Characterization of the transcriptional response of Candida parapsilosis to the antifungal peptide MAF-1A
- Published
- Accepted
- Received
- Academic Editor
- Timothy Read
- Subject Areas
- Bioinformatics, Genomics, Microbiology, Molecular Biology, Mycology
- Keywords
- Antimicrobial peptide, Antifungal, MAF-1A, Candida parapsilosis, Transcriptional response, RNA-seq
- Copyright
- © 2020 Cheng et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Characterization of the transcriptional response of Candida parapsilosis to the antifungal peptide MAF-1A. PeerJ 8:e9767 https://doi.org/10.7717/peerj.9767
Abstract
Candida parapsilosis is a major fungal pathogen that leads to sepsis. New and more effective antifungal agents are required due to the emergence of resistant fungal strains. MAF-1A is a cationic antifungal peptide isolated from Musca domestica that is effective against a variety of Candida species. However, the mechanism(s) of its antifungal activity remains undefined. Here, we used RNA-seq to identify differentially expressed genes (DEGs) in Candida parapsilosis following MAF-1A exposure. The early (6 h) response included 1,122 upregulated and 1,065 downregulated genes. Late (18 h) responses were associated with the increased expression of 101 genes and the decreased expression of 151 genes. Upon MAF-1A treatment for 18 h, 42 genes were upregulated and 25 genes were downregulated. KEGG enrichment showed that the DEGs in response to MAF-1A were mainly involved in amino acid synthesis and metabolism, oxidative phosphorylation, sterol synthesis, and apoptosis. These results indicate that MAF-1A exerts antifungal activity through interference with Candida parapsilosis cell membrane integrity and organelle function. This provides new insight into the interaction between Candida parapsilosis and this antimicrobial peptide and serves as a reference for future Candida parapsilosis therapies.
Introduction
Immunosuppressed patients are at a high risk of hospital-acquired fungal infections. Candida albicans (C. albicans) is the most common pathogen of Candida species, its dominance has decreased as the incidence of non-albicans Candida (NAC) species have increased (Vieira de Melo et al., 2019). Over the last two decades, epidemiological studies of fungal pathogens have shown that NAC has surpassed C. albicans as the most prevalent cause of invasive Candida (Sular et al., 2018). New anti-NAC treatment regimens are therefore urgently required.
Amongst NAC infections, Candida parapsilosis (C. parapsilosis) is particularly problematic due to its propensity to form biofilms on central venous catheters and other medically implanted devices (Fais et al., 2017; Vieira de Melo et al., 2019). Additionally, patients in the intensive care unit (ICU) who have undergone total parenteral nutrition are highly susceptible to C. parapsilosis infection, including undernourished children and neonates of low-birth-weights. Recent epidemiological studies have shown that C. parapsilosis is the second most commonly isolated species following only C. albicans in southern Europe, some regions of Asia, and Latin America (Toth et al., 2019). When immunosuppressed patients are exposed to C. parapsilosis, the rate of infection is high. The biological characteristics of infection, include toxicity, immune regulation, and drug resistance are in contrast with those of C. albicans (Toth et al., 2019). These interspecies specificities affect recognition by the host, clearance, and antifungal drug efficacy.
Candida pathogens have developed varying degrees of drug resistance, with some representing a serious threat to human health (Robbins, Caplan & Cowen, 2017). The currently available antifungal agents inhibit cell wall synthesis (echinocandins), destroy cell membrane components (azoles), or bind to ergosterol and perforate the cell membrane (amphotericin B). With the widespread use of antifungal drugs, the presence of drug resistance-related genes has increased. Antimicrobial peptides (AMPs) form a key arm of the innate immune response of a variety of organisms including plants, insects, and humans (Moravej et al., 2018). It is uncommon for microbial infections to be resistant to AMPs which are an emerging source of novel antifungal drugs (Ghosh et al., 2019; Nuti et al., 2017; Patocka et al., 2018), making these molecules a potential alternative to fungemia therapies.
AMPs can exhibit both cationic and amphiphilic properties. Cationic AMPs are amphipathic permitting their interaction with negatively charged cell membranes, leading to cell membrane disruption and cell death (Kobbi et al., 2018). AMPs are diverse with respect to length (20–100 amino acids), sequence and structure, and are produced by almost all organisms. Filamentous fungi produce a wide spectrum of AMPs that serve as defense and/or host signaling molecules. Penicillium chrysogenum secretes PAF and PAFB that possess complex tertiary structures and activity centers. PAF and PAFB are produced as 92 amino acid preproproteins that are active against a variety of pathogenic fungi, bacteria, and viruses (Huber et al., 2020). Insects are extremely resistant to microbial infections, which are an important source of AMPs. Insect AMPs are smaller (between 12 and 50 amino acids) with secondary structures formed predominantly of α-helices and β-sheets. Whilst membrane damage is the canonical mechanism through which AMPs act, other mechanisms exist. AMPs have specific subcellular targets, including the inhibition of DNA synthesis, RNA synthesis, protein synthesis, and cell wall integrity (Guilhelmelli et al., 2013; Li et al., 2016). However, their mechanism(s) of action at the molecular level remain unclear. The Musca domestica antifungal peptide-1 (MAF-1) is a novel cationic AMP isolated from the instar larvae of houseflies (Fu, Wu & Guo, 2009). We previously cloned the full-length MAF-1 gene and derived 26 amino acid MAF-1A peptides from the MAF-1 structural domain. Despite the established antifungal effects of MAF-1A, the molecular mechanism(s) governing its activity remain largely undefined (Zhou et al., 2016).
In recent years, the development of high throughput sequencing technologies has facilitated research on both antimicrobial drug function and drug-resistance. For example, HAC1 (CPAR2_103720) is a key mediator of endoplasmic reticulum stress in C. parapsilosis identified through RNA-seq (Iracane et al., 2018). In our previous studies, we showed that MAF-1A inhibits C. albicans through its effects on the cell wall, plasma membrane, protein synthesis, and energy metabolism (Wang et al., 2017). However, the mechanism(s) through which C. albicans responds to MAF-1A were not fully defined. Here, we have expanded our knowledge on how MAF-1A acts on C. parapsilosis and investigated differences in the responses of C. albicans and C. parapsilosis to MAF-1A treatment. RNA-seq was used to investigate changes in gene expression at early (6 h) and late (18 h) time points, according to time-kill curves of C. parapsilosis growth.
Materials and Methods
Strains and growth conditions
Transcriptional profiling was performed on the C. parapsilosis reference strain ATCC22019. The strain was preserved in goat blood and stored at −80 °C. C. parapsilosis was streaked on Sabouraud Dextrose Agar (SDA) plates (Sangon, Shanghai, China) at 35 °C as described by Lis et al. (2010). MAF-1A treatments were performed in Sabouraud Dextrose Broth (SDB) (Sangon, Shanghai, China).
Peptide synthesis
MAF-1A was synthesized by Sangon Biotech (Shanghai, Shanghai, China) as a linear peptide of 26 amino acids: KKFKETADKLIESAKQQLESLAKEMK. Analytical high-performance liquid chromatography (HPLC) was used to confirm purity ≥ 95%. The peptide was dissolved in sterile ultrapure water at 5 mg/mL and stored at −20 °C.
Minimum inhibitory concentration (MIC) and time-kill curves
Antifungal assays were performed as per the requirements of the Clinical and Laboratory Standards Institute (CLSI) M27-A3. Briefly, cultures were grown for 24 h at 35 °C and resuspended in SDB. Concentrations were adjusted to approximately 0.5 × 103–2.5 × 103 CFU/mL and 100 µl of the suspension was added to each well of 96-well polypropylene microplates (NEST, Wuxi, China). MAF-1A was added at concentration ranging from 0.1 mg/mL to 1.2 mg/mL. All experiments were performed in triplicates. After incubation at 35 °C for 24 h, absorbances were measured at 492 nm on a Microplate Reader (BioTek Synergy H1, Vermont, USA). MIC was defined as the lowest drug concentration showing 80% growth inhibition compared to the drug-free controls. The following formulas were used (Li et al., 2008):
(1) Percentage Fungal Growth = (Treatment Well A Value − Control Well A Value)/(Growth in Control Well A Value − Control Well A Value) × 100%.
(2) Percentage Inhibition of Fungal Growth = 1 − Percentage Fungal Growth.
Time-kill curves were performed according to the literature (Li et al., 2008; Sun et al., 2008). C. parapsilosis suspensions were mixed with MAF-1A (MIC) in triplicate and cultured at 35 °C. Aliquots of 100 µl were removed from each test solution at pre-determined time points (0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 h). Dilutions were produced (1:100) and streaked in triplicate onto SDA agar plates for colony counts after incubation at 35 °C for 24 h. Sterile ultrapure water was used as a control.
Transcriptome sequencing
C. parapsilosis was inoculated into SDB medium (Sangon, Shanghai, China) at 35 °C for 24 h. C. parapsilosis was treated with MAF-1A at MIC for 6 h (CPAS) and 18 h (CPBS), before RNA extraction. Untreated cultures served as controls (6 h, CPAC; 18 h, CPBC). Total RNA was extracted using RNAiso Plus (Takara, Dalian, China) according to the manufacturer’s instructions. RNA concentration and quality were determined on a NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA) and Agilent 2100 bioanalyzer (Agilent Technologies, CA, USA). Libraries were prepared using NEBNext® UltraTM RNA Library Prep Kit (NEB, USA) as per the manufacturer’s recommendations. Purified libraries were quantified on an Agilent 2100 bioanalyzer Effective concentrations were determined through qRT-PCR analysis. Libraries were prepared and sequenced using a Novoseq sequencer (Illumina, USA) to produce 150 bp paired-end reads.
Differential expression analysis
Raw reads were filtered to obtain high-quality clean reads for subsequent analysis. All reads were mapped to the reference genome of C. parapsilosis (assembly ASM18276v2) from the National Center for Biotechnology Information (NCBI) using HISAT2 v2.0.5 (Kim, Langmead & Salzberg, 2015). Differential expression analysis between the conditions was assessed using the Bioconductor software package DESeq2 in R 1.16.1 (Love, Huber & Anders, 2014). Relative gene expression was assessed using FPKM (Fragments Per Kilobase of transcript sequence per Millions of base pairs sequenced) and compared using log2 FC. P-values were adjusted to generate false discovery rates (padj) as described by Benjamini-Yekutieli, assigning the significance threshold for DEGs as padj < 0.05 (Benjamini et al., 2001; Mortazavi et al., 2008).
Enrichment and interaction network analysis of the differentially expressed genes
To further understand the functions of the DEGs, Gene Ontology (GO) enrichment was performed using the Bioconductor software clusterProfiler 3.4.4 in R package (Yu et al., 2012). Statistical enrichment of the DEGs was also performed in the Kyoto Encyclopedia of Genes and Genomes (KEGG) for pathway enrichment (Kanehisa et al., 2019; Ogata et al., 1999). PPI analysis of the DEGs was performed based on the STRING database to define key protein-protein interactions (Yu et al., 2012). The network was constructed using Cytoscape 3.6.1 (Shannon et al., 2003).
Validation of RNA-seq by quantitative RT-PCR (qRT-PCR)
To confirm the RNA-seq data, 20 DEGs (10 with increased expression and 10 with decreased expression) were selected for qRT-PCR validations. Reactions were performed using SYBR Premix Ex Taq TM Kit (Takara) according to the manufacturer’s protocol. Reaction conditions were as follows: 40 cycles of 95 °C for 30 s; 95 °C for 5 s; and 60 °C for 30 s. PCRs were performed on a BIO-RAD CFX-Connect Real-Time System. Relative gene expression was determined using the 2−ΔΔCt method normalized to 18S rRNA (Livak & Schmittgen, 2001). Significant differences were determined using a t-test with a threshold of p < 0.05. Primers are listed in Table S1. Primer efficiency and melting curves are listed in Table S2 and Figs. S1–S5.
Results
MIC assays and time-kill curves
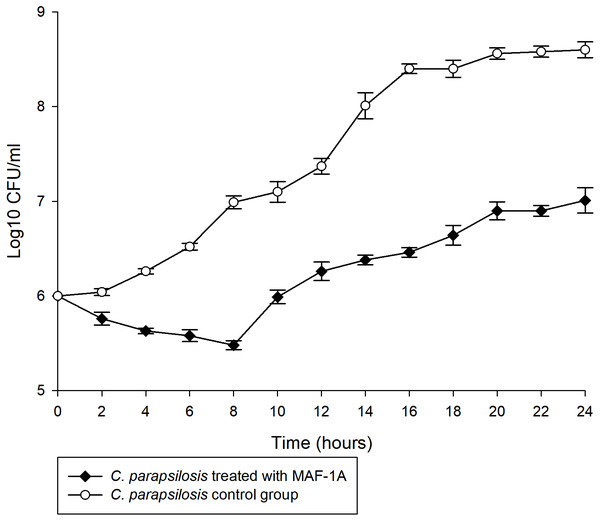
The MIC of MAF-1A against C. parapsilosis was determined as 0.6 mg/mL. Time-kill curves of MAF-1A at MIC showed a gradual antifungal effect during the first 8 h of C. parapsilosis culture (Fig. 1). After 8 h, cell numbers increased but remained lower than those of the control group.
Figure 1: Time-kill curves of MAF-1A under MIC for C. parapsilosis.
The mean growth of three C. parapsilosis cultures were recorded (Log10 CFU/ml) every 2 h for 24 h.Transcriptional stress responses and enrichment analysis of MAF-1A treated C. parapsilosis
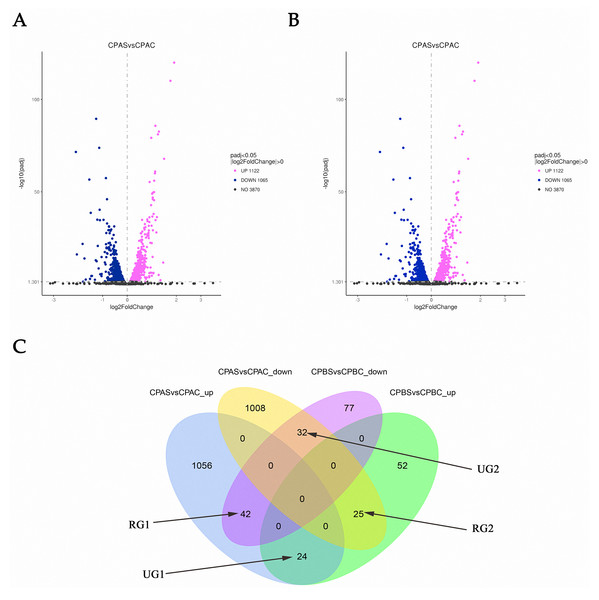
RNA-seq analysis in C. parapsilosis treated with MAF-1A for 6 and 18 h showed 5,747 DEGs. Sequence reads were deposited in the NCBI Sequence Read Archive (SRA) under accession number PRJNA638006. A total of 2,439 DEGs were detected. Out of these genes, 2187 were identified at 6 h, representing 38.05% of the total detectable genes. A total of 252 genes were differentially expressed after 18 h and accounted for 4.38% of the total expressed genes. After 6 and 18 h of MAF-1A treatment, 67 DEGs with opposite trends were observed (reversed genes 1, RG1 and reversed genes 2, RG2). In total, 56 DEGs were upregulated, whilst down-regulated genes remained unchanged (one unchanged genes: UG1; two unchanged genes: UG2) (Fig. 2).
Figure 2: Gene expression changes in C. parapsilosis following MAF-1A treatment.
Volcano plots of the DEGs. (A) Volcano plots depicting log2 FC (fold change) in expression after 6 h of treatment with MAF-1A, (C) parapsilosis was treated with MAF-1A at MIC for 6 h (CPAS), without MAF-1A as a control (CPAC). The expression of 1122 genes significantly increased; 1065 genes were significantly downregulated (padj < 0.05). (B) Volcano plot depicting the log2 FC expression after 18 h of treatment with MAF-1A. (C) parapsilosis was treated with MAF-1A at MIC for 18 h (CPBS). Controls lacked MAF-1A treatment (CPBC). The expression of 101 genes significantly increased in contrast to 151 genes whose expression decreased (padj < 0.05). (C) Gene expression Venn diagrams revealing two gene groups with opposite trends, labeled as RG1, RG2, UG1 and UG2; CPAS vs. CPAC_up: genes with increased expression after 6 h; CPAS vs. CPAC down: genes with decreased expression after 6 h; CPBS vs. CPBC_up: genes with increased expression after 18 h; CPBS vs. CPBC down: genes with decreased expression after 18 h.DEG enrichment analysis
Amongst the DEGs at 6 h, 1122 showing increased expression were enriched in 85 KEGG pathways, 20 of which were significant with padj < 0.05. The most significant pathways with increased expression in C. parapsilosis following MAF-1A treatment were: oxidative phosphorylation, peroxisome, citrate cycle (TCA cycle), carbon metabolism, cell cycle-yeast, MAPK signaling-yeast, meiosis-yeast, fatty acid degradation, and autophagy (Table 1). Genes of decreased expression were enriched in steroid biosynthesis, biosynthesis of amino acids, cysteine and methionine metabolism, biosynthesis of antibiotics, ribosome, RNA polymerase, biosynthesis of secondary metabolites, RNA transport, ribosome biogenesis in eukaryotes, lysine biosynthesis, 2-oxocarboxylic acid metabolism, pyrimidine metabolism, glycine, serine and threonine metabolism and purine metabolism (Table 2). At 18 h, 101 genes were upregulated and enriched in 24 KEGG pathways, two of which were significant (Table 3). A total of 151 genes were downregulated and significantly enriched in carbon metabolism, biosynthesis of antibiotics, oxidative phosphorylation, the biosynthesis of secondary metabolites, and the biosynthesis of amino acids (Table 4).
| KEGG ID | Description | p value | padj |
|---|---|---|---|
| cdu00190 | Oxidative phosphorylation | 3.34 × 10−12 | 2.84 × 10−10 |
| cdu04146 | Peroxisome | 2.02 × 10−8 | 8.56 × 10−7 |
| cdu00020 | Citrate cycle (TCA cycle) | 2.21 × 10−5 | 6.27 × 10−4 |
| cdu01200 | Carbon metabolism | 3.50 × 10−5 | 7.44 × 10−4 |
| cdu04111 | Cell cycle—yeast | 9.20 × 10−5 | 1.56 × 10−3 |
| cdu04011 | MAPK signaling pathway—yeast | 8.17 × 10−4 | 1.16 × 10−2 |
| cdu04113 | Meiosis—yeast | 2.35 × 10−3 | 2.80 × 10−2 |
| cdu00071 | Fatty acid degradation | 2.63 × 10−3 | 2.80 × 10−2 |
| cdu04136 | Autophagy—other | 4.03 × 10−3 | 3.80 × 10−2 |
Notes:
padj of < 0.05 set as the significance threshold.
| KEGG ID | Description | p value | padj |
|---|---|---|---|
| cdu00100 | Steroid biosynthesis | 1.64 × 10−8 | 1.40 × 10−6 |
| cdu01230 | Biosynthesis of amino acids | 5.95 × 10−7 | 2.53 × 10−5 |
| cdu00270 | Cysteine and methionine metabolism | 4.80 × 10−6 | 1.36 × 10−4 |
| cdu01130 | Biosynthesis of antibiotics | 1.07 × 10−5 | 2.27 × 10−4 |
| cdu03010 | Ribosome | 3.01 × 10−5 | 5.12 × 10−4 |
| cdu03020 | RNA polymerase | 2.14 × 10−4 | 3.03 × 10−3 |
| cdu01110 | Biosynthesis of secondary metabolites | 3.68 × 10−4 | 4.47 × 10−3 |
| cdu03013 | RNA transport | 9.34 × 10−4 | 8.83 × 10−3 |
| cdu03008 | Ribosome biogenesis in eukaryotes | 9.35 × 10−4 | 8.83 × 10−3 |
| cdu00300 | Lysine biosynthesis | 2.31 × 10−3 | 1.96 × 10−2 |
| cdu01210 | 2-Oxocarboxylic acid metabolism | 2.66 × 10−3 | 2.05 × 10−2 |
| cdu00240 | Pyrimidine metabolism | 3.54 × 10−3 | 2.40 × 10−2 |
| cdu00260 | Glycine, serine and threonine metabolism | 3.67 × 10−3 | 2.40 × 10−2 |
| cdu00230 | Purine metabolism | 5.08 × 10−3 | 3.09 × 10−2 |
Notes:
padj of < 0.05 set as the significance threshold.
| KEGG ID | Description | p-value | padj |
|---|---|---|---|
| cdu00220 | Arginine biosynthesis | 3.01 × 10−4 | 7.21 × 10−3 |
| cdu00250 | Alanine, aspartate and glutamate metabolism | 1.48 × 10−3 | 1.77 × 10−2 |
Notes:
padj of < 0.05 were set as the significance threshold.
| KEGG ID | Description | p-value | padj |
|---|---|---|---|
| cdu01200 | Carbon metabolism | 1.65 × 10−8 | 7.61 × 10−7 |
| cdu01130 | Biosynthesis of antibiotics | 6.21 × 10−7 | 1.43 × 10−5 |
| cdu00190 | Oxidative phosphorylation | 5.58 × 10−6 | 6.91 × 10−5 |
| cdu01110 | Biosynthesis of secondary metabolites | 6.01 × 10−6 | 6.91 × 10−5 |
| cdu00010 | Glycolysis/Gluconeogenesis | 1.71 × 10−5 | 1.57 × 10−4 |
| cdu00260 | Glycine, serine and threonine metabolism | 5.83 × 10−4 | 4.47 × 10−3 |
| cdu01230 | Biosynthesis of amino acids | 1.77 × 10−3 | 1.16 × 10−2 |
| cdu00680 | Methane metabolism | 3.62 × 10−3 | 2.05 × 10−2 |
| cdu00520 | Amino sugar and nucleotide sugar metabolism | 4.02 × 10−3 | 2.05 × 10−2 |
| cdu00052 | Galactose metabolism | 6.24 × 10−3 | 2.87 × 10−2 |
| cdu00730 | Thiamine metabolism | 7.93 × 10−3 | 3.20 × 10−2 |
| cdu00630 | Glyoxylate and dicarboxylate metabolism | 8.34 × 10−3 | 3.20 × 10−2 |
| cdu00330 | Arginine and proline metabolism | 9.61 × 10−3 | 3.24 × 10−2 |
| cdu00670 | One carbon pool by folate | 9.85 × 10−3 | 3.24 × 10−2 |
Notes:
padj of < 0.05 set as the significance threshold.
RG1, RG2, UG1, and UG2 gene enrichment analysis
The 42 genes in RG1 were enriched in 17 KEGG pathways, of which oxidative phosphorylation was most significant. Additionally, 25 genes in RG2 were enriched in 13 KEGG pathways, of which arginine biosynthesis, the biosynthesis of antibiotics, the biosynthesis of amino acids, and the biosynthesis of secondary metabolites were enriched. A total of 24 genes in UG1 and 32 genes in UG2 were enriched in 8 and 20 KEGG pathways, respectively. In UG1, the genes were enriched in butanoate metabolism, propionate metabolism, beta-alanine metabolism, valine, leucine, and isoleucine degradation. No pathways were significantly enriched in UG2 at a padj < 0.05 (Table 5).
| Sort | KEGG ID | Description | p-value | padj |
|---|---|---|---|---|
| RG1 | cdu00190 | Oxidative phosphorylation | 1.34 × 10−5 | 2.27 × 10−4 |
| RG2 | cdu00220 | Arginine biosynthesis | 1.97 × 10−5 | 2.56 × 10−4 |
| cdu01130 | Biosynthesis of antibiotics | 4.09 × 10−3 | 2.39 × 10−2 | |
| cdu01230 | Biosynthesis of amino acids | 5.52 × 10−3 | 2.39 × 10−2 | |
| cdu01110 | Biosynthesis of secondary metabolites | 1.21 × 10−2 | 3.92 × 10−2 | |
| UG1 | cdu00650 | Butanoate metabolism | 1.82 × 10−2 | 4.91 × 10−2 |
| cdu00640 | Propanoate metabolism | 2.48 × 10−2 | 4.91 × 10−2 | |
| cdu00410 | beta-Alanine metabolism | 2.64 × 10−2 | 4.91 × 10−2 | |
| cdu00280 | Valine, leucine and isoleucine degradation | 2.81 × 10−2 | 4.91 × 10−2 |
Notes:
padj of < 0.05 set as the significance threshold.
Genes in RG1, RG2, UG1, and UG2 were enriched in 535 GO terms, a total of 9 of which were significant (Table 6). Genes in RG1 were involved in energy production and redox processes. Genes in RG2 were associated with the anabolic processes of various organic acids. Genes in UG2 were involved in oxidation–reduction processes.
| Sort | Category | GO ID | Description | p-value | padj |
|---|---|---|---|---|---|
| RG1 | BP | GO:0006091 | Generation of precursor metabolites and energy | 8.71 × 10−5 | 7.14 × 10−3 |
| BP | GO:0055114 | Oxidation–reduction process | 8.49 × 10−4 | 3.48 × 10−2 | |
| RG2 | BP | GO:0016053 | Organic acid biosynthetic process | 5.27 × 10−4 | 2.11 × 10−2 |
| BP | GO:0046394 | Carboxylic acid biosynthetic process | 5.27 × 10−4 | 2.11 × 10−2 | |
| BP | GO:0044283 | Small molecule biosynthetic process | 1.56 × 10−3 | 3.12 × 10−2 | |
| BP | GO:0006082 | Organic acid metabolic process | 2.34 × 10−3 | 3.12 × 10−2 | |
| BP | GO:0019752 | Carboxylic acid metabolic process | 2.34 × 10−3 | 3.12 × 10−2 | |
| BP | GO:0043436 | Oxoacid metabolic process | 2.34 × 10−3 | 3.12 × 10−2 | |
| UG2 | BP | GO:0055114 | Oxidation–reduction process | 3.53 × 10−4 | 2.15 × 10−2 |
Notes:
padj of < 0.05 set as the significance threshold for enrichment.
Verification of differentially expressed genes
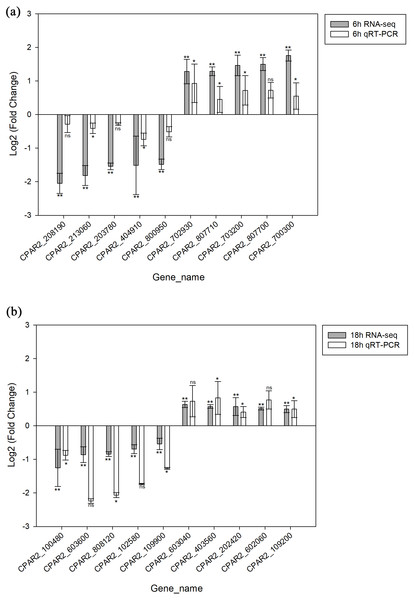
A total of 20 genes were selected, including 10 with increased expression and 10 with decreased expression. Genes were evenly selected from 6 h and 18 h time points to validate the RNA-seq data by qRT-PCR. The results indicated the expression levels have a consistent change for both RNASeq and qRT-PCR. Hence, the qRT-PCR results confirmed the reliability of our RNA-Seq data (Fig. 3).
Figure 3: Validation of the RNA-seq data via qRT-PCR analysis.
(A) 6 h timepoint. (B) 18 hour timepoint. * p < 0.05, ** p < 0.01, ns, not-significant.Protein-protein interaction (PPI) network analysis
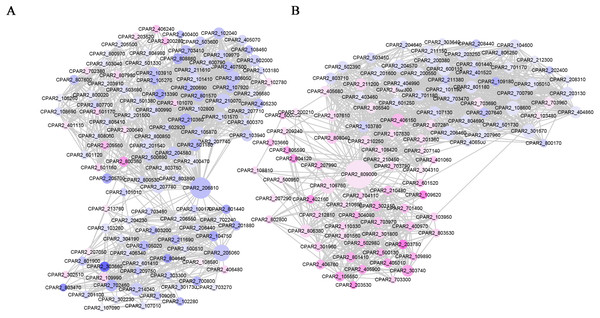
We constructed a PPI network based on the STRING database of the DEGs after 6 h of treatment. The PPI network contained 624 nodes and 6264 edges, with a degree filter of ≥ 10 (Fig. 4). The connectivity degree (dg) of multiple nodes in the PPI network were high, including: CpUbi1 (dg = 146), CpGlt1 (dg = 82), CpCdc28 (dg = 54), CpCys4 (dg = 50), CpCyt1 (dg = 45), CpRpc40 (dg = 42), CpArx1 (dg = 42), CpDim1 (dg = 41), CpYtm1 (dg = 41), CpRip1 (dg = 41). Upon enrichment analyses the identified genes were associated with oxidative phosphorylation (Fig. S6).
Figure 4: PPI network of the DEGs following MAF-1A treatment of C. parapsilosis for 6 h.
Node sizes correlate with node importance; purple nodes denote genes with increased expression, and blue denote decreased expression genes.Discussion
C. parapsilosis is one of the most prevalent fungal species in many regions. In addition to its high rates of infection, its etiology differs from that of C. albicans (Holland et al., 2014). Specific C. parapsilosis isolates are resistant to conventional antifungal drugs including echinocandins, azoles, and amphotericin B (Lotfali et al., 2016; Maria et al., 2018; Thomaz et al., 2018). Antimicrobial peptides lead to cell lysis and death through cell membrane leakage (Papo & Shai, 2003; Paterson et al., 2017; Shai, 1999; Utesch et al., 2018). However, mechanistic studies of antimicrobial peptides have determined that their membrane interactions are complex. Park, Kim & Kim (1998) showed that buforin II prevents microorganisms entry into cells. Lee et al. (2019) found that antifungal β-peptides cause cell death by entering cells and causing nuclear and vacuole dysfunction. Chileveru et al. (2015) showed that human alpha-defensin 5 enters the cytoplasm of Escherichia coli and interferes with cell division.
AMPs work through various mechanisms. In our previous studies, we showed that MAF-1A inhibits C. albicans through its effects on the cell wall, cell membrane, and ribosomes (Wang et al., 2017). In this study, we found that MAF-1A alters gene expression in several important biological pathways in C. parapsilosis, including oxidation–reduction processes and alternative energy sources. We further compared the response of C. albicans and C. parapsilosis to MAF-1A, most DEGs have the same expression trend (upregulated/downregulated), and identified changes in both stress and energy metabolism pathways (carbon metabolism, cell cycle, peroxisome, carbon metabolism, fatty acid degradation) (Tables S3 and S4). We hypothesized that the antifungal peptide MAF-1A exerts antifungal effect and disrupts energy metabolism by affecting oxidation–reduction processes, due to its effects on the mitochondria. Whilst antimicrobial peptides have multiple modes of action, these remain undetermined for MAF-1A. Our findings suggest that intracellular targets may be the key sites of MAF-1A activity, with enrichment analysis of the DEGs suggesting that MAF-1A exerts antimicrobial activity through a variety of mechanisms.
Membrane destruction
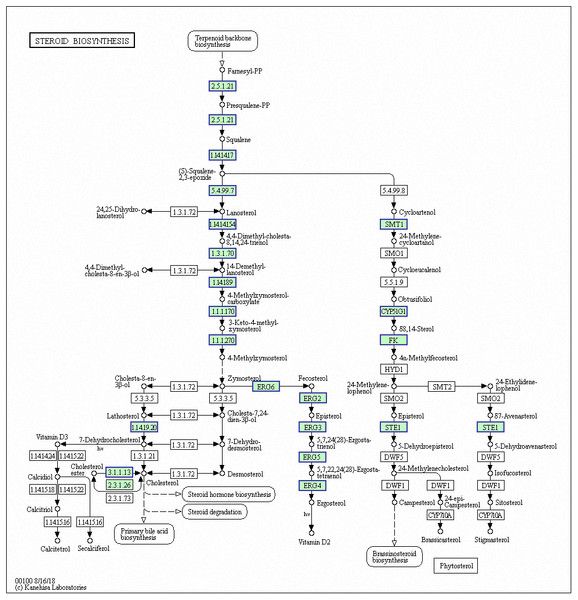
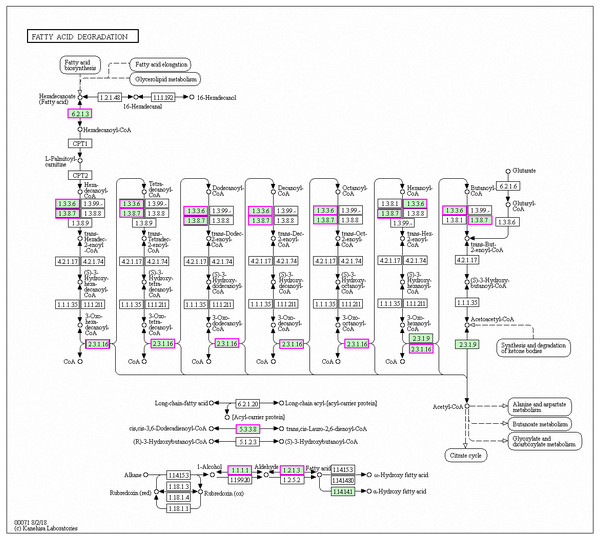
Genes with decreased expression after 6 h were significantly enriched in steroid biosynthesis (Fig. 5) including CpERG1, CpERG3, CpERG6, CpERG7, CpERG9, CpERG11, CpERG25, CpERG26, CpERG27, CpERG2, CpERG4, CpERG5, CpERG24, and CpSPBC16A3.12c (Kanehisa et al., 2019; Ogata et al., 1999). Azole agents exert antifungal activity by inhibiting the synthesis of ergosterol, a major component of fungal cell membranes (Ermakova & Zuev, 2017). The overexpression of ERG11 (encoding lanosterol 14-demethylase) is a major cause of azole resistance, often mediated by point mutations in the ERG11 gene. Members of the ERG gene family encode proteins involved in ergosterol biosynthesis, of which lanosterol 14-demethylase is critical. In this study, MAF-1A decreased the expression of 14 genes related to sterol synthesis including CpERG11, suggesting it interferes with ergosterol synthesis. Additionally, 10 genes showed increased expression after 6 h and were enriched in fatty acid degradation pathways (Fig. 6) (Kanehisa et al., 2019; Ogata et al., 1999). Of these, the expression of CpPOX4 (CPAR2-807700) significantly increased, (log2 FC = 1.503). We further verified the upregulation of these genes through qRT-PCR (log2FC = 2.834). CpPOX4 encodes a component of fatty acid biosynthesis, which indicates that the composition of the cell membrane was affected by MAF-1A.
Figure 5: Significantly enriched KEGG pathways in steroid biosynthesis.
DEGs with decreased expression are shown in blue. Permission for publication granted by the Kyoto Encyclopedia of Genes and Genomes, Kyoto University, Japan.Figure 6: Significantly enriched KEGG pathways in fatty acid degradation.
DEGs with increased expression are marked in purple. Permission for publication was granted by the Kyoto Encyclopedia of Genes and Genomes, Kyoto University, Japan.Mitochondrial function
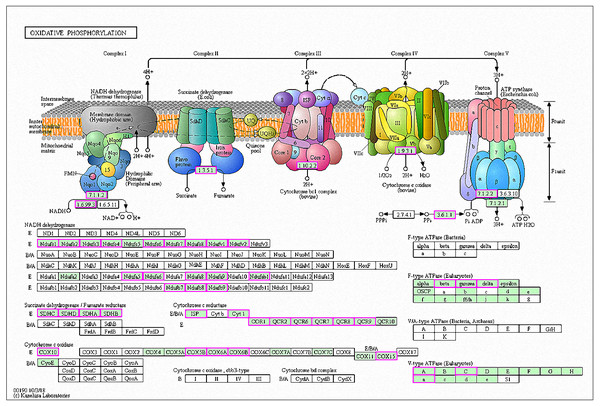
A total of 42 genes showed increased expression at 6 h and were enriched in oxidative phosphorylation pathways. The genes were mainly involved in respiratory chain electron transport processes in the mitochondrial inner membrane, including NHD release of H+ and ATP synthase (Fig. 7) (Kanehisa et al., 2019; Ogata et al., 1999). A total of six genes in RG1 were also enriched in this pathway (CpCOX15, Cpnuo-21, CpQCR2, CpQCR8, CpQCR7, and CpCOR1). Previous studies showed that COX15 encodes an indispensable mitochondrial protein for Saccharomyces cerevisiae cytochrome oxidase (Glerum et al., 1997). Cytochrome oxidase is a terminal enzyme in the respiratory electron transport chain that is essential for ATP synthesis. Reactive oxygen species (ROS) are produced by the oxidative phosphorylation of ATP and can disrupt the electron transport chain in mitochondria (Piippo et al., 2018). ROS production induces damage to lipids, proteins, lipids, and nucleic acids, leading to cell death. Eukaryotes prevent cell damage through oxidative stress detoxification and the prevention of ROS accumulation. In this study, GO enrichment analysis of the RG1 genes showed that 7 that were highly expressed were associated with redox processes (CpPOX9, CpCOX15, Cpnuo-21, CpAAEL001134, CpQCR7, CpRGI1, and CpnamA). These processes help cells to remove accumulated ROS. Due to the increased expression of these genes in response to MAF-1A, it is possible that MAF-1A promotes oxidative phosphorylation, which disrupts electron transfer in the mitochondria, enhancing ROS production and subsequent cell damage.
Figure 7: Significantly enriched KEGG pathways in oxidative phosphorylation.
DEGs with increased expression are marked in purple. Permission for publication was granted by the Kyoto Encyclopedia of Genes and Genomes, Kyoto University, Japan.C.parapsilosis has an unusual mitochondrial genome architecture, consisting of linear DNA molecules of 30.9-Kbp, terminating with specific telomeric structures on both sides (738-Kbp long). This differs from telomeres at the ends of eukaryotic nuclear chromosomes, particularly in humans (Kovac, Lazowska & Slonimski, 1984). MAF-1A interferes with the expression of multiple genes related to the mitochondrial functions of C. parapsilosis, It is, therefore, feasible that MAF-1A interferes with the normal function of C. parapsilosis mitochondria.
Conclusions
In summary, MAF-1A has a complex response in C. parapsilosis. Most DEGs identified through RNA-seq analysis were related to oxidation–reduction processes and the use of alternative energy sources, Mitochondria are important target for the anti-fungal peptide MAF-1A to exert anti-C. parapsilosis. RNA-seq data therefore provide future direction to study the antifungal mechanisms of MAF-1A and highlight the potential pathways that contribute to resistance.
Supplemental Information
The primer efficiency and melting curves
CPAR2_208190 (a1, a2), CPAR2_213060 (b1, b2), CPAR2_203780 (c1, c2), CPAR2_404910 (d1, d2), CPAR2_800950 (e1, e2).
The primer efficiency and melting curves
CPAR2_702930 (a1, a2), CPAR2_807710 (b1, b2), CPAR2_703200 (c1, c2), CPAR2_807700 (d1, d2), CPAR2_700300 (e1, e2).
The primer efficiency and melting curves
CPAR2_100480(a1, a2), CPAR2_603600 (b1, b2), CPAR2_808120 (c1, c2), CPAR2_102580 (d1, d2), CPAR2_109900 (e1, e2).
The primer efficiency and melting curves
CPAR2_603040 (a1, a2), CPAR2_403560 (b1, b2), CPAR2_202420 (c1, c2), CPAR2_602060 (d1, d2), CPAR2_109200 (e1, e2).
The primer efficiency and melting curves of reference gene
18S rRNA (a1, a2).
Results of KEGG enrichment analysis for genes in PPI network with higher degree (dg ¿40)
X axis is the ratio of differential genes, and Y axis is the KEGG pathway.
The primer efficiency of 20 differentially expressed genes and reference
reference gene: 18S rRNA.
A comparison between the DEGs of C. albicans and C. parapsilosis to MAF-1A
the C. parapsilosis were treated with MAF-1A at MIC for 6 h (CPAS) and 18h (CPBS). The untreated cultures CPAC and CPBC as control; CA_DT: the C. albicans were treated with MAF-1A at MIC for 2 h, CA_D: untreated cultures.
A comparison between the significantly enriched KEGG pathways for DEGs of C. albicans and C. parapsilosis to MAF-1A
The C. parapsilosis were treated with MAF-1A at MIC for 6 h (CPAS) and 18h (CPBS), untreated cultures CPAC and CPBC as control; CA_DT: the C. albicans were treated with MAF-1A at MIC for 2 h, CA_D: untreated cultures.