fully evaluate this.
Additionally, we observed more than a 2-fold increase in FGF-7 expression in cells treated with the extract, which is in accord with the cell viability results that showed an increase in cell proliferation following extract treatment.
Interestingly, TIMP-1, HGF, and FGF-7 expression was lower in the non-treated control group, and BDNF was undetectable. This clearly indicates that E. umbellata extract influences the expression of these signalling factors, regardless of oxidative stress.
Conclusion
This study was the first to explore the antioxidant effects of E. umbellata fruit methanol-acetone extract on normal human fibroblasts subjected to oxidative stress. We found that the extracts did not show any toxicity toward HFFF-2 cells at the concentrations used, but instead increased the viability of cells. Additionally, the studied extract reduced cell mortality caused by H2O2 treatment. We also showed that the extract reduced chemokine expression in H2O2-induced fibroblast cells. Collectively, our obtained data suggested that E. umbellata fruit extract has anti-inflammatory properties and could be used as a nutraceutical (or functional additive) because of its inflammatory-regulating properties. Moreover, the extract was demonstrated to increase the expression of TIMP-1, an MPP-9 inhibitor, indicating that collagen may be protected from degradation during oxidative stress. These properties, combined with the increase in BDNF expression, suggest that E. umbellata extracts may be effective in the treatment of neuro-inflammatory disorders, such as Alzheimer’s or Parkinson’s diseases. However, further studies are necessary to delineate the precise mechanisms underlying these activities.