Identification of a pathogen causing fruiting body rot of Sanghuangporus vaninii
- Published
- Accepted
- Received
- Academic Editor
- Aristóteles Góes-Neto
- Subject Areas
- Agricultural Science, Microbiology, Mycology
- Keywords
- Sanghuangporus vaninii, Green mould disease, Classification, Trichoderma virens
- Copyright
- © 2023 Yuan et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Identification of a pathogen causing fruiting body rot of Sanghuangporus vaninii. PeerJ 11:e15983 https://doi.org/10.7717/peerj.15983
Abstract
Sanghuangporus vaninii is a medicinal macrofungus that is increasingly cultivated in China. During cultivation, it was found that the fruiting body of S. vaninii was susceptible to pathogenic fungi, resulting in significant economic losses to the industry. The symptoms of the disease occur in the initial stage of fruiting body development. The isolate YZB-1 was obtained from the junction of the diseased and healthy areas of the fruiting body. In order to verify the pathogenicity of YZB-1, its purified spore suspension was inoculated into the exposed area nearby the developing fruiting body of S. vaninii. After 10 days, the same disease symptoms appeared in the inoculated area. Morphological identification and molecular analysis of rDNA ITS region confirmed that the isolate YZB-1 was identified as Trichoderma virens. The temperature stability assay revealed that the mycelia of YZB-1 grew the fastest at 25 °C, with growth slowing down gradually as the temperature increased or decreased. Dual-culture tests of T. virens and S. vaninii showed that the inhibition rate of T. virens on S. vaninii mycelium was the highest (79.01 ± 2.79%) at 25 °C, and more green spores were produced at the intersection of T. virens and S. vaninii.
Introduction
Sanghuangporus vaninii (Ljub.) Zhu et al. (2019) and Wu & Dai (2020) is a species of Basidiomycota, Hymenochaetales, Hymenochaetacae, Sanghuangporus, of which fruiting body is commonly known as “Sanghuang” in China. Sanghuang has been recorded in historical studies such as “On Medicinal Properties” and “Compendium of Materia Medica” (Kim et al., 2004; Sun et al., 2006; Song et al., 2019). Sanghuangporus vaninii is considered as one of the medicinal macrofungi due to its excellent efficiency in treating dysentery and blood insidiousness, anti-tumor, hypoglycemic, anti-oxidative, and immune-enhancing effects (Song et al., 2020). It has been a hot topic in the research and development of pharmaceutical preparations and health products industries in China and some other countries (Che et al., 2005; Gao, Zhang & Yu, 2014). The development of Sanghuang industry promotes the revitalization of rural economy in China. In 2021, the production of Sanghuang increased to 300 t, and the industry was attached great importance by the government (Yang et al., 2023a).
In China, Sanghuang and other mushrooms are grown using facilities cultivation techniques. Once the facilities are built, the same variety of mushroom is cultivated every year. Some even achieve annual cultivation in facilities by controlling temperature or rotating mushrooms suitable for different seasons, to improve facility utilization and obtain higher economic benefits (Yang et al., 2023b). However, as the cultivation years increase, the occurrence of diseases has a great impact on mushroom cultivation, reducing the quality and yield. A large number of diseases have been reported in mushroom cultivation, such as wet bubble disease caused by Mycogone perniciosa in white button mushrooms (Agaricus bisporus) (McGee, 2018; Yang et al., 2021), dry bubble disease caused by Verticillium fungicola in white button mushrooms and oyster mushroom (Murmu, Maurya & John, 2020), cobweb disease caused by Cladosporium spp. in oyster mushrooms (Oyebamiji et al., 2018; Gea, Navarro & Suz, 2019), and white mold disease caused by Paecilomyces penicillatus in morels (Yu et al., 2022). In addition to fungal pathogens, Pseudomonas tolaasii is consistently associated with mushroom brown blotch disease (Ghasemi et al., 2021), while Ewingella americana has been reported as a pathogenic bacterium of brown rot disease on shiitake mushroom (Na, Luo & Yu, 2021). However, despite the history of more than 2000 years of Sanghuang in China, diseases occurring during the process of S. vaninii cultivation have not been reported so far due to its short time of artificial cultivation.
In recent years, artificial cultivation of S. vaninii has made great progress and the cultivation scale is expanding (Yang et al., 2023a). However, the disease problem is becoming more prominent. From 2018 to 2021, we investigated cultivation companies where the disease occurred and found that the incidence of fungal disease in the cultivation bags of S. vaninii was as high as 30–70% in Hangzhou city, Zhejiang province of China. The symptoms of these diseases are basically the same, occurring in the initial or developing stage of S. vaninii fruiting bodies, preventing fruiting body formation, or causing brown to dark brown lesions on the fruiting body. The occurrence of this disease influences the quality and yield of Sanghuang, causing great economic losses to producers and becoming an important restriction factor of the Sanghuang industry.
In this study, we observed and described the symptoms of diseases in S. vaninii cultivation bags, isolated and identified pathogens using morphological characteristics and phylogenetic analysis with a combination of rDNA ITS genetic regions. The temperature stability of the pathogen was analyzed by in vitro test.
Materials and Methods
Isolation and purification of pathogens
Disease symptoms of S. vaninii were observed in a greenhouse at Hangzhou Academy of Agricultural Sciences, located in Zhejiang province, China (120°0′88″E, 30°1′63″N) between late June and late July 2020. Ten diseased cultivation bags were collected, and samples were taken from the junction of the diseased and healthy areas of each bag and plated onto potato dextrose agar (PDA) containing 0.25 g chloramphenicol. The plates were then incubated at 25 °C. After 7 days of incubation, agar blocks (5 mm in diameter) were cut from the growing edge of colonies and inoculated onto fresh PDA, and this process was repeated several times to obtain putative pure pathogens.
Pathogenicity assay
To conduct the pathogenicity assay, we prepared a conidial suspension (1 × 106 spores/mL) using five representative isolates. At the end of the vegetative growth stage of S. vaninii, a semicircle was cut in the middle of the plastic bags to somatic part of the mycelia in the air. Then, 500 μL of the pathogen’s conidial suspension was inoculated into the areas surrounding the initial fruiting bodies of S. vaninii. The bags were incubated for 10 days at 25 °C and a relative humidity of 98%, and each isolate was tested in triplicate. Uninoculated bags were used as controls. Disease symptoms were observed and recorded, and the pathogens were isolated again from the diseased sites to confirm their morphological characteristics.
Morphological identification
To identify the fungal pathogens, ten representative isolates were cultured on potato dextrose agar (PDA), CMD (cornmeal agar 20 g, dextrose 20 g, agar 20 g with 1 L distilled water) and SNA (KH2PO4 1 g, KNO3 1 g, MgSO4•7H2O 0.5 g, KCl 0.5 g, glucose 0.2 g, sucrose 0.2 g, agar 15 g with 1 L distilled water) (Jaklitsch, 2009), and incubated at 23 °C under a 12-h light/dark cycle. The structure of conidiophores, phialides, and conidia were observed and measured using a Zeiss Axiophot 2 microscope equipped with an Axiocam CCD camera and Axiovision digital imaging software (Axio-Vision Software Release 3.1., v.3–2002; Carl Zeiss Vision Imaging Systems, Jena, Germany), as previously described (Tomah et al., 2020).
Molecular analysis
To analyze the ITS region and the genes involved in taxonomy, ten isolates of pathogens were grown in 100 mL potato dextrose broth (PDB) on a shaker at 180 rpm, 25 ± 1 °C for 3 days. Genomic DNA was extracted using the Ezup Column Bacteria Genomic DNA Purification Kit (Sangon Biotech Co., Shanghai, China) according to the manufacturer’s instructions. The ITS rDNA regions were amplified using the primer pairs ITS5 (5′GGAAG TAAAAGTCGTAACAAGG3′) and ITS4 (5’TCCTCCGCTTATTGATATGC3′) (Jiang et al., 2016). The purified PCR product was sequenced in both directions and edited by BioEdit 7.1.3.0. and compared with homologous sequences available in the NCBI databases using BLAST.
Multiple alignment of the ITS rDNA sequences of this study and sequences from NCBI database (type strains of Trichoderma species, containing some species reported to be harmful to edible mushroom and some species closely related to the isolated strains) was carried out using Clustal W and a phylogenetic tree was constructed using MEGA 6. The evolutionary history was inferred by using the maximum likelihood (ML) method based on the Jukes-Cantor model (da Silva et al., 2017). The ML method was used to construct the phylogenetic tree with 1,000 bootstrap frequency. The type strain Sphaerostilbella lutea CBS 405.59 was used as the outgroup (Perera et al., 2023).
Temperature stability assay
Temperature stability was assessed by investigating in vitro mycelial growth at different temperatures. Isolate disks (5 mm diameter) were cultured on PDA plates and incubated in the dark at 5 °C, 15 °C, 25 °C, 30 °C, and 35 °C, each temperature treatment three replicates respectively. After 48 h, the diameters of the mycelial colonies were measured. Through diameter comparison, the temperature range suitable for the growth of the isolate was selected to continue the next experiment.
The inhibition of pathogenic isolate on mycelial growth of S. vaninii at different temperatures was observed by dual-culture test (Zang et al., 2023). Disks (5 mm diameter) of S. vaninii were placed on one side of PDA plates and incubated in the dark at 15 °C, 25 °C, and 30 °C, each temperature treatment three replicates respectively. Seven days later (to compensate for the slower growth of S. vaninii), disks of pathogenic isolate were placed on the other side and continued to incubate at the same temperature. The plates with only one disk of S. vaninii without pathogenic isolate were used as controls. After another 9 days, the radius of the mycelial colonies of S. vaninii was measured.
Analysis of variance (ANOVA) was done using SPSS 20.0 software program (SPSS Inc., Chicago, IL, USA). Mean value and standard deviation of each experiment were grouped according to S-N-K multiple range test with significance level of 5%. Dunnett’s test (P < 0.05) was also used to compare treatment plots with positive and negative control plots in the experiments.
Results
Disease symptoms and pathogen isolation
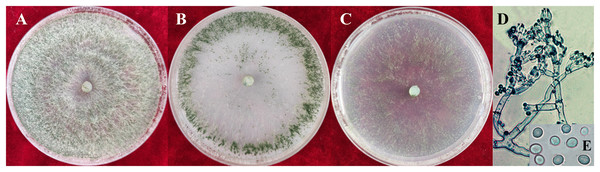
During the process of artificial cultivation, disease symptoms typically occurred around the timing of fruiting body production of S. vaninii. After the somatic growth of S. vaninii in a cultivation bag ended, a semi-circular area in the middle of the bag was cut to expose a part of mycelia for the development of fruiting bodies. Pathogen contamination manifested as white hyphae covering the surface of the exposed area or by infecting the initial small fruiting body. Subsequently, green spores appeared on the white mycelium (Fig. 1A). The entire exposed substrate or the fruiting body could be covered by the pathogen mycelium (Fig. 1B), thus preventing development or further development of the fruiting body. The disease symptoms were similar to those caused by Tricoderma spp. in green mold disease on other mushrooms. After purification, five representative single-spore isolates (YZB-1 to YZB-5) were collected for pathogenicity testing and identification.
Figure 1: Disease symptoms during the cultivation of S. vaninii and after artificial inoculation.
(A and B) During the cultivation of S. vaninii. Pathogen hyphae covering the surface of the initial fruiting body and exposed substrate. (C) After inoculation. Hyphae inoculated with isolate YZB-1 covering the surface of the substrate and surrounding fruiting body. (D) Normally growing S. vaninii fruiting body.Pathogenicity tests
A spore suspension of the five isolates was inoculated into the exposed area nearby the developing fruiting body of S. vaninii, and white hyphae developed rapidly. Ten days after inoculation, a lot of hyphae with a green mold layer covered the exposed substrate and surrounded the developing fruiting body (Fig. 1C). All of the inoculated bags showed the same symptoms as the natural incidence, whereas the control treatment remained symptomless. The five isolates were separated from the inoculated bag again (YZB-1-P to YZB-5-P).
Morphological identification of pathogens
The colony characteristics of all ten isolates were similar. On PDA, the colonies were floccose with massive conidiation covering the whole surface of the plate (Fig. 2A). On CMD, isolates had a flat colony with aerial mycelium (Fig. 2B). Conidiophores and conidia were produced concentrically or near the margin of the plate. On SNA, they were relatively sparse (Fig. 2C). Conidiophores were gliocladium-like, arising from aerial hyphae, straight, 42–75 μm long (n = 30), generally unbranched (Fig. 2D), and sterile near the base, branching irregularly near the tip, with each branch terminating in a whorl of 3–6 phialides; metulae and phialides arose at narrow angles. Phialides were lageniform or ampulliform, 8.5–9.0 × 3.9–4.2 μm at the widest point. Conidia were green, smooth, subglobose, 4.2–4.5 × 3.9–4.0 μm (Fig. 2E). The isolates were similar to T. virens Gli 21, as described by Chaverri, Samuels & Stewart (2001). They are markedly different from the reported Trichoderma species in terms of spore size, color and location of colonization, phialides morphology and number of branches, and so on (Tomah et al., 2020; An et al., 2022).
Figure 2: Colonies and microscopic photographs of pathogenic fungi.
YZB-1 grown on PDA, CMD or SNA in 9-cm-diam Petri dishes under 12 h darkness/12 h light for 7 d. (A) On PDA. (B) On CMD. (C) On SNA. (D and E) Conidiophores and phialides conidia. D = 100 μm; E = 10 μm.Molecular analysis
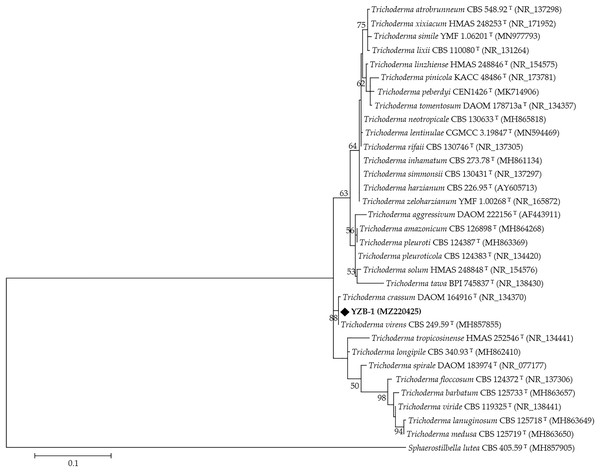
The DNA from ten isolates was amplified using the primer pairs ITS5/ITS4. Sequence alignment results showed that the ITS nucleotide identity of all isolates was 100%. One isolate, YZB-1, was selected for subsequent analysis, and the ITS fragments were approximately 630 bp in length. The accession number in GenBank is MZ220425.1. Phylogenetic analysis was performed using ITS sequences from 31 type strains of Trichoderma species and one outgroup type strain Sphaerostilbella lutea. The resulting phylogenetic tree showed that all strains were separated into different clades (Fig. 3), and most reference strains could be distinguished on the species level. Strain YZB-1 was clustered together with T. virens. These data confirmed that YZB-1 is a member of T. virens.
Figure 3: The phylogenetic tree generated from the ITS sequences of Trichoderma spp.
Branch values lower than 50% were omitted.Temperature stability assay
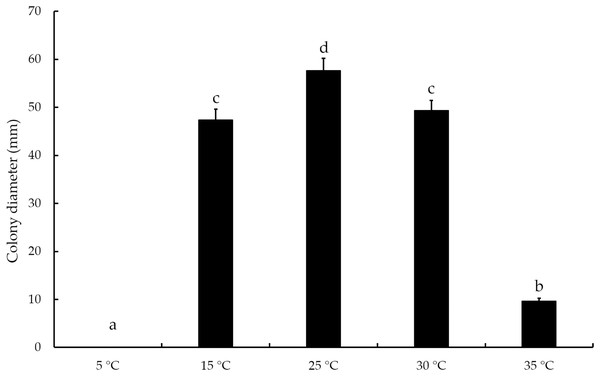
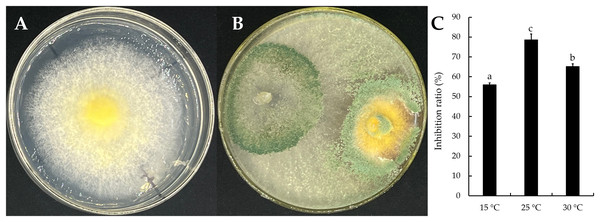
The mycelial growth of T. virens strain YZB-1 was significantly affected by different incubation temperatures (Fig. 4). The mycelia grew fastest at 25 °C, with an average colony diameter of 57.67 ± 2.52 mm. At temperatures above or below 25 °C, mycelium growth gradually slowed down. At 5 °C, the mycelia stopped growing. Dual-cultures of T. virens and S. vaninii were performed at temperatures suitable for pathogen growth (15 °C, 25 °C, and 30 °C). The inhibition rate of T. virens on S. vaninii mycelium was highest when incubated at 25 °C (79.01 ± 2.79%), with significant differences in inhibition rates at the three temperatures (Fig. 5C). Trichoderma virens not only occupied the medium surface more quickly with mycelial growth but also produced more green spores at the intersection of T. virens and S. vaninii (Fig. 5B).
Figure 4: The diameters of T. virens strain YZB-1 at different temperatures.
The error bars indicate the standard deviation, and different letters indicate significantly different values (P < 0.05).Figure 5: The dual-culture of T. virens strain YZB-1 and S. vaninii.
(A and B) S. vaninii (A) and T. virens × S. vaninii (B) were incubated at 25 °C. (C) The inhibition ratios of S. vaninii by T. virens at different temperatures. The error bars indicate the standard deviation, and different letters indicate significantly different values (P < 0.05).Discussion
Sanghuangporus vaninii is a renowned oriental medicinal mushroom, known in China as “Sanghuang,” in Japan as “Meshimakobu,” and in Korea as “Sangwhang” (Chen et al., 2019). Its fruiting body, also called yellow medicinal polyporus or basidiocarp, grows on the trunk of Populus sp. Linn., and is prized for its anti-tumor activity due to the bioactive protein-polysaccharide complex it contains (Oh & Han, 1993). However, Sanghuang occurs naturally in rare instances, making it highly valued. As a result, there has been extensive research on the artificial cultivation of S. vaninii (Wang et al., 2018; Hur, 2008). To achieve the formation of fruiting bodies, indoor temperature ranging from 31–35 °C and over 96% relative humidity are ideal, conditions that are also suitable for the occurrence of diseases (Hong, Sung & Nam, 2004).
Trichoderma green mold in edible basidiomycetes has been well known for some time (Hatvani et al., 2012). Among the most significant diseases affecting the most commonly cultivated mushrooms worldwide, such as P. ostreatus and A. bisporus, are those caused by some Trichoderma species, including T. guizhouense, T. harzianum, T. pleuroticola, and T. aggressivum (Bisset et al., 2015; Chaverri et al., 2015; Kosanovic, Grogan & Kavanagh, 2020; Turgay et al., 2023). However, T. virens has been rarely reported to infect edible basidiomycetes. In this study, we found that T. virens colonized the mycelium of S. vaninii, with the infection being limited to the fruiting body stage. To our knowledge, this is the first report of green mold disease caused by T. virens in S. vaninii cultivation.
The antifungal mechanism of Trichoderma spp. against fungi has been reported because of their biocontrol functions. Trichoderma spp. control microorganisms through competition, parasitism, antibiotic action, synergistic antagonism, and other mechanisms (Contreras-Cornejo et al., 2016). Compared to pathogenic microorganisms, Trichoderma spp. have faster growth and reproduction rates, stronger decay ability, and wider adaptability. The optimal growth temperature for Trichoderma spp. for biocontrol is 25–30 °C (Daryaei et al., 2016). They achieve a fungistatic effect by competing for the living space and nutrient resources of pathogens (Alwathnani et al., 2012). When T. harzianum and Fusarium solani were co-cultured, T. harzianum parasitized F. solani from multiple contact points and led to its death (Amira et al., 2017). Additionally, the Trichoderma group can degrade the cell wall of pathogens and absorb their nutrients by secreting a series of hydrolases, such as cellulase, glucanase, chitinase, and protease (Mukherjee et al., 2013). Trichoderma is beneficial in plant cultivation, but harmful in edible mushroom cultivation (Kredics et al., 2021).
As macroscopic fungi, the growth of edible mushrooms is also inhibited by Trichoderma species as aforementioned antifungal mechanism (Velázquez-Cedeño et al., 2007; Abubaker, Sjaarda & Castle, 2013). The optimal growth environment for Trichoderma is consistent with the mycelia growth and fruiting body formation environment of most edible fungi, which leads to its infection and harm to edible fungi during the mycelium and fruiting body stages (Kosanovic et al., 2020; Ponnusamy et al., 2022). This was confirmed by the results of both fruiting body inoculation and hyphal dual-culture experiments in the present study. There are few reports on the pathogenic mechanism of T. virens infecting the fruiting body of edible mushrooms, which may be related to parasitism and antibiotic action. The control of Trichoderma mainly relies on environmental control methods for prevention. Some safe agents (Innocenti et al., 2019) or biocontrol microorganisms (Ma et al., 2019) can be used to control Trichoderma during the hypha growth stage. However, the agent may have the potential to cause phytotoxicity (Kwon et al., 2021) or residues (Li et al., 2022) during the fruiting body growth stage.
Conclusions
This study has confirmed that the pathogen responsible for fruiting body rot in S. vaninii is the isolate YZB-1 through pathogenicity assays. Based on morphological identification and molecular analysis of the rDNA ITS region, the isolate YZB-1 was identified as T. virens. Trichoderma virens not only infects the fruiting body and causes abnormal growth but also inhibits hyphal growth. Further confirmation is required to determine whether its infection process and pathogenesis are consistent with the above mechanism. Finding safe and effective control methods for Trichoderma disease in S. vaninii is crucial for future studies.
Supplemental Information
The sequence information of type strains in phylogenetic analysis.
The species, strains and GenBank accession numbers of 31 Trichoderma and 1 Sphaerostilbella lutea. The ITS sequences of these strains were used in the phylogenetic analysis.
Raw data for temperature stability assay.
The diameters of T. virens and S. vaninii on the single plates and the semi diameters of two fungi on the dual-culture plates. These data were used for statistical analysis to obtain the inhibition ratio.