Genome-wide analysis of the heat shock transcription factor family reveals saline-alkali stress responses in Xanthoceras sorbifolium
- Published
- Accepted
- Received
- Academic Editor
- Diaa Abd El-Moneim
- Subject Areas
- Molecular Biology, Plant Science
- Keywords
- Xanthoceras sorbifolium, Saline-alkali stress, Genetic evolution, Expression pattern, Heat shock transcription factor
- Copyright
- © 2023 Li et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Genome-wide analysis of the heat shock transcription factor family reveals saline-alkali stress responses in Xanthoceras sorbifolium. PeerJ 11:e15929 https://doi.org/10.7717/peerj.15929
Abstract
The heat shock transcription factor (HSF) family is involved in regulating growth, development, and abiotic stress. The characteristics and biological functions of HSF family member in X. sorbifolium, an important oil and ornamental plant, have never been reported. In this study, 21 XsHSF genes were identified from the genome of X. sorbifolium and named XsHSF1-XsHSF21 based on their chromosomal positions. Those genes were divided into three groups, A, B, and C, containing 12, one, and eight genes, respectively. Among them, 20 XsHSF genes are located on 11 chromosomes. Protein structure analysis suggested that XsHSF proteins were conserved, displaying typical DNA binding domains (DBD) and oligomerization domains (OD). Moreover, HSF proteins within the same group contain specific motifs, such as motif 5 in the HSFC group. All XsHSF genes have one intron in the CDS region, except XsHSF1 which has two introns. Promoter analysis revealed that in addition to defense and stress responsiveness elements, some promoters also contained a MYB binding site and elements involved in multiple hormones responsiveness and anaerobic induction. Duplication analysis revealed that XsHSF1 and XsHSF4 genes were segmentally duplicated while XsHSF2, XsHSF9, and XsHSF13 genes might have arisen from transposition. Expression pattern analysis of leaves and roots following salt-alkali treatment using qRT-PCR indicated that five XsHSF genes were upregulated and one XsHSF gene was downregulated in leaves upon NaCl treatment suggesting these genes may play important roles in salt response. Additionally, the expression levels of most XsHSFs were decreased in leaves and roots following alkali-induced stress, indicating that those XsHSFs may function as negative regulators in alkali tolerance. MicroRNA target site prediction indicated that 16 of the XsHSF genes may be regulated by multiple microRNAs, for example XsHSF2 might be regulated by miR156, miR394, miR395, miR408, miR7129, and miR854. And miR164 may effect the mRNA levels of XsHSF3 and XsHSF17, XsHSF9 gene may be regulated by miR172. The expression trends of miR172 and miR164 in leaves and roots on salt treatments were opposite to the expression trend of XsHSF9 and XsHSF3 genes, respectively. Promoter analysis showed that XsHSFs might be involved in light and hormone responses, plant development, as well as abiotic stress responses. Our results thus provide an overview of the HSF family in X. sorbifolium and lay a foundation for future functional studies to reveal its roles in saline-alkali response.
Introduction
Heat shock transcription factors (HSFs) are widely expressed, including in bacteria, fungi, yeast, animals, and plants (Wiederrecht, Seto & Parker, 1988; Clos et al., 1990; Rabindran et al., 1991; Sarge et al., 1991; Scharf et al., 1991; Ghorbani, Alemzadeh & Razi, 2019). However, the number of HSF genes varies greatly in various classes of organisms, including between animals and sessile plants. Drosophila and vertebrates have only one and three HSF genes, respectively, while Arabidopsis, rice, tomato, and soybean contain 21, 18, 23, and 38 HSF genes, respectively (Guo et al., 2008; Hübel & Schöffl, 1994; Yang et al., 2016; Li et al., 2014). The HSFs possess the general structure of transcription factors: a DNA binding domain (DBD), an oligomerization domain (OD), and a nuclear localization signal (NLS). Furthermore, some HSFs are endowed with a nuclear export signal (NES) and a C-terminal activation domain (CTAD) (Harrison, Bohm & Nelson, 1994; Schultheiss et al., 1996; Kotak et al., 2004). Among these structures, the DBD and OD are the most conserved domains. The DBD is located at the N-terminus and has a helix-turn-helix hydrophobic structure, which is required to recognize and bind the heat shock element (HSE) motif in the promoters of target genes (Schultheiss et al., 1996). The OD domain is composed of two hydrophobic heptapeptide repeat regions A and B (HR-A and HR-B), forming a coiled-coil structure that helps HSF proteins form homotrimers (Peteranderl et al., 1999). Based on the number of amino acid residues inserted between HR-A and HR-B, HSFs can be divided into three types: HSFA with 21 amino acid residues inserted, HSFC with seven amino acid residues inserted, and HSFB with no amino acid residue inserted in this region (Nover et al., 1996). The NLS and NES domains guide the nuclear import and export, respectively, of HSF proteins (Lyck et al., 1997; Heerklotz et al., 2001; Kotak et al., 2004). The combined effect of the NLS and NES determines the subcellular localization of HSFs in different states.
The first report on plant HSFs dealt with tomatoes under heat stress, but subsequent studies have shown that HSFs are involved in a variety of stress responses including responses to freezing, drought, and salinity-alkalinity tolerance (Nover et al., 2001; Garg et al., 2002; Scharf et al., 1991). There are many studies on the role of HSF genes to heat stress in plant. Notably, the members of the HSF A class play important roles in heat stress response in Arabidopsis (Busch, Wunderlich & Schöffl, 2005), tomato (Li et al., 2013), wheat (Harsh et al., 2013) and strawberry (Hu et al., 2015). HSFs also play important roles in regulating the resistance of plants to low temperatures, such as AtHSFA6a, AtHSFA6b, AtHSFA9, AtHSFC1, OsHSFC1, OsHSFC2b, OsHSFA3, OsHSFA4d, OsHSFA7, OsHSFA9, BraHSF043, and BraHSF039 from Arabidopsis, rice, and cabbage (Miller & Mittler, 2006; Liu et al., 2010; Huang et al., 2015), (Scharf et al., 1991; Ma et al., 2014). Moreover, several HSF genes are involved in the adaptive response of plants to drought (Wiederrecht, Seto & Parker, 1988; Yoshida, Nishizawa & Yabuta, 2006; Guo, Wang & Zhang, 2017; Ghorbani, Alemzadeh & Razi, 2019). Overexpression of either AtHSFA6a or AtHSFA1b enhances tolerance for drought stress in Arabidopsis (Bechtold et al., 2013). but some HSF genes were identified that negatively impact drought resistance in plants, such as OsHSFB2b in rice (Xiang et al., 2013).
Similar to drought stress, the expression patterns of HSF family members varied during different stages of salt treatment. In the strawberry leaves treated with 300 mmol L−1 NaCl, the expression of FvHsfA2a increased during the early stage of stress, while the expression of FvHsfA3a, FvHsfA5a, and FvHsfA9a increased during the middle or late stages (Hu et al., 2015). Likewise, different members of the HSF family can act as positive or negative regulators in salt stress response. Notably, expression of AtHsfA2, OsHsfA7, OsHsfA2e, and TaHsfA2d enhances salt tolerance in Arabidopsis, rice, and wheat, respectively (Ogawa, Yamaguchi & Nishiuchi, 2007; Yokotani et al., 2008; Liu et al., 2013). However, over expression of the OsHsfB2b gene reduced the salt tolerance of rice (Xiang et al., 2013).
Due to the important roles of HSFs in responding to both biological and abiotic stresses in plants, numerous studies have concentrated on their regulatory mechanism. The HSPs, whose transcription is regulated by HSFs, accumulate rapidly in plants to help related proteins fold, intracellular distribution and degrade under environmental stress, when stress causes the occurrence of protein damage or inactivation (Fragkostefanakis et al., 2014). Although HSFs can participate in a variety of stresses, its transcriptional regulation mechanism in response to heat stress is relatively well studied. HSFs can regulate plant responses to stress by interacting with HSP proteins, other HSFs, or other substances. In a normal environment, the AtHSFA2 protein is distributed in the cytoplasm and nucleus with a low activity. In Arabidopsis AtROF1 and AtHSP90.1 form a complex that is stored in the cytoplasm. Under heat stress, the ROF-HSP90.1 complex interacts with HSFA2, facilitating its translocation to the nucleus, thereby regulating the expression of heat-responsive genes (Yokotani et al., 2008). Similarly, SlHSFA1 can interact with SlHSFA2 to activate the transcriptional expression of SlHSP90 and SlHSP70 in potato (Solanum lycopersicum) (Scharf et al., 1998; Kwan et al., 2009). Regardless of the fact that SlHSFB1 protein is not active, it can help SlHSFA1 to play a regulatory role in the heat shock process (Bharti et al., 2004). In addition, SlHSP90 can, in turn, regulate the expression of SlHSFA2 and SlHSFB1 proteins, thus forming a negative feedback loop (Hahn et al., 2011). HSF transcription factors form complexes to participate in stress signal perception and transmission processes. Light and other stresses can induce the accumulation of reactive oxygen species (ROS) in Arabidopsis. HSFA4, as a receptor of the H2O2 signal, can transmit the ROS signal to downstream transcription factors, including ZATs and WRKYs, through the mitogen-activated protein kinase (MAPK) pathway, thus triggering the cellular response network (Davletova et al., 2005; Qu et al., 2013). Furthermore, the transcriptional activity of several HSFs is regulated by calcium signals, triggered by heat in plasma membrane of the cell (Liu et al., 2008; Suzuki et al., 2011). The HSFs are functionally diverse and display different expression levels in different plant tissues and under different stress conditions. However, little is known about the regulatory mechanisms of HSFs in numerous species except model plants such as tomato and Arabidopsis. Furthermore, most of the studies on HSFs focus on heat stress, and their roles in various abiotic stresses, such as salt-alkali, freezing, and humidity, are still lacking.
Xanthoceras sorbifolium is an endangered and single species of the genus Xanthopanax in the Sapindaceae. The wild population is distributed in a ravine of the Loess Plateau in China, having developed root system with resistance to barrenness, drought, and saline-alkali environments (Bi & Guan, 2014). Due to its oil-rich seeds as well as its dense and beautiful flowers, the domesticated population had been grown in northern China (Yu et al., 2017). However, an excessive salt concentration in the soil remains a limiting environment factor for the cultivation of X. sorbifolium. How much saline-alkali stress it can withstand and the mechanism of salt-alkali resistance in X. sorbifolium remains unclear. The HSF transcriptional factors not only control plant growth and development but also enhance the resistance to abiotic stress. However, HSFs in X. sorbifolium have not been analyzed in detail. A high-quality sequence of the X. sorbifolium genome has been published, which provides the foundation for genome-wide analysis of the HSF genes (Bi et al., 2019). In this study, 21 HSF genes from X. sorbifolium were identified and their gene and protein features were characterized in detail. To reveal the mechanism of the role the HSF transcription factor plays in salt-alkali tolerance regulation in X. sorbifolium, the expression profiles of 15 XsHSFs were examined in the roots and leaves under saline-alkali stress. Therefore, this study provides the functional characteristics of the HSF family in X. sorbifolium and lays the foundation for their application in molecular breeding.
Materials & Methods
HSF genes identification
The genomes of Arabidopsis thaliana and rice (Oryza sativa) were downloaded from Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) (Nover et al., 2001; Lamesch et al., 2012). The genomic data of X. sorbifolium (Bi et al., 2019), Dimocarpus longan (Lin et al., 2017), and https://www.ncbi.nlm.nih.gov/genome/83651 (Yang et al., 2019) were collected from the Giga database (http://gigadb.org/). The candidate genes of the HSF family were extracted from the X. sorbifolium, D. longan, and https://www.ncbi.nlm.nih.gov/genome/83651 genome databases using the software HMMER (Johnson, Eddy & Portugaly, 2010) based on a Hidden Markov Model (HMM) of the DBD-domain profile (PF00447), obtained from the Pfam database (https://pfam-legacy.xfam.org/). The e-value cut-off was 0.01. To confirm the presence of the DBD domain in candidate XsHSF family members, all of the obtained protein sequences were analyzed by the online CD-search software (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The putative XsHSF genes were named depending on their genomic location. The subcellular location of HSF proteins was predicted by the Cell PLoc2 software (Chou & Shen, 2010).
Chromosomal location and gene duplication analyses
The chromosomal location information of HSF genes was obtained from X. sorbifolium genome database, and the chromosomal location map was produced by TBtools (Chen et al., 2018). Tandom duplicated genes were identified and paralogs were separated by five or fewer genes in a 100 kb region, as proposed by Tu et al. (2010). Identification of segmentally duplicated genes was conducted based on the plant genome duplication database (Lee et al., 2017; Lee et al., 2013). The non-synonymous substitution (Ka) and synonymous substitution (Ks) values of duplicated pairs were calculated on PAL2NAL (http://www.bork.embl.de/pal2nal) (Suyama, Torrents & Bork, 2006) after alignment of protein sequences. The duplication time of duplicated genes was calculated by T = Ks/2λ × 10−6 Mya (λ = 1.5 ×10-8 for dicots) (Blanc & Wolfe, 2004).
Gene characteristics, structure, and cis-elements in promoter
Physical parameters of putative XsHSF proteins, including the protein length, molecular weight (kDa), theoretical isoelectric point (pI), and grand average of hydropathicity (GRAVY), were calculated by the ExPASy tool (http://www.expasy.org/tools/protparam.html). The exon-intron structures in each XsHSF genes were displayed by alignment of the CDS with DNA sequences using Gene structure display server (GSDS) program (http://gsds.cbi.pku.edu.cn/). The orthologous gene of XsHSF in Arabidopsis were annotated using the Blast2GO (Conesa et al., 2005). The online database PLACE (https://www.dna.affrc.go.jp/PLACE/?action=newplace) was employed to investigate putative cis-regulatory elements in the sequences about 1,500 bp upstream of the transcription start site (ATG).
Protein domains and conserved motif analyses
The online MEME program was used to display the motif structure of HSF proteins (http://meme-suite.org/tools/meme) on the default parameters except for the optimum motif widths with 6 to 200 residues. SMART (http://smart.embl-heidelberg.de) and CD-search (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) web servers were used for structural motif annotation.
Sequence alignment and phylogenetic analysis
The Clustal X program was used to conduct the multiple sequence alignment of the full-length HSF protein sequences from X. sorbifolium, D. longan, A. yangbiense, Populus trichocarpa, A. thaliana, and O. sativa (Thompson et al., 1997). The Minimum Evolution (ME) phylogenetic tree was constructed by MEGA 6.0 software under the Jones-Taylor-Thornton (JTT) amino acid and Gamma Distributed (G) substitution model. Bootstrap values from 500 replicates were indicated at each node. Finally, the phylogenetic tree was beautified using online software iTOL https://itol.embl.de/itol.cgi.
MicroRNA target site prediction
The PsRNATarget tool (https://www.zhaolab.org/psRNATarget/) was used to predict microRNA target sites in each full-length XsHSFs sequence according to published microRNA sequences from Citrus sinensis, belonging to the Sapindales (Song et al., 2012).
Preparation of plant materials
X. sorbifolium was cultivated in the greenhouse in Qingdao Argriculture University. To explore the expression of XsHSFs, seedlings that had been germinating for 30 days were treated with water, 200 mM NaHCO 3, or 200 mM NaCl. After 0.5, 6 h, and 7 days of treatments, the roots and leaves of seedlings with different treatments were sampled and immediately frozen in liquid nitrogen for total RNA extraction and real-time RT-PCR assays. All samples were obtained in three biological replicates. All samples were collected and frozen in liquid nitrogen immediately, then stored in −80 °C for RNA extraction.
RNA extraction and qRT-PCR
Total RNA was extracted from mature leaves and young roots, separately, on different treatment using the RNA extraction Kit (Tiangen, Beijing, China). A total of 1 µg of total RNA was reverse-transcribed to first-strand cDNA by the PrimerScript RT Reagent Kit (TaKaRa Biotechnology, Dalian, China). Subsequently, qRT-PCR was performed using fluorescent dye SYBR Green I (TaKaRa, Dalian, China) according to the instructions. GADPH gene was selected as reference genes. Three biological replicates were carried out and expression levels were calculated by the 2−ΔΔCt method (Schmittgen & Livak, 2008). In addition, 500 ng total RNA was used to synthesis miRNA first-strand cDNA by the miRNA 1st strand cDNA synthesis kit (by stem-loop) (Vazyme, China, Nanjing). The 5.8s gene was used as reference gene for miRNA qRT-PCR. Gene primer sequences were listed in Table S1.
Results
Identification and characteristic analysis
A total of 21 gene sequences that are part of the HSF family were identified in the X. sorbifolium genome using the PF00447 Hidden Markov Model (HMM) sequence as a query (E < 1e−5). These sequences were named XsHSF1-XsHSF21 based on their chromosomal positions from top to bottom successively (Table 1). The number of HSF genes in X. sorbifolium was similar to Arabidopsis (21), D. longan (20), rice (25), Poplar (27), whereas it was 0.50-fold less than that in Soybean (52) and 1.4-fold greater than that in Acer yangbiense (15) (Nover et al., 2001; Baniwal et al., 2004; Wang, Zhang & Shou, 2009; Li et al., 2014). Gene lengths were between 796 to 6,687 bp. The corresponding protein sequences were submitted to CD-search (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) web servers to confirm the presence of the DBD domain. The XsHSF proteins ranged in length from 211 and 525 amino acids (aa) with an average of 371.4 aa and the isoelectric points (pI) ranged from 4.75 to 9.03. According to the prediction of the protein subcellular localization by Cell-PLoc 2.0 package, all HSF proteins were located in nucleus (Table 1).
| Gene name | Gene ID | Locus | Gene_start | Gene_stop | pI | MW | Strand | Subcellular localization | Gene length (bp) | Protein length (aa) | Arabidopsis ortholog |
|---|---|---|---|---|---|---|---|---|---|---|---|
| XsHSF1 | EVM0009602 | LG1 | 10952517 | 10954672 | 5.6 | 42673.27 | – | Nucleus | 2,156 | 376 | HSFA7A |
| XsHSF2 | EVM0018288 | LG1 | 30144743 | 30149941 | 4.93 | 56493.58 | + | Nucleus | 5,199 | 516 | HSFA1D |
| XsHSF3 | EVM0023941 | LG3 | 5587982 | 5591971 | 5.59 | 56276.6 | + | Nucleus | 3,990 | 502 | HSFA5 |
| XsHSF4 | EVM0020189 | LG3 | 25844286 | 25846001 | 5.08 | 43276.34 | + | Nucleus | 1,716 | 375 | HSF1 |
| XsHSF5 | EVM0003212 | LG4 | 28823754 | 28824973 | 8.17 | 40264.4 | + | Nucleus | 1,220 | 360 | HSFB2B |
| XsHSF6 | EVM0024360 | LG4 | 28899606 | 28900824 | 8.17 | 40264.4 | + | Nucleus | 1,219 | 360 | HSFB2B |
| XsHSF7 | EVM0006645 | LG5 | 3463810 | 3466132 | 5.34 | 45586.96 | + | Nucleus | 2,323 | 399 | HSFA4A |
| XsHSF8 | EVM0016425 | LG5 | 6794741 | 6795536 | 9.03 | 24596.02 | + | Nucleus | 796 | 211 | HSF4 |
| XsHSF9 | EVM0007574 | LG6 | 8861249 | 8867935 | 5.07 | 55755.26 | – | Nucleus | 6,687 | 502 | HSF3 |
| XsHSF10 | EVM0007442 | LG6 | 11510725 | 11512056 | 5.55 | 38923.54 | – | Nucleus | 1,332 | 336 | HSFA1D |
| XsHSF11 | EVM0007788 | LG6 | 26645262 | 26646978 | 5.12 | 51340.27 | – | Nucleus | 1,717 | 459 | HSFA2 |
| XsHSF12 | EVM0014424 | LG6 | 29759584 | 29760880 | 5.78 | 35230.91 | – | Nucleus | 1,297 | 314 | HSFC1 |
| XsHSF13 | EVM0005619 | LG6 | 31542679 | 31546019 | 4.95 | 43166.13 | + | Nucleus | 3,341 | 380 | HSFA2 |
| XsHSF14 | EVM0011404 | LG7 | 16687883 | 16688947 | 7.18 | 30429.3 | – | Nucleus | 1,065 | 260 | HSFB4 |
| XsHSF15 | EVM0016821 | LG8 | 25452697 | 25456928 | 4.75 | 44388.03 | – | Nucleus | 4,232 | 387 | HSFA8 |
| XsHSF16 | EVM0010398 | G10 | 23276109 | 23279230 | 7.01 | 30593.96 | – | Nucleus | 3,122 | 273 | HSFB2B |
| XsHSF17 | EVM0009694 | LG11 | 25864478 | 25866125 | 4.86 | 34933.52 | – | Nucleus | 1,648 | 315 | HSFB4 |
| XsHSF18 | EVM0007349 | LG12 | 9980293 | 9981938 | 5.14 | 25548.49 | – | Nucleus | 1,646 | 220 | HSFB2A |
| XsHSF19 | EVM0014086 | LG12 | 24748556 | 24750234 | 5.87 | 36903.25 | – | Nucleus | 1,679 | 330 | HSFB2B |
| XsHSF20 | EVM0010871 | LG13 | 27658322 | 27660823 | 5.15 | 57777.74 | – | Nucleus | 2,502 | 525 | HSFA3 |
| XsHSF21 | EVM0015064 | ctg1165 | 8512 | 10872 | 5.34 | 45613.98 | – | Nucleus | 2,361 | 399 | HSFA4A |
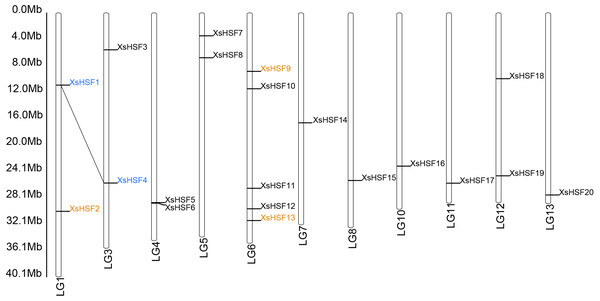
Chromosomal location and gene duplication
The 20 XsHSF genes were distributed on 11 chromosomes (Chr) and no HSF genes were found on Chr2, Chr9, Chr14, and Chr15 (Fig. 1). The XsHSF21 gene was the only 1 found on an unmapped scaffold. Gene duplication events, leading to gene functional diversity and plant adaptations, are mainly divided into three categories: segmental duplication, tandem duplication, and transposition (Kong et al., 2007). We identified 1 pair (XsHSF1 and XsHSF4) in the segmental duplication category and 3 genes (XsHSF2, XsHSF9, and XsHSF13) that might have arisen from transposition in the X. sorbifolium genome, accounting for 23.81% of the genes in the family (Data S1). The Ka/Ks ratios of gene pairs were calculated to investigate the gene divergence trend. A Ka/Ks ratio <1 indicates purifying selection. None of the XsHSF were randomly distributed.
Figure 1: Chromosomal location and duplicated gene pairs of XsHSF genes.
Blue letters connected with dotted black lines indicate segmental gene pairs, while the orange letters show genes arising from transposition.Phylogenetic analysis and conservative domain alignment
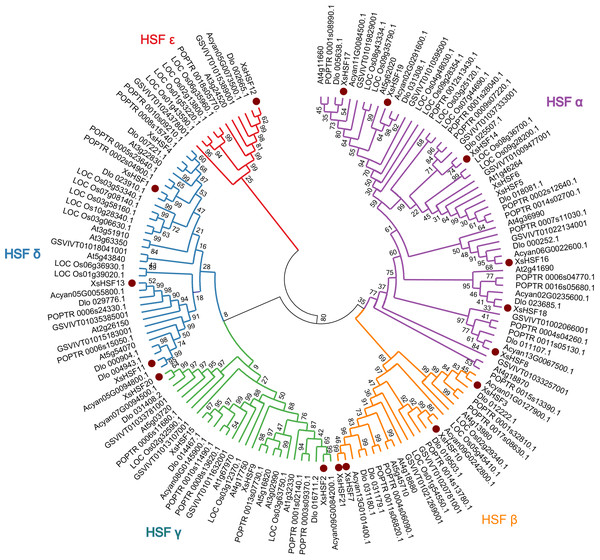
To reveal the classification of HSF proteins from X. sorbifolium and their evolutionary relationships, full-length HSF proteins in X. sorbifolium, D. longan, Acer yangbiense, Populus trichocarpa, O. sativa, and A. thalian were selected to construct a phylogenetic tree (Fig. 2). The full-length sequences of all HSF proteins identified in this study are presented in Data S2. The HSF proteins of X. sorbifolium are allocated into five major groups (Group α, β, γ, δ, and ɛ). Group α included eight genes (XsHSF5, XsHSF6, XsHSF14, XsHSF8, XsHSF16, XsHSF17, XsHSF18, and XsHSF19), group β included four genes (XsHSF3, XsHSF7, XsHSF10, and XsHSF21), group γ and δ both have four members, and group ɛ only included XsHSF12.
Figure 2: Phylogenetic tree of HSF transcription factors from Xanthoceras sorbifolium, Dimocarpus longan, Acer yangbiense, Populus trichocarpa, Oryza sativa, and Arabidopsis thaliana.
HSF protein sequences were aligned using Clustal X and the phylogenetic tree was produced using MEGA 6.0 software with the Minimum Evolution method. Bootstrap values are based on 500 replicates. The phylogenetic tree was divided into five groups ( α, β, γ, δ, and ɛ ). Each group is distinguished by a different color.Gene structure and promoter Cis-acting element analysis of XsHSFs
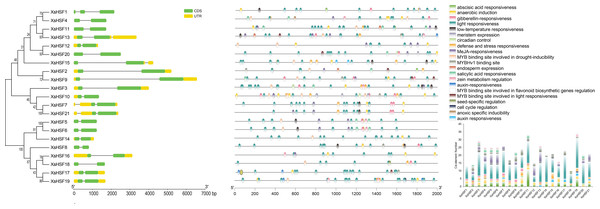
The intron/exon arrangements (Fig. 3, left) illustrated the structural similarities of the various XsHSF genes. Most of the XsHSF genes have one intron in their CDS region except for XsHSF1 with two introns. The cis-acting elements of the promoter play a leading role in regulating the expression of genes involved in phytohormone and environmental response. The 2,000 bp upstream sequences from ATG of XsHSF genes were extracted from X. sorbifolium genome and predicted by Plant CARE to identify cis-acting elements. A total of 21 cis-acting elements were identified in the different XsHSF promoters (Fig. 3 right, Table S2). Among these predicted cis-acting elements, most were related to light responsiveness, including ACE, G-Box, GT1, 3-AF1 binding site, Sp1, MRE, Box 4, LAMP-element, TCCC-motif, and AE-box. Moreover, a large number of cis-elements related to phytohormones were present on XsHSF promoters. For instance, the TGA-element and AuxRR-motif related to auxin responsiveness, the P-box, GARE-motif, and TATC-box related to gibberellin responsiveness, as well as ABRE, which were present in almost all XsHSF promoters except those of XsHSF13, XsHSF15, and XsHSF20, were activated in response to abscisic acid, implying that XsHSF genes could be induced by phytohormone signals. Various elements, including LTR, MBS, ARE, and TC-rich repeats, that are activated in response to stress responsiveness, were found in XsHSF promoters, suggesting that XsHSFs may participate in the response to environmental stimulation. Among these, ARE was found in all XsHSF promoters except for XsHSF2, XsHSF13, and XsHSF18. In addition, the RY-element, involved in seed-specific regulation, O2-sit, involved in the regulation of zein metabolism, CAT-box, involved in meristem development, and the GCN4-motif, involved in endosperm development, were found in several XsHSF promoters, indicating their essential function in plant development.
Figure 3: Gene structure and promoter cis-element analysis of XsHSF in X. sorbifolium.
Left, Exon and intron structure of HSFs in X. sorbifolium. The orange and green round-corner rectangles represent untranslated region (UTR) regions and exons, respectively, and the black line shows introns. Right, Cis-acting elements of the XsHSF promoters.Conserved domains and motif analysis
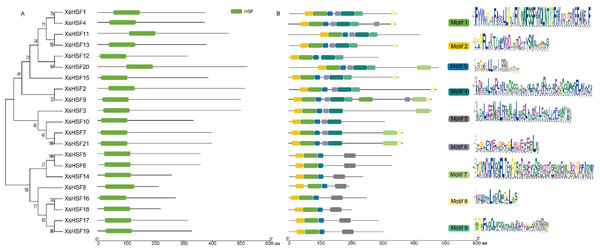
The alignment of the two conservative domains (DBD and OD) is shown in Fig. 4. DBD and OD domains were present in all XsHSF proteins. And XsHSF transcription factors were divided into three classes according to the number of amino acids between HR-A and HR-B in OD domain, XsHSF A subfamily includes members in group β, γ, and δ, and XsHSF B and C include the members in group α and ɛ, respectively. The conserved motifs, analyzed by MEME online tool, are shown in Fig. 5. Each putative motif was annotated using Pfam and SMART softwares. The motifs 1 and 2 in the DBD domain as well as motif 3 in the OD domain were relatively conserved in all XsHSF proteins. However, motif 4 encode NES and are extant only in A class. Motif 6 was present only in the HSF A and B classes but not in the C class. Motif 8 and motif 9 were present in the A and C classes while motif 5 was seen in B class.
Figure 4: Multiple sequence alignment of HSF proteins in X. sorbifolium.
(A) Multiple sequence alignment and protein sequence logo of the typical DNA binding domain (DBD). (B) Multiple sequence alignment of the oligomerization domain (OD).Figure 5: Conserved domains and motif analysis of XsHSF proteins in X. sorbifolium.
Left, Conserved domains analysis; Middle, Conserved motif analysis. The sequence logo (right) was created in MEME online software.Expression pattern analysis of XsHSF genes during salt and alkaline stress
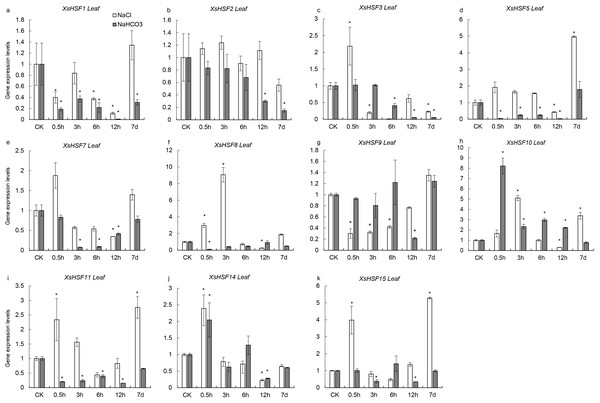
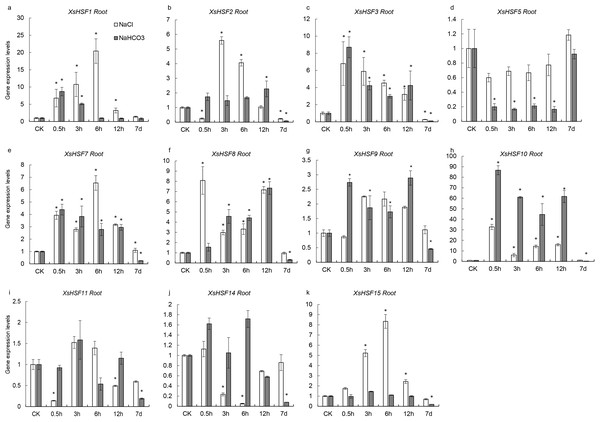
To investigate the roles of XsHSFs in response to salt and alkali stress, qRT-PCR analysis was conducted in leaves (Fig. 6) and roots (Fig. 7) and raw data were showed in Data S3. Diverse expression profiles indicated that under normal growth conditions all XsHSFs were detected in leaves and roots but that their expression patterns significantly varied. In leaves, the expression of XsHSF11 and XsHSF15 was induced by NaCl treatment, while the XsHSF1, XsHSF9, and XsHSF7 expression levels were significantly decreased (Figs. 6A, 6E, 6G, 6I, 6K). The trend of XsHSF3, XsHSF8 and XsHSF14 genes expression was first up and then down, while the expression of XsHSF5 showed the opposite trend on NaCl stress. Upon NaHCO3 treatment, except of XsHSF9 and XsHSF10, the expression levels of most genes, including XsHSF1, XsHSF2, XsHSF3, XsHSF5, XsHSF7, XsHSF11, XsHSF14, and XsHSF15, were significantly decreased in leaves (Fig. 6). In roots, the expression trend of several genes (XsHSF1, XsHSF2, XsHSF3, XsHSF10, XsHSF11 and XsHSF14 gene) under NaCl treatment was the same as that under NaHCO3 treatment, XsHSF1 and XsHSF10 were up-regulated, XsHSF11 and XsHSF14 were down-regulated (Figs. 7A, 7B, 7C, 7H, 7J). What is noteworthy is that the expression of XsHSF10 was up-regulated more than 80 times after 0.5 h treatment with NaHCO3 solution. Moreover, XsHSF7, XsHSF8, and XsHSF11 were up-regulated on NaCl treatment, while their expression displayed first up and then down in 7d under NaHCO3 treatment (Figs. 7E, 7F, 7G).
Figure 6: Expression analysis of ten selected XsHSFs in leaves upon different treatments.
XsGADPH was used as an internal control. Vertical lines represent S.E. (n = 3), * letters indicate significant differences (P < 0.05 by LSD test and fold change ≥ 2) among treatments. CK, leaf of seedling; Y-axis indicate leaf of seedling with 200 mM NaCl or 200 mM NaHCO3 treatment 0.5 h, 3 h, 6 h, 12 h, and 7d, respectively.Figure 7: Expression analysis of fifteen selected XsHSFs in roots upon different treatments.
XsGADPH was used as an internal control. Vertical lines represent S.E. (n = 3), * letters indicate significant differences (P < 0.05 by LSD test and fold change ≥ 2) among treatments. CK, leaf of seedling; Y-axis indicate leaf of seedling with 200 mM NaCl or 200 mM NaHCO3 treatment 0.5 h, 3 h, 6 h, 12 h, and 7d, respectively.Prediction of microRNA target sites in XsHSFs
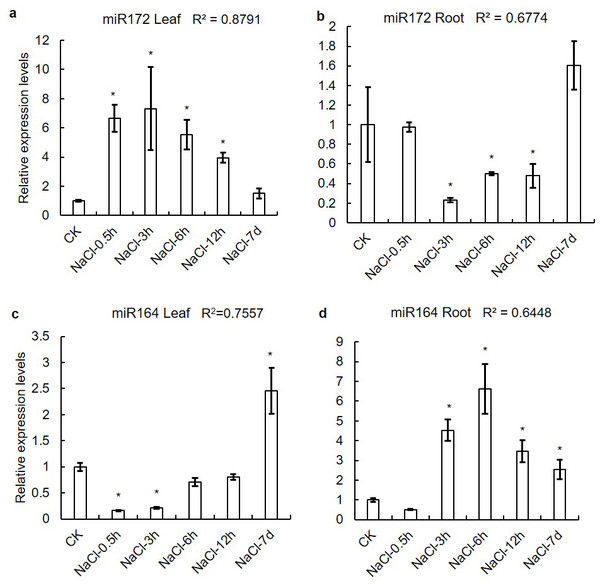
The prediction of microRNA target sites uncovered that the activity of several XsHSF proteins was regulated by microRNAs and that some genes might be regulated by mutiple microRNAs (Data S4). For example, the HSFA group gene XsHSF2 might be regulated by miR156, miR394, miR395, miR408, miR7129, and miR854, while the miR156, miR171, and miR390 target sites were found in the HSF B group XsHSF19 mRNA (Table 2). Due to XsHSF3 and XsHSF9 have the target sites of miR164 and miR172, respectively. qRT-PCR was performed to determine the expression levels of miR172 and miR164 in the leaves and roots treated with NaCl (Fig. 8). With the increase of NaCl concentration, the expression levels of miR172 firstly increased and then decreased in leaves, and decreased and then increased in roots, which was opposite to the expression level of XsHSF9 gene, and the correlation coefficient in leaves and root were 0.8791 and 0.6774, respectively. Similarly, the miR172 expression trends in leaves and roots following NaCl concentration were also opposite to those of XsHSF3.
| Target gene | miRNA |
|---|---|
| XsHSF1 | miR156, miR167 |
| XsHSF2 | miR156, miR394, miR395, miR408, miR7129, miR854 |
| XsHSF3 | miR164, miR477 |
| XsHSF4 | miR395 |
| XsHSF7 | miR397, miR858 |
| XsHSF9 | miR172 |
| XsHSF15 | miR393, miR530 |
| XsHSF20 | miR159 |
| XsHSF21 | miR156 |
| XsHSF5 | miR156 |
| XsHSF6 | miR156 |
| XsHSF8 | miR395 |
| XsHSF16 | miR156, miR390 |
| XsHSF17 | miR164 |
| XsHSF18 | miR854 |
| XsHSF19 | miR156, miR171, miR390 |
Figure 8: Expression levels of miR164 and miR172 in leaves and roots on saline-alkali stress.
5.8s was used as an internal control. Vertical lines represent S.E. (n = 3), * letters indicate significant differences (P < 0.05 by LSD test and fold change ≥ 2) among treatments. CK, root of seedling; Y-axis indicate leaf of seedling with 200 mM NaCl or 200 mM NaHCO3 treatment 0.5 h, 3 h, 6 h, 12 h, and 7d, respectively.Interaction network of HSF proteins between X. sorbifolium and A. thaliana
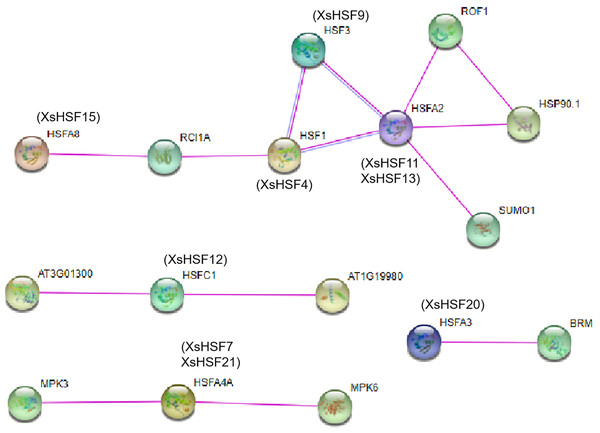
The protein–protein interactions of HSF proteins between X. sorbifolium and A. thaliana were analyzed using the online software STRING (http://string-db.org) (Fig. 8). The orthologous protein was matched with the highest bitscore, and the lines with different color represented different types of evidence for the interaction. The XsHSF11 and XsHSF13 proteins had highly similar amino sequences to AtHSFA2, and XsHSF9, XsHSF15, XsHSF4, XsHSF12, XsHSF20 had similar sequences to AtHSF3, AtHSFA8, AtHSF1, AtHSFC1, AtHSFA3, respectively (Table 1, Fig. 9). In Arabidopsis, AtHSFA8, AtHSFA3, AtHSFA2, AtHSFA4A, and AtHSF1C were reported to regulate heat stress (Giesguth et al., 2015; Friedrich et al., 2021). AtHSFA3 binds AtHSFA2 and other proteins form heteromeric complexes to acquire thermotolerance. In addition to heat stress, the HSFA4A gene was also reported to confer salt tolerance and is regulated by the MAP kinases MPK3 and MPK6 (Nover et al., 2001). AtHSF1a is the orthologous gene of XsHSF4 in Arabidopsis, and overexpressing the AtHSF1a gene can promote the tolerance to diverse stressors, including high pH, by promoting inducible HSP expression (Qian et al., 2014). Those results showed that XsHSF genes might regulate the diverse stresses in X. sorbifolium.
Figure 9: Interaction networks of seven XsHSF proteins in X. sorbifolium according to the orthologs in Arabidopsis.
The nodes represent different proteins and different colored lines indicate different types of evidence for the interaction. Light blue and rose red lines indicate known interactions originating from curated databases and experimental determinations, respectively.Discussion
X. sorbifolium is a special woody oil crop in China. Its fruit oil is a high quality edible oil and the main raw material for biodiesel production. X. sorbifolium has a certain tolerance to salt and alkali. However, with the aggravation of soil salinization, it is important to study the salt and alkali tolerance of X. sorbifolium to expand the scope of the cultivated region of saline-alkali threat, to relieve the pressure of agricultural production, improve soil quality and restore the ecological environment.
Characteristics of the HSF family in X. sorbifolium
The HSF transcriptional factors play a major role in plant development, biotic, and abiotic stress. The number of HSF genes is species-specific, for example, 21, 25, 27, 52, and 56 HSF genes were identified in Arabidopsis, rice (Guo et al., 2008), poplar (Wang et al., 2012), soybean (Li et al., 2014), and wheat (Ye et al., 2020), respectively. But the number of HSF family members did not correlate with the genome size of the species, for example, the number of HSF genes was similar in Arabidopsis, rice, and X. sorbifolia, but the genome sizes of Arabidopsis, rice, popular and X. sorbifolia are vastly different, at 135 Mb (Goodman, Ecker & Dean, 1995), 466 Mb (Yu et al., 2002), and 420 Mb, respectively. Previous studies showed that the number of plant HSF genes may be related to selective pressure during plant evolution in addition to genome replication events (Klaus-Dieter et al., 2012). Compared with soybean (52) and wheat (56), our result indicated a decrease of HSF members in X. sorbifolium, which may be caused by a lack of whole-genome duplication events in X. sorbifolium (Bi et al., 2019). In the present study, genes of the HSF family were also identified in X. sorbifolia, https://www.ncbi.nlm.nih.gov/genome/83651 and https://www.ncbi.nlm.nih.gov/genome/13043, three Sapindaceae trees. No recent whole-genome-wide duplication event was detected in Sapindaceae (Lin et al., 2017), but the number of HSF family members in these species ranged from 15 to 21, indicating that HSF genes in X. sorbifolia, https://www.ncbi.nlm.nih.gov/genome/83651, and https://www.ncbi.nlm.nih.gov/genome/13043 experienced different selection pressure.
Based on the phylogenetic analysis and the result of the multiple sequences alignment, 21 XsHSFs were further clustered into three classes, similar to HSF gene division in other plants (Busch, Wunderlich & Schöffl, 2005; Guo et al., 2008; Ma et al., 2014; Ma et al., 2015; Hu et al., 2015). The DBD domains of XsHSF s were highly conserved, suggesting that the DBD domains were founded before gene functional divergences occurred. Moreover, the number of amino acid residues inserted between HR-A and HR-B in OD domain was different among HSFA, B, and C classes, which is in agreement with the results of previous studies (Fig. 4). Several distinct motifs were observed in the three classes, suggested that three class genes underwent different selective constraints and function divergence.
Response of XsHSF genes to saline-alkali stress in X. sorbifolium
The HSPs play an important role in response to various biological and abiotic stresses by promoting cell repair, protein folding and degradation, and signal transduction. Moreover, the HSFs are the principal regulatory factors that directly activate the expression of HSPs. The expression of HSFs can be regulated by abiotic stresses, including heat, salt, drought, and cold. But the mechanism of HSF gene response to saline-alkali stress is less studied. Various studies have indicated that different HSF family members have varied functions in regulating plant salinity tolerance. Several HSF genes can enhance the salt tolerance in Arabidopsis (Ogawa, Yamaguchi & Nishiuchi, 2007), maize (Jiang et al., 2018), and Populus (Shen et al., 2015). AtHsfA6a acts as a transcriptional activator of stress-responsive genes and their overexpression can be induced by exogenous ABA, NaCl, and drought stress (Hwang et al., 2014). AtHSFA7b regulates its target genes, containing E-box-like and heat shock element motifs, to trigger serial physiological changes to improve salt tolerance, including adjusting osmotic potential to reduce water loss rate, removing reactive oxygen species (ROS), and maintaining cellular ion homeostasis (Zang et al., 2019). AtHSF4A mediates tolerance to salt and oxidative stress by interacting with mitogen-activated protein kinases MPK3 and MPK6, leading to transcriptional activation of HSP17.6A (Pérez-Salamó et al., 2014). The expression of maize ZmHsf04 can increase salt tolerance in Arabidopsis (Jiang et al., 2018). In addition, ssome HSFs are functioning as a negative regulator, such as ZmHsf08 in maize (Wang et al., 2021) and OsHsfB2b in rice (Xiang et al., 2013).
The salt tolerant response of XsHSF genes in X. Sorbifolium is still unclear. In this study, to explore the role of XsHSFs in regulating saline-alkali stress in X. sorbifolium, the seedlings were treated with neutral salt NaCl and alkaline salt NaHCO3. The expression trends of different XsHSF gene varied in seedlings treated with a neutral salt and an alkaline salt. The expression profile of 11 genes with low sequence similarity was detected in leaves and roots following salt or alkali treatment. In all, four class A members (XsHSF10, XsHSF11, XsHSF15) and class B members (XsHSF5, XsHSF8, XsHSF14) exhibited high expression levels in leaves on salt treatment, suggesting those genes mainly participated in salt tolerance in leaf (Fig. 6). This is in accord with that the orthologous genes of XsHSF10 and XsHSF11, AtHSFA2 and AtHSFA1D, were essential positive regulator for salt tolerance (Ogawa, Yamaguchi & Nishiuchi, 2007; Liu, Liao & Charng, 2011). On alkaline stress, the expression of most XsHSFs were supressed in leaves, including XsHSF1, XsHSF2, XsHSF3, XsHSF5, XsHSF7, XsHSF11, and XsHSF15, but only two genes (XsHSF5 and XsHSF15) were also down-regulated in both leaves and roots under alkali stress, suggesting that XsHSF5 and XsHSF15 may function as negative regulators in alkali tolerance (Figs. 6 and 7). Most XsHSFs expression levels can be induced by alkali stress in less than 12 h, but were suppressed after seven days, indicating that these genes may respond to alkali stress in a short time, but the long-term high concentration of alkali inhibited their expression. Moreover, the functions of XsHSF genes in X. Sorbifolium are worthy of further investigation.
Conclusions
Analysis of the X. sorbifolium genome identified 21 HSF family genes that were characterized, according to their gene structure and protein conserved domains and an expression profile during salinity-alkalinity stress was provided. Based on the phylogenetic tree, these HSF members were classified into three groups and their evolutionary relationships were further analyzed. Furthermore, the expression of 11 XsHSFs in different organs (root and leaf) and salinity-alkalinity conditions were changed. Given the likely importance of the HSF family in X. sorbifolium, our study provides a valuable basis for the subsequent elucidation of the functions of XsHSF genes.