Taxonomic identification and biological traits of Platystethynium triclavatum (Donev & Huber, 2002), comb. n. (Hymenoptera, Mymaridae), a newly recorded egg parasitoid of the Italian endemic pest Barbitistes vicetinus (Orthoptera, Tettigoniidae)
- Published
- Accepted
- Received
- Academic Editor
- Joseph Gillespie
- Subject Areas
- Entomology, Parasitology, Taxonomy, Zoology
- Keywords
- Taxonomy, Mymaridae, Biological control, Barbitistes vicetinus, Parasitoid
- Copyright
- © 2020 Ortis et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Taxonomic identification and biological traits of Platystethynium triclavatum (Donev & Huber, 2002), comb. n. (Hymenoptera, Mymaridae), a newly recorded egg parasitoid of the Italian endemic pest Barbitistes vicetinus (Orthoptera, Tettigoniidae) PeerJ 8:e9667 https://doi.org/10.7717/peerj.9667
Abstract
The little known fairyfly (Hymenoptera, Mymaridae), Platystethynium (Platystethynium) triclavatum (Donev & Huber, 2002), comb. n. from Pseudocleruchus Donev & Huber, 2002, is newly recorded as an egg parasitoid of Barbitistes vicetinus Galvagni & Fontana, 1993 (Orthoptera, Tettigoniidae). This bush-cricket is endemic to northeastern Italy (mainly Euganean Hills of Veneto Region), where it has recently become an economically significant agricultural and forest pest. Data on discovery, distribution, and some remarkable biological traits of this gregarious egg parasitoid are presented. Its identification and availability of many well-preserved fresh specimens have made possible to re-define Pseudocleruchus Donev & Huber, 2002 syn. n., with type and the only described species Pseudocleruchus triclavatus Donev & Huber, 2002, as a synonym of Platystethynium Ogloblin, 1946 and its nominate subgenus, P. (Platystethynium), and also to describe the brachypterous male of P. (Platystethynium) triclavatum. It is the first known male for the entire genus. Enlarged mandibles of the megacephalous males are used to chew holes in the hard chorion of the host egg, allowing fully winged females, whose mandibles are strongly reduced and do not cross over, to emerge after mating with the males inside it. Up to 136 individual parasitoids (about 77 on average) can hatch from a single egg of B. vicetinus, with their sex ratio being strongly female biased (80–97% females per egg).
Introduction
Occasional outbreaks have been reported in some species of bush-crickets (Orthoptera, Tettigoniidae), causing severe damage to crop and forest plants (Escherich, 1928; Laussmann, 1994; Holuša, Heralt & Drapela, 2006). During these demographic fluctuations, the population density rises to values much higher than those usually presented by a species (Lorch & Gwynne, 2000; Bailey et al., 2007; Bailey, Gwynne & Ritchie, 2007; Srygley, 2014). A significant impact on a particular life-history stage of Tettigoniidae could be associated with the activity of predators and other natural enemies (Gwynne, 2001). There are stressors, such as parasitoids (Hunt & Allen, 1998; Umbanhowar & Hastings, 2002), that could adversely affect both fitness and developmental stability of a pest species. The behavior of individual parasitoids in response to an increasing prey density is thought to be an important attribute related with parasitoid success (Fernández & Corley, 2003). To date, interactions between parasitoids and bush-crickets during years of demographic explosion have been virtually unknown. In a large number of temperate species the egg stage persists for longer than a year, being adapted to withstand all the seasonal and annual adversities (Bailey & Rentz, 1990). Although Orthoptera eggs are available to natural enemies for such long periods, few of them are recorded at the egg stage, probably because finding and collecting eggs in the soil is a lengthy and difficult task (ElSayed, ElEla & Eesa, 2016). The best collection methods, such as by Malaise traps, yellow pan traps, sweep netting or Berlese funnels, are not suitable to detect these parasitoids in associations with their hosts, especially for egg parasitoids that search for their hosts in the ground (Chiappini & Huber, 2008). Most of the studies were conducted on egg-pods of Celifera, and they identified members of the family Scelionidae (Hymenoptera, Platygastroidea) as the most important parasitoids of grasshopper eggs (Grissell, 1997; Ghahari et al., 2009). In addition, some chalcidoid wasps (Hymenoptera, Chalcidoidea) were also reported to parasitize eggs of Tettigoniidae (Gupta et al., 2015).
The occurrence of severe outbreaks (since 2008) in the endemic area of northeastern Italy (Euganean Hills of Veneto Region) of the recently described bush-cricket Barbitistes vicetinus Galvagni & Fontana, 1993 (Orthoptera, Tettigoniidae), prompted interest in studying its biology; this bush-cricket is a pest which causes severe defoliation across sub-Mediterranean forests and neighboring crops (Cavaletto et al., 2018; Cavaletto et al., 2019). Like many other Tettigoniidae, this species is univoltine and overwinters as eggs laid in the forest soil. Egg hatching generally begins at the end of March, and the life cycle lasts until the first half of July when the last adults die after oviposition. The eggs of B. vicetinus can remain in the soil for one or more years in a diapause state, but since the outbreaks occurred only in the last decade, no data are currently available (Cavaletto et al., 2019). Damage in forests and crops caused by the outbreaks, and the environmental impact caused by conventional control strategies have stimulated search for alternative control methods such as potential use of natural enemies.
Here, we taxonomically identify the newly recorded egg parasitoid of B. vicetinus and provide data on its biological traits and geographical distribution data.
Materials and Methods
Study area
The study was carried out during 2018–2019 in the Euganean Hills (Province of Padua, Veneto Region, northeastern Italy). The area is characterized by about 100 volcanic hills covering an elliptical area of approximately 22,000 ha and with elevation ranges from sea level to 600 m a.s.l. This area corresponds with a small part of B. vicetinus distribution range, most affected by the outbreaks of the pest. The climate is characterized by an annual average temperature of about 12 °C and 700–900 mm of precipitation (Kaltenrieder et al., 2009). The landscape is fragmented and consists of forest patches, interspersed with crop fields and rural settlements.
Eggs of B. vicetinus sampling procedures
During autumn 2018, we collected eggs laid by B. vicetinus in the soil from 40 outbreak forest sites across the Euganean Hills, where the nymph density had been monitored in the previous study (Cavaletto et al., 2019). In each site, we identified six circular areas (sub-replicates) of 15 cm radius and five cm deep, free from branches, roots, stones and coarse litter. The six circular areas were spaced by no less than one m from each other. In total, from each site, the soil was collected from a surface of 0.42 square meters. The soil sample for each sub-replicate was collected with a small shovel and carried to the laboratory in sturdy plastic bags. Each sample of soil was washed in a hydraulic sieving-centrifugation device with different sieves of progressively smaller mesh (weaves decreasing from 20 to 1 mm) to facilitate separation of the eggs from organic and inorganic matter. Eggs were washed carefully from the remaining soil with running water, collected using soft tweezers, and placed in Petri dishes.
Eggs of B. vicetinus analysis
The eggs (length: 4,5 mm; width: 2,50 mm) (Galvagni & Fontana, 1999) were then recognized under a stereomicroscope and divided for each sub-replicate in (i) hatched eggs, (ii) flattened eggs, (iii) eggs with small holes in the chorion, and (iv) fertile eggs. The hatched eggs displayed a rupture in correspondence of the hatching line. Small pieces of chorion were not included in the count. The flattened eggs had no broken chorion and were recognized by the flat profile probably due to the lack of yolk inside. Eggs with small circular emergence holes, across the surface of the chorion, were assumed to be parasitized in the past seasons. The apparently fertile eggs, recognized by their turgescence sensu Warne (1972), were kept outdoors, in Petri dishes on filter paper and moistened weekly, to allow overwintering. The impact of parasitoids in the previous seasons was assessed, for each site, by counting eggs with small holes. Moreover, to evaluate efficacy of the egg parasitoid, the index of “discovery efficiency”, proposed by Bin & Vinson (1990), was applied. For each site, discovery efficiency was calculated as the number of sub-replicates discovered by the parasitoid over the six sub-replicates sampled. A sub-replicate was considered discovered when it included at least one egg with holes. Sub-replicates not containing any typology of eggs were not included in the count.
To describe the relationship between activity of the parasitoid and density of the bush-cricket, we related, for each site, the number of the previously parasitized eggs with the sum of all collected eggs.
Identification of the egg parasitoid and taxonomic studies
Morphological terms, mounting and photography techniques, measurements (in mm), and abbreviations (F = funicle segment of female antenna or flagellomere segment of male antenna; mps = multiporous plate sensillum or sensilla) follow those described in Triapitsyn et al. (2019). Specimens sent to the second author were labeled including a UCRC ENT database number (unique identifier for each individual).
Most voucher specimens of the parasitoids were deposited at DAFNAE; additional specimens were deposited in CNC (Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, ON, Canada) and UCRC (Entomology Research Museum, Department of Entomology, University of California, Riverside, CA, USA), as indicated under “Material examined” section below.
Statistical analysis
All analyses were conducted using R (version 3.6.2) (R Core Team). To relate the activity of the parasitoid during the past with the oviposition density of the bush-cricket we used a linear model (LM). For each site, the number of total eggs (hatched eggs, flattened eggs, fertile eggs, eggs with holes) was included in the model as fixed effect, while the response variable (number of eggs with holes) was log-transformed to improve linearity.
The impact of parasitoids was assessed, for each site, as the number of previously parasitized eggs divided by the total number of eggs collected. In addition, we calculated the sex ratio by dividing, for each parasitized egg, the number of hatched females with the total number of individuals.
Results
Distribution and biology of the egg parasitoid
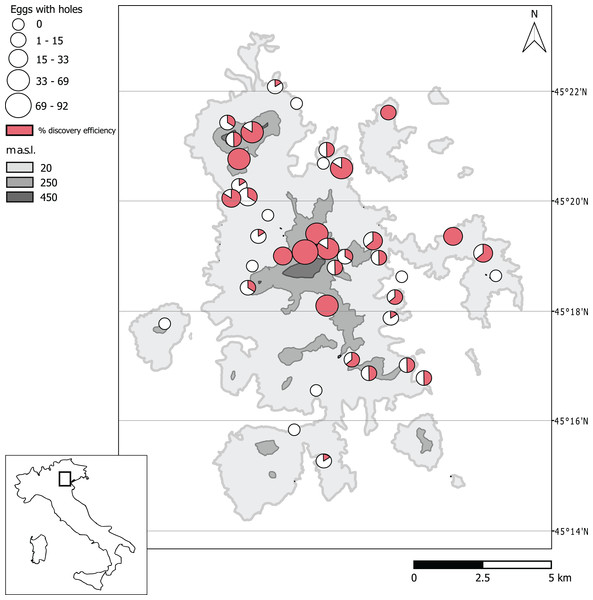
From the 40 sampling sites considered, in a total of 240 samples of soil (sub-replicates), we collected and analyzed 0.847 cubic meters of soil. A total of 24,329 eggs of B. vicetinus were identified from which 18,965 were recognized as hatched eggs, 3,132 flattened eggs, 1,669 fertile eggs and 559 eggs with small emergence holes. The average number of hatched eggs of B. vicetinus per site was 474 ± 85 SE, while that of the other Orthoptera was 10 ± 3 SE. We recorded activity of the parasitoids in 31 of the 40 sites studied. From 231 sub-replicates containing eggs of the bush-cricket, eggs with small holes were found in 113 sub-replicates (index of discovery efficiency = 48%) (Table S1; Fig. 1).
Figure 1: Distribution of Platystethynium triclavatum in the sampling sites of Euganean Hills, based on the presence of eggs of Barbitistes vicetinus with emergence holes.
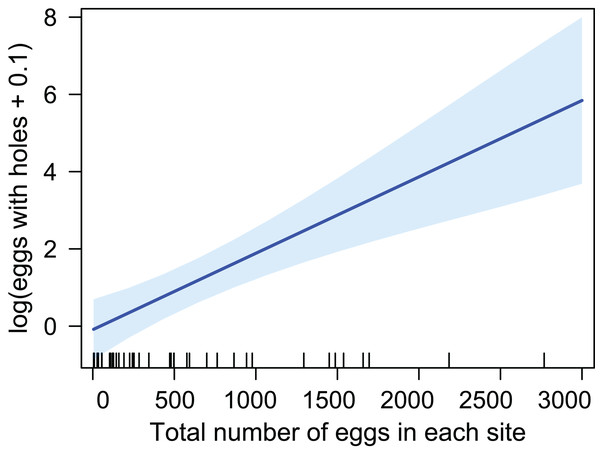
The abundance is indicated by white circles, elevation is indicated by gray color. The discovery efficiency for the six sub-replicates is represented by red color. Latitude is indicated by gray lines. Map Source: Curve di livello della Regione Veneto, IODL 2.0.The number of eggs with emergence holes was considered as number of the previously parasitized eggs. This number was positively related to the total number of eggs collected for each site, that is, higher number of eggs with emergence holes was found in sites where the oviposition density of the host was elevated (Fig. 2) (LM; R-squared = 0.35; df = 38, p-value = 4.41E−05).
Figure 2: Relation between previously parasitized eggs and the amount of eggs in each site.
At the end of spring, a portion of about 70 fertile eggs, which were not hatched, was monitored to verify the presence of parasitoids. Approximately the 20% of these eggs was randomly chosen and dissected. Among them, eight eggs showed numerous parasitoids in the embryonic or larval stages (Fig. 3). Subsequently, presence of parasitoids inside the eggs was detected without dissection, exploiting transparency of the chorion, increased by the treatment with xylene as described by Warne (1972) (Fig. 3). All these eggs were monitored and placed individually within single transparent plastic vials covered on top with cotton, provided with water weekly, and placed in a climatic chamber (T 26 ± 1 °C, RH 75 ± 5%, photoperiod 12:12). In June, we obtained a total of 537 samples of parasitoid wasps which emerged from seven eggs across an average number of holes per egg of 2.5. On average, 76.7 parasitoids emerged per egg. Up to 136 individuals hatched from a single egg (Table 1). We estimated a mean of previously parasitized eggs of 2.3% ± 0.5 SE among the 40 sites. The sex ratio of the parasitoid was strongly female biased, with a mean of 92% ± 2.4 SE of females per egg.
Figure 3: Eggs of Barbitistes vicetinus.
(A) Fertile egg treated with xylene to enhance the transparency of the chorion; (B) dissected egg with embryos of the parasitoid; (C) larval stages of the parasitoids visible in transparency in an egg treated with xylene; (D) emergence holes of Platystethynium triclavatum; (E) eggs of B. vicetinus.| Site | Latitude–Longitude | Elevation (m) | Holes | Total | F | M |
|---|---|---|---|---|---|---|
| 12 | 45°19′3.99″N–11°41′20.10″E | 447 | 4 | 136 | 132 | 4 |
| 12 | 45°19′3.99″N–11°41′20.10″E | 447 | 2 | 124 | 121 | 3 |
| 12 | 45°19′3.99″N–11°41′20.10″E | 447 | 2 | 120 | 115 | 5 |
| 12 | 45°19′3.99″N–11°41′20.10″E | 447 | 2 | 95 | 89 | 6 |
| 6 | 45°21′0.80″N–11°39′35.90″E | 295 | 4 | 40 | 35 | 5 |
| 3 | 45°19′19.68″N–11°43′20.67″E | 140 | 3 | 11 | 9 | 2 |
| 13 | 45°21′37.05″N–11°39′56.92″E | 367 | 2 | 11 | 10 | 1 |
| 3 | 45°19′19.68″N–11°43′20.67″E | 140 | Dissected | 6 | Na | Na |
| 4 | 45°19′22.60″N–11°45′19.70″E | 44 | Dissected | 50 | Na | Na |
| 17 | 45°19′17.1″N–11°41′33.3″E | 459 | Dissected | 50 | Na | Na |
| 4 | 45°19′22.60″N–11°45′19.70″E | 54 | Dissected | 30 | Na | Na |
| 4 | 45°19′22.60″N–11°45′19.70″E | 54 | Dissected | 30 | Na | Na |
| 4 | 45°19′22.60″N–11°45′19.70″E | 54 | Dissected | 10 | Na | Na |
| 4 | 45°19′22.60″N–11°45′19.70″E | 54 | Dissected | 13 | Na | Na |
Note:
Holes: number of exit holes; Total: total number of hatched individuals; F: number of females; M: number of males.
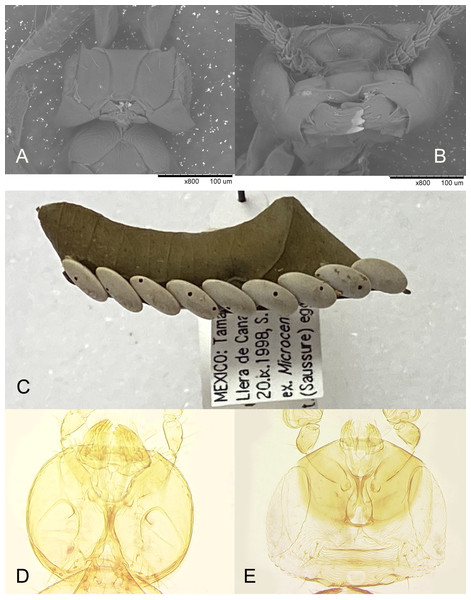
A stereomicroscope with an eyepiece micrometer was used to measure and compare the maximum diameter of the parasitoid emergence holes observed in these fertile eggs and in the previously parasitized eggs (Fig. 4A). No differences were found between the diameter means of the two groups (t-test, n = 19, n = 32, p-value = 0.66). The average diameter of holes was 0.33 mm ± 0.15 SE.
Figure 4: Emergence holes and females of P. triclavatum.
(A) Eggs of Barbitistes vicetinus with numerous emergence holes of Platystethynium triclavatum; (B) newly emerged females of Platystethynium triclavatum preserved in ethanol.The emerged parasitoids were preserved in 96% ethanol awaiting to be identified. All the samples of parasitoid wasps emerged were initially identified by the second author as Pseudocleruchus triclavatus Donev & Huber, 2002 (Hymenoptera, Mymaridae).
Identification and taxonomy of the egg parasitoid
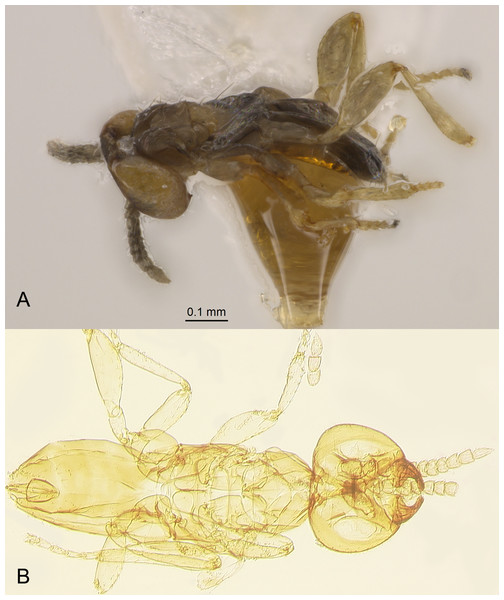
Identification. Females of the egg parasitoid (Figs. 4B and 5) were compared morphologically with the original description and illustrations of Pseudocleruchus triclavatus from Bulgaria (Donev & Huber, 2002) and with the illustrations of the non-type specimens from Czech Republic (Samková, Janšta & Huber, 2020), and also with taxonomic notes on the females of the two undescribed Pseudocleruchus spp., one from Romania (Pricop, 2011) and the other (examined by the second author) from Finland (Samková, Janšta & Huber, 2020). The reared females from Italy were found to be almost identical to those of Pseudocleruchus triclavatus from Bulgaria, besides the slight differences in having the fore wing slightly wider (the length:width ratio is slightly lower) and having a few more rows of discal microtrichia; that might be due to the fact that the Italian specimens are from a much lower elevation whereas the Bulgarian ones came from higher elevations and thus might have a slight degree of brachyptery. The length:width ratio of the scape of the female antenna is either the same or slightly higher in the Bulgarian specimens (as described), but that could be probably due to the way specimens of the type series of P. triclavatus were slide-mounted by Atanas Donev (in a somewhat different orientation). Even if these minor differences were correctly assessed, they are, undoubtedly, due to intraspecific variation, likely both geographical and host-induced. Indeed, because B. vicetinus is endemic only to the two hill areas of Veneto Region in Italy (Eugenean and Berici Hills), Platystethynium (Platystethynium) triclavatum (Donev & Huber, 2002), comb. n. from Pseudocleruchus Donev & Huber, 2002, has to have different tettigoniid hosts (likely bush-crickets) in Bulgaria and Czech Republic, which are yet to be identified. Like many other fairyflies, it is unlikely that P. triclavatum is very narrowly host specific; rather, it might be able to parasitize eggs of several, likely more or less related, genera of Tettigoniidae that lay eggs in soil. A molecular comparison and analysis of specimens morphologically identified as P. triclavatum could clarify the topic, but unfortunately no other properly preserved specimens of this species from other countries in Europe are currently available for DNA extraction.
Figure 5: Dry-mounted female of Platystethynium triclavatum.
(A) Habitus in dorsal view; (B) habitus in lateral view.Taxonomy
Genus Platystethynium Ogloblin, 1946
Platystethynium Ogloblin, 1946: 290. Type species: Platystethynium onomarchicidum Ogloblin, 1946, by original designation.
Platypatasson Ogloblin, 1946: 293. Type species: Platypatasson fransseni Ogloblin, 1946, by original designation. Synonymy by Donev & Huber, 2002: 118; given subgeneric status as Platystethynium (Platypatasson) by Huber, Read & Triapitsyn, 2020: 289–292.
Pseudocleruchus Donev & Huber, 2002: 118–120. Type species: Pseudocleruchus triclavatus Donev & Huber, 2002, by original designation. Syn. n.
Pseudocleruchus: Pricop, 2011: 25–26 (comparison with the nominal subgenus of Cleruchus Enock, 1909, updated diagnosis); Samková, Janšta & Huber, 2020: 205 (key to females), 207 (key to males).
Platystethynium: Sankararaman, Manickavasagam & Palanivel, 2019: 10 (key to world species).
Platystethynium (Platypatasson): Huber, Read & Triapitsyn, 2020: 289–292 (diagnosis, discussion, illustrations).
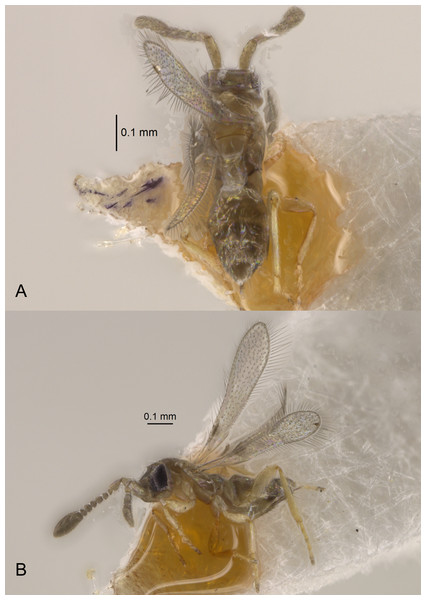
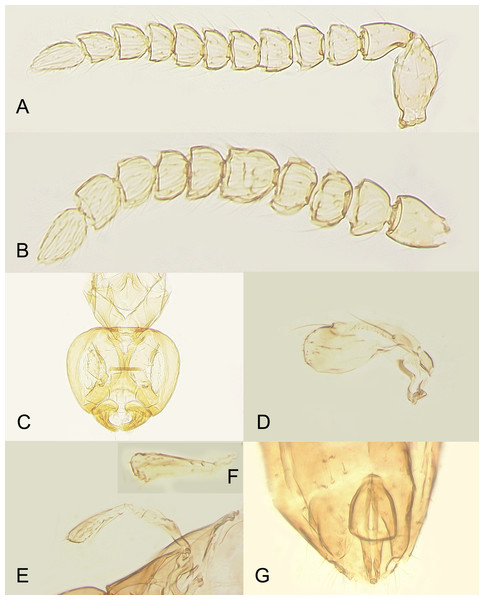
Brief diagnosis of the subgenus Platystethynium (Platystethynium Ogloblin). Female: head in lateral view rectangular, at least a little longer than high, with face usually strongly projecting anteriorly; mandibles strongly reduced, not crossing over; funicle 6-segmented, with at least some segments transverse; clava 3-segmented; only macropterous individuals are known; frenum longitudinally divided medially; gaster sessile; tarsi 4-segmented. Male (so far known only for one species, P. (Platystethynium) triclavatum): megacephalous (Figs. 6C and 7), with enlarged, 3-toothed mandibles crossing over (Figs. 8C and 9B); antenna much shorter than body, with flagellum normally 10-segmented (Figs. 7B, 8A and 8B); strongly brachypterous.
Figure 6: Platystethynium triclavatum.
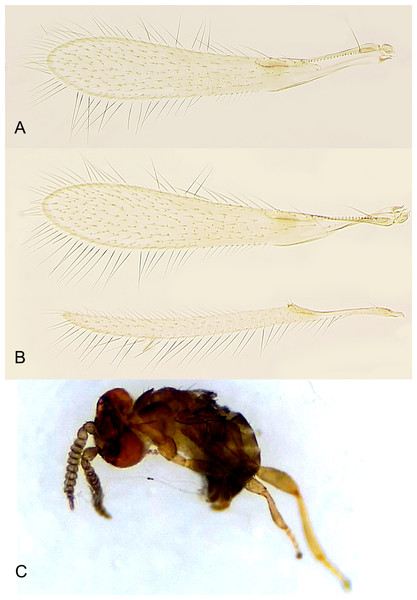
(A) Female fore wing; (B) female fore and hind wings; (C) habitus of male in lateral view (preserved in ethanol upon emergence).Figure 7: Male of Platystethynium triclavatum.
(A) Habitus in dorsolateral view (dry-mounted specimen); (B) body (slide-mounted specimen).Figure 8: Male of Platystethynium triclavatum.
(A) Antenna (flagellum 10-segmented); (B) antenna (flagellum nine-segmented); (C) head; (D) fore wing; (E and F) hind wings; (G) genitalia.Figure 9: P. triclavatum compared to B. mexicana.
(A and B) Mandibles of Platystethynium triclavatum (scanning electron micrographs): (A) female; (B) male; (C) eggs of Microcentrum rhombifolium with exit holes of Burksiella mexicana; (D and E) mandibles of Burksiella mexicana: (D) male; (E) female.Hosts. Orthoptera, Tettigoniidae (Ogloblin, 1946).
Comments. Platystethynium belongs to the Cleruchus group of genera (Huber, Read & Triapitsyn, 2020). Its nominate subgenus occurs only in the Old World, while P. (Platypatasson Ogloblin, 1946) occurs in both New and Old Worlds. In Europe, females of the genus and its nominate subgenus (as Pseudocleruchus) can be identified using a key in Samková, Janšta & Huber (2020), who incorrectly guessed (p. 207, in the key to males of the European genera of Mymaridae), that males of Pseudocleruchus, then unknown, could be macropterous like conspecific females.
Recently, three species were transferred from Cleruchus to Platystethynium (Donev & Huber, 2002; Triapitsyn, 2014), so the genus currently contains six described, valid species worldwide including P. triclavatum (Sankararaman, Manickavasagam & Palanivel, 2019; Huber, Read & Triapitsyn, 2020). Among them, three species belong to the subgenus P. (Platypatasson): P. (Platypatasson) fransseni (Ogloblin, 1946) from Indonesia, P. (Platypatasson) terebrator (Ogloblin, 1959) and P. (Platypatasson) vagatus (Ogloblin, 1959) from Argentina. Supporting molecular evidence, which is now lacking, would be needed to confirm the subgeneric concepts within Platystethynium, as suggested by Huber, Read & Triapitsyn (2020).
Pseudocleruchus is synonymized herein under Platystethynium and its nominate subgenus P. (Platystethynium) because all of their important generic-level morphological features, including the reduced female mandibles, are very similar, and the known host of P. (Platystethynium) onomarchicidum Ogloblin, 1946 is also a bush-cricket, Onomarchus uninotatus (Serville, 1838) (Tettigoniidae) (Ogloblin, 1946). Females of the European species of the former genus Pseudocleruchus (1 described and 2 undescribed species, which for the time being, until genetic data becomes available, can be grouped in the informal triclavatum species group), have shorter heads, less flattened and shorter bodies, and wider fore wings than the two described Oriental species of the nominate subgenus of Platystethynium, P. (Platystethynium) onomarchicidum, known from Indonesia (Ogloblin, 1946), and P. (Platystethynium) glabrum Jin & Li, 2016, known from China (Jin & Li, 2016) including Taiwan (Triapitsyn, 2018), and also from India (Sankararaman, Manickavasagam & Palanivel, 2019). The latter two nominal species can be grouped in the informal onomarchicidum species group and are possibly synonymous (Triapitsyn, 2018), although both Jin & Li (2016) and Sankararaman, Manickavasagam & Palanivel (2019) indicated some of their apparent differences, but their validity requires further study and confirmation. A key to females of the described species of Platystethynium (Platystethynium) follows.
Key to species groups and described species of the subgenus Platystethynium (Platystethynium Ogloblin, 1946), females
1 Head in dorsal view at least as long as wide; body strongly flattened (onomarchicidum species group)2
—Head in dorsal view wider than long; body at most a little flattened (triclavatum species group)P. (Platystethynium) triclavatum (Donev & Huber, 2002), comb. n.
2 (1) Ovipositor about 3.0 times length of metatibiaP. (Platystethynium) onomarchicidum Ogloblin, 1946
—Ovipositor about 1.8 times length of metatibiaP. (Platystethynium) glabrum Jin & Li, 2016
Platystethynium (Platystethynium) triclavatum (Donev & Huber, 2002), comb. n.
(Figs. 4B, 5, 6, 7, 8, 9A, 9B and 10)
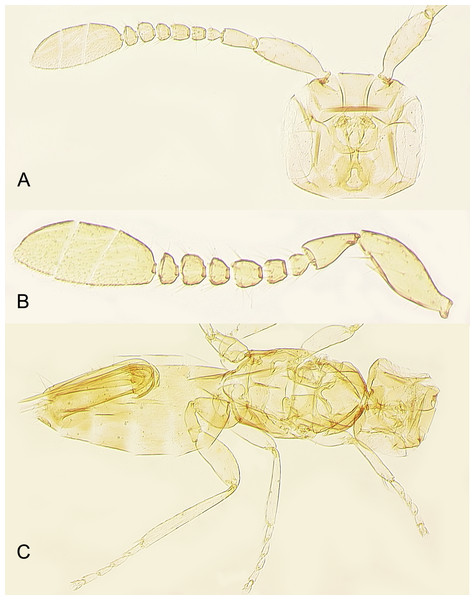
Figure 10: Female of Platystethynium triclavatum.
(A) Head and antenna; (B) antenna; (C) body.Pseudocleruchus triclavatus Donev & Huber, 2002: 121–122. Type locality: 1,470 m, mountain hostel Martsiganitsa (Martsiganitsa Chalet), Rhodope (Rodopi) Mountains, Plovdiv Province, Bulgaria. Holotype female, in private collection, currently with Mrs. Doneva, Dr. Atanas Donev’s widow, Asenovgrad, Bulgaria (not examined); it will be donated to the Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, Sofia, Bulgaria (Peter S. Boyadzhiev, personal communication to S. V. Triapitsyn).
Pseudocleruchus triclavatus: Procop, 2011: 26–27 (compared with Pseudocleruchus sp. from Romania); Samková, Janšta & Huber, 2020: 226 (distribution in Czech Republic).
Material examined. Italy, Veneto, Euganean Hills: 45°19′19.68″N 11°43′20.67″E, 140 m, parasitized egg of Barbitistes vicetinus collected in soil 20.x.2018, G. Ortis: parasitoids emerged 7.vi.2019 (5 females, CNC, UCRC [UCRC ENT 541215–541219]); host egg dissected 1.vii.2019 (5 males, UCRC [UCRC ENT 541220–541224]). 45°19′3.99″N 11°41′20.10″E, 448 m, parasitized egg of B. vicetinus collected in soil 20.x.2018, G. Ortis, parasitoids emerged 3–7.vi.2019 (25 females, CNC, UCRC [UCRC ENT 542125–541249]). Also numerous additional, unmounted, specimens of both sexes (DAFNAE).
Redescription. FEMALE. Body length of critical point dried, point-mounted specimens 0.53–0.66 mm; of slide-mounted specimens 0.8–0.88 mm. Body and appendages (Figs. 4B and 5) mostly light brown to brown except pronotum and apex of gaster darker. Ocelli present, on a well-defined stemmaticum; subantennal grooves prominent (Fig. 10A). Mandibles strongly reduced, not crossing over (Fig. 9A). Antenna (Figs. 10A and 10B) with short radicle fuzed to the rest of scape, scape smooth, 2.8–3.2 times as long as wide; pedicel longer than any funicular segment; F1 slightly shorter than F2, F1–F3 each about as long as wide (but sometimes F3 clearly longer than wide), F3 the longest funicular segment, F4–F6 each wider than long, mps only on F3 (2) and F5 (1); clava either about as long as or a little shorter than funicle, 2.3–2.5 times as long as wide, with 7 mps (2 on first, 2 on second, and 3 on third claval segments). Body (Fig. 10C) slightly compressed dorsoventrally. Mesosoma shorter than metasoma, smooth; pronotum longitudinally divided; midlobe of mesoscutum with a pair of adnotaular setae close to posterior margin; scutellum wider than long; frenum subquadrate. Macropterous; fore wing (Figs. 6A and 6B) 5.5–5.8 times as long as wide (0.6–0.65 mm long), disc notably infuscate throughout, with about 10 rows of microtrichia in the broadest part; longest marginal seta 0.8–0.9 times greatest width of wing. Hind wing (Fig. 6B) 17–18 times as long as wide; longest marginal seta 2.5–3.0 times greatest width of wing; disc slightly infuscate and with about 3 rows of microtrichia. Legs (Fig. 10C) with femora not as prominently enlarged as in the male (Figs. 7A and 7B). Ovipositor (Fig. 10C) about 0.3 mm long, occupying about 0.6 length of gaster, 1.9–2.0 times length of metatibia, slightly exserted beyond gastral apex (by at most 0.06 times own length).
Description. MALE. Body length of air-dried, point-mounted, shriveled specimen (Fig. 7A) 0.66 mm; of slide-mounted specimens 0.99–1.08 mm. Body mostly brown to dark brown except lower face, eyes, mesoscutum, axillae and frenum light brown; antenna brown, legs light brown. Larger than female, megacephalous (Figs. 6C and 7), head 1.5 times wider than mesosoma, with mandibles 3-toothed, strongly enlarged and crossing over (Figs. 7B, 8C and 9B). Antenna (Fig. 7B) much shorter than body (length of antenna of one slide-mounted specimen 0.47 mm), with scape 2.1–2.2 times as long as wide; flagellum normally 10-segmented (Fig. 8A) but occasionally with F4 and F5 fuzed, so then antenna 9-segmented (Fig. 8B); flagellar segments without a distinct clava, either subquadrate or transverse except F9 slightly longer than wide and F10 notably so (the longest flagellomere); all flagellar segments with several mps. Strongly brachypterous, only short stubs with very little membrane remain of the wings, these do not extend beyond posterior margin of propodeum; fore wing (Fig. 8D) in one specimen 2.9 times as long as wide; hind wing length varies (Figs. 8F and 8E) from about as long as that of fore wing or notably shorter, or, occasionally, slightly longer; genitalia (Fig. 8G) length 0.13–0.15 mm, with phallobase rather wide, aedeagal apodemes almost extending to its base, and with volsellar digiti straight.
Distribution. Bulgaria (Donev & Huber, 2002), Czech Republic (Samková, Janšta & Huber, 2020), and Italy (new record).
Host. Orthoptera, Tettigoniidae: Barbitistes vicetinus Galvagni & Fontana, 1993 (new record).
Discussion
In this study we reported for the first time the discovery of a parasitoid wasp Platystethynium triclavatum belonging to the family Mymaridae, from eggs of B. vicetinus. The species, initially described as Pseudocleruchus triclavatus Donev & Huber, 2002, was previously known only from Bulgaria (Donev & Huber, 2002) and Czech Republic (Samková, Janšta & Huber, 2020), while the genus Pseudocleruchus was also known from Romania (Pricop, 2011) as well as Finland and Sweden (Samková, Janšta & Huber, 2020). The high number of individuals hatched from field-collected host eggs gave us the opportunity to evaluate the genus placement of the parasitoid and propose the synonymy of Pseudocleruchus with Platystethynium, to redescribe the female of P. triclavatum, and also to describe its previously unknown male. In fact, these are the first known males for the entire genus. That is, not that surprising given their peculiar biology, as described herein. Only fully winged females of this species can be collected by the traditional methods such as sweeping with a net or by various traps. Even those are very rarely collected in Europe, and prior to this study have never been collected in Italy. The strongly brachypterous, megacephalous males with enlarged mandibles can only be found inside the host eggs in the ground, while females are fully winged, with normal sized heads, and with strongly reduced mandibles. Thus, females depend on the males to be able to hatch from host eggs; as common for the minute egg parasitoids, males are likely to hatch first and wait for their sisters to emerge; mating occurs inside the egg.
However, we still do not know when and how oviposition of the egg parasitoid takes place; one of the reasonable assumptions is that it occurs during oviposition of a female bush-cricket host, while the egg chorion is still relatively soft. An interesting oviposition behavior was described in the gregarious Ufens spp. (Hymenoptera, Trichogrammatidae), egg parasitoids of Homalodisca spp. sharpshooters (Hemiptera, Cicadellidae, Cicadellinae, Proconiini) in North America (Al-Wahaibi, Owen & Morse, 2005). We suspect that a generally similar, albeit likely a somewhat different ovipositional strategy (given the fact that host eggs are laid in soil) might be in P. triclavatum, when females of the egg parasitoid, aggregating at the ovipositional sites of their hosts (probably at ground surface in this case) would respond rapidly and aggressively to the chemical or other, unknown, cues by the ovipositing female bush-crickets, landing on their ovipositors and then walking (squeezing) down to parasitize host eggs. That would also explain why parasitized eggs increase with the higher density of the host bush-crickets: their response time to the narrow opportunity for oviposition would be much shorter.
We assume that P. triclavatum is a gregarious, arrhenotokous species because gregarious behavior during oviposition could explain high number of the embryos found inside the host egg (Chiappini & Huber, 2008); however, egg production in most mymarid species probably does not exceed 100 (Sahad, 1984; Cronin & Strong, 1990; Huber et al., 2006). If this parasitoid is indeed a gregarious species, it is likely that one mated female (who, as many other fairyflies, is likely to be born with a full content of mature eggs and thus ready for ovipositing immediately, without additional feeding) would be able to parasitize just one host egg, using her entire supply of eggs. However, we do not exclude a possibility that more than one female could oviposit in a single host egg. Unmated females of the egg parasitoid would produce only males in the progeny. Otherwise, albeit unlikely because this species seems to be arrhenotokous, polyembryony could be suspected, but to our knowledge it has never been reported to occur in this family.
Apparently a partially similar trait, regarding sexual morphological dimorphism necessary for chewing exit holes through a very hard egg chorion of the katydid host, is evident in the recently described egg parasitoid of an unidentified species of Tettigoniidae, Burksiella mexicana Ávila-Rodríguez & Myartseva, 2011 (Hymenoptera, Trichogrammatidae) from Tamaulipas, Mexico (Ávila-Rodríguez, Myartseva & González-Hernández, 2011). In its original description it is only mentioned that males of this gregarious species are larger, lighter colored than females, and megacephalous, but without noticing the striking peculiarity of the male mandibles. In fact, while the mandibles of the females are of normal size for the genus (Fig. 9E), those of the conspecific males are enlarged (Fig. 9D); these are used to chew out 1 or 2 round holes in each host egg (Fig. 9C) through which numerous smaller females emerge. Unlike in Platystethynium triclavatum, however, in Burksiella mexicana female mandibles can cross over, and males are fully winged. A similar sexual dimorphism occurs in Burksiella benefica (Dozier, 1932), originally described from Haiti; both (along with several undescribed species from the New World in UCRC) belong to the benefica species group of the genus Burksiella De Santis, 1957, as defined by Pinto (2006), members of which parasitize eggs of those Tettigoniidae that lay egg masses openly on leaves and possibly other substrates above the ground. The following non-type specimens of Burksiella mexicana were identified and examined by the second author: Mexico, Tamaulipas: Ciudad Victoria, 1999 or 2000, S. N. Myartseva, from eggs of an unidentified katydid (Tettigoniidae) on citrus leaf (three females and two males, UCRC (UCRC_ENT 00536185, 00536187–00536190)); Llera de Canales, Rancho La Purísima, 20.ix.1998, S. N. Myartseva, from eggs of the broad-winged katydid, Microcentrum rhombifolium (Saussure, 1859), on leaf of a lemon (222 females and 22 males, UCRC (UCRC_ENT 00541280–00541370)). Thus, in the latter sample from Llera de Canales, males constituted about 9% of the total number of B. mexicana individuals which emerged from 8 host eggs (those with parasitoid emergence holes); about 30 wasps of both sexes emerged per host egg on average.
Data on this parasitoid were obtained through a study to assess the egg density of B. vicetinus after the oviposition period. Eggs of B. vicetinus were collected 10 years after the first outbreak reported in the Euganean Hills; during this period of time, this forest and agricultural pest has spread throughout the area of study. In this work we found that the diameter of the emergence holes of the eggs collected in the forest soil is comparable with diameter of the holes from which P. triclavatum emerged in the laboratory. Therefore, we assumed that all the eggs with these emergence holes across the surface of the chorion collected in the forest soil belong to this mymarid wasp. Our reconstruction of the distribution area of P. triclavatum is based on eggs with holes, showing that this species is spread in almost all the sites sampled and along all the elevation gradients. Since the index of discovery efficiency reached almost 50% among all the sub-replicates, fitness of this parasitoid could be related to the ability to find eggs in the soil. It is important to note that higher rates of discovery efficiency were found in sites with greater total amount of eggs.
During outbreaks of B. vicetinus, the presence of other Orthoptera is in overwhelming minority, particularly in the canopy. As we reported, the numbers of eggs belonging to other species were much lower compared to those for the eggs of B. vicetinus. From this point of view, the different abundance of this parasitoid among sites could be the result of the local level of infestation of the pest during the years. A higher number of the previously parasitized eggs can be explained by the oviposition density of the bush-cricket. Our results indicate that this wasp could take a reproductive advantage when large numbers of host eggs of B. vicetinus are present in the area. Probably, a major volume of eggs could be fundamental for the searching behavior, identification and later acceptance of the host (Moreau et al., 2009). Because outbreaks occurred only in the last decade, the low parasitism rate reported in this study probably is the effect of a time lag in the parasitoid-host system. A portion of eggs collected in this study could date back to the first period of the outbreaks, when the abundance of this parasitoid was lower.
Although P. triclavatum hatched from numerous eggs of B. vicetinus, we cannot rule out that this wasp could parasitize other hosts. The parasitoid has been already recorded, even though without any host data, also in other European countries, where the geographical distribution area of other species of the genus Barbitistes Charpentier, 1825 overlaps (e.g., B. serricauda Fabricius, 1798, B. ocskayi Charpentier, 1850, B. constrictus Brunner von Wattenwyl, 1878) (Massa et al., 2012), suggesting a possible host-specificity with bush-crickets of this genus, at least. It will be important, in perspective, to explore host specificity of this parasitoid both on B. vicetinus eggs and those of other species and genera of bush-crickets.
The activity of this mymarid could be increased by the repeated outbreaks of B. vicetinus, whose eggs represent suitable hosts. The high number of individuals that can develop from a single host egg demonstrates that this host provides enough nutritional material for the development of more than one hundred larvae of the parasitoid. Despite the low parasitism rate found in this study, the total number of P. triclavatum individuals that can hatch from a single egg can better reflect the potential impact of this discovered parasitoid in the Euganean Hills.
Our results provide new insights into the biology of P. triclavatum that could be useful regarding the potential role in biological control against the pest B. vicetinus. Undoubtedly, our findings will be also useful for discovering the currently unknown males and determining hosts of some other described members of Platystethynium, particularly of the two known but still undescribed species from Europe.
Supplemental Information
Mean and standard error for each category of eggs found in each site.
The mean and standard error for each site is calculated on the 6 sub-replicates. The mean indicated in the last row is calculated on the sum of eggs of the 6 sub-replicates for each site.
Holes measurements.
Mean and standard error of the two groups (laboratory and field)