Modeling the influence of temperature and water potential on seed germination of Allium tenuissimum L.
- Published
- Accepted
- Received
- Academic Editor
- Kun Lu
- Subject Areas
- Agricultural Science, Plant Science
- Keywords
- Seed germination, Allium tenuissimum, Drought tolerant, Crop production, Hydrothermal time model
- Copyright
- © 2020 Xiao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Modeling the influence of temperature and water potential on seed germination of Allium tenuissimum L. PeerJ 8:e8866 https://doi.org/10.7717/peerj.8866
Abstract
Allium tenuissimum L. is a widely distributed perennial herbaceous species in temperate and desert steppes. Relative to other wild Allium species, it produces unique sweet flavors, more biomass in arid and cold environments, and has generated greater interest for crop production. Successful crop establishment, however, will depend on rapid and uniform seed germination. Our study aimed to characterize seed germination of A. tenuissimum under various temperature regimes (11, 15, 20, 24 and 28 °C) and water potential levels (0, −0.2, −0.4 and −0.6 MPa), and model germination by hydrotime (HT) and hydrothermal time (HTT) analysis. Final germination percentage (FGP) increased within the range of 11 to 20 °C, yet it declined within the range of 24 to 28 °C and generally decreased as water potential became more negative within each temperature setting. Maximum FGP was observed at 20 °C at all water potential settings and ranged from 55.0 ± 5.3 to 94.8 ± 1.4%. According to HT and HTT models, the base (Tb) and optimum temperatures (To) for seed germination were 7.0 and 20.5 °C, respectively. In addition, base water potential for the fraction of germination within the seed lot (Ψb(g)) shifted to 0 MPa as temperature increased from Tb to ceiling temperature (Tc). For obtaining 50 % seed germination, Ψb(50) and Tc(50) were estimated to be −0.67 MPa and 27.2 °C, respectively. These values for Tb and Ψb(50) suggest seed germination of A. tenuissimum is both cold and drought tolerant and suitable for production in semi-arid regions. Our characterization of the ideal sowing conditions for A. tenuissimum, i.e., 20.5 °C and soil water potential less negative than −0.67 MPa offers information to forecast suitable settings to enhance crop production.
Introduction
Seed germination is an essential process in establishing stable plant populations and is regulated by many environmental factors (Atashi et al., 2015; Juan-Vicedo et al., 2016). Among these factors, temperature and moisture conditions strongly regulate germination dynamics (Bakhshandeh & Gholamhossieni, 2019), and characterizing the base, optimum, and ceiling temperatures is one way of describing how temperature influences seed germination dynamics (Bakhshandeh et al., 2013; Bewley et al., 2013). Furthermore, thermal time (TT) models can be applied to predict seed germination dynamics across a range of temperatures (Mccartan, Jinks & Barsoum, 2015; Bidgolya et al., 2018; Trudgill et al., 2005) and have proven useful in estimating germination under the dynamic temperatures of field seedbeds (Rawlins et al., 2012; Izquierdo et al., 2013). However, TT models may inaccurately predict seed germination responses under supra-optimal temperature ranges (Bakhshandeh et al., 2015; Bradford, 2002).
Germination is also very sensitive to moisture conditions and water deficiency-induced osmotic stress is known to prevent seed germination or slow germination rates (Tobe et al., 2001). Accordingly, water status is typically reported as osmotic water potential, and hydrotime (HT) models are typically applied to simultaneously account for changes in both final germination percentage and germination rate across variable water potential levels (Soltani et al., 2017; Bakhshandeh & Gholamhossieni, 2018). Furthermore, the combination of HT and TT models have produced hydrothermal time (HTT) models (Alvarado & Bradford, 2002; Gummerson, 1986) capable of determining the hydrothermal accumulation for seed germination at various temperature and water potential conditions and predict the time course of seed germination even across sub-optimal (Gummerson, 1986) and supra-optimal temperature ranges (Alvarado & Bradford, 2002). Consequently, parameters of HTT models can be used to characterize the physiological status of seed populations in response to variable temperature and water potential and have been widely applied to predict germination dynamics in numerous crops such as safflower (Carthamus tinctorius) (Bidgolya et al., 2018; Torabi et al., 2016), sesame (Sesamum indicum) (Bakhshandeh et al., 2017), zucchini (Cucurbita pepo) (Atashi et al., 2015), and watermelon (Citrullus vulgaris cv. ‘Crimson sweet’) (Bakhshandeh et al., 2015) and other wild species (Abdellaoui et al., 2019; Fakhfakh, Anjum & Chaieb, 2018; Horn, Nettles & Clair, 2015).
Allium tenuissimum L. is a perennial herbaceous species distributed in temperate and desert steppes of north-central Asia (He, 2008; Li & Zhang, 2011). Within these ecosystems, it plays a critical role in sand fixation and conservation of water and soil due to having a well-developed fibrous root system and high tolerance of environmental stresses (Zhao, 2010). It is also recognized as a high-quality forage for herbivores (Song, Niu & Wan, 2016) and has economic importance due to its distinctive and tasty flavor (Li & Zhang, 2011; Zhang & Liu, 2012). Consequently, it is highly valued as a vegetable or food seasoning (Li & Zhang, 2011; Liu et al., 2016) and has generated interest in exploring new harvesting methods (Zhang, Li & Zheng, 2014), potential ways to extract essential oil from its flowers (Zhang & Liu, 2012), and identifying the unique volatile flavor compounds in flowers of A. tenuissimum (Xu et al., 2017). Despite these favorable qualities, establishing crops of A. tenuissimum on large-scales is not feasible by propagation of bulb tillers, but may become economically viable through propagation by seeds (Li et al., 2013; Zhao, Li & Badema, 2011). Therefore, exploring seed germination dynamics under variable environmental conditions is an important step toward industrialized production. In this study, we aimed to focus on the response of seed germination to various temperatures and water potential conditions in A. tenuissimum: (i) characterizing seed germination dynamics of A. tenuissimum using HT and HTT models, and (ii) defining cardinal temperatures and base water potentials for A. tenuissimum seeds based on model parameters.
Materials & Methods
Seed germination
Seeds of A. teniussimum were collected from a temperate desert steppe area in Dongsu County (43°51′36″N, 113°40′02″E, 1,060 m a.s.l. (meters above sea level)), Inner Mongolia during October 2015 (approved by Yuping Rong, China Agricultural University). After that, the seeds were naturally dried, surface cleaned and put into a sealed glass container, and then stored at 4 °C in a refrigerator until needed for experimentation (April 2016).
Germination assays were conducted in a growth chamber (SPX-250-GB, Hengyu, China) located at the Grassland Science Department, China Agricultural University, Beijing, China with an 8 h light/16 h dark day/night lighting pattern. The light intensity and relative humidity were set as 6000 lx and 50%. Mature Seeds were sterilized for 5 min with 10% NaClO and then washed with distilled water (Rong, Li & Johnson, 2015). For the germination tests, 100 seeds were placed in glass Petri dishes (90 mm inner diameter) containing two layers of filter paper (1001-090, Whatman, UK) saturated with distilled water (Ψ = 0 MPa) or solutions of different water potential levels (Ψ = −0.2, −0.4 and −0.6 MPa). Petri dishes were then transferred to the chamber and germination was characterized at four constant temperature settings (11, 15, 20, 24 and 28 °C), each replicated four times for each water potential level. Different water potential levels were produced by mixing aqueous solutions of polyethylene glycol (PEG) 6000 with distilled water according to Michel & Kaufmann (1973). A vapour pressure osmometer (Model 5100C, Wescor, Inc., Logan, UT, USA) was used to measure Ψ of solutions and create desired levels for all temperature settings. To maintain constant Ψ and avoid fungal attack, seeds incubated on PEG 6000 solutions were transferred to fresh solutions every 2 d. Germination was scored daily by observing radicle protrusion. Seed germination was defined depend on the length of radicle. Normally seeds were regarded as germinated when the length of radicle was more than 2 mm (Saleem et al., 2019). To avoid errors in recording germination, germinated seeds were removed after being counted. Furthermore, germination tests were terminated when no new germinated seeds were counted for three consecutive days.
Germination analysis
Germination rates (GRg) were calculated using the equation: GRg = 1/tg, where tg is the duration to radicle emergence. Estimations of germination rate for the 50th percentile (GR50) in each replicate were calculated by interpolation using curves fit to the time course data. To determine the optimal germination temperature, germination rates at 20 and 24 °C were compared. If final germination percentage at 20 °C was significantly greater than germination at 24 °C, the optimal temperature was assumed to be 20 °C.
HT model
The relationship between GRg and Ψ was described by using the following equation: (1) where θH is the hydrotime (MPa d) constant of the seeds required for germination, Ψb(g) is the theoretical threshold or base Ψ that will prevent the germination of fraction g. The parameters in the HT model were estimated according to the equation: (2) where Ψb(50) is the base water potential for attain the 50th percentile of germination, and σΨb is the standard deviation of Ψb(g) in seed lots. To examine whether parameters of this model can be accurately used to quantify the sensitivity of seed populations to the variation of Ψ, the [1-(Ψ/Ψ(g))] tg factor derived from Bradford (1990) was applied to normalize germination time courses at reduced Ψ in this study. Germination time course can be normalized by this factor at any Ψ to the corresponding time course that would occur in water for the seed population using the parameters from the HT model. All normalized data from different temperature conditions were plotted on a common thermal time scale, using the estimated Tb at 0 MPa.
HTT model
The HTT model describes seed germination patterns when T and Ψ both vary; Alvarado & Bradford, 2002. The relationship between GRg and variable conditions of T and Ψ are described by Eq. (3) for sub-optimal T, and modified Eq. (4) for supra-optimal T:
(3) (4) where θHT is the hydrothermal constant and Tb is the base temperature. In Eq. (4), [KT(T −To)] applies only in the supra-optimal range of T and KT is the slope of the relationship between Ψb(50) and temperatures when T > To; To is the optimum temperature. The value of Ψb(g) is set equal to the distribution of Ψb(g) at To, and (T −Tb) is equal to (To − Tb). Parameter values in above models can be obtained by probit analysis according to Eqs. (5) and (6) for sub- and supra-optimal T, respectively.
(5) (6)
As described in Alvarado & Bradford (2002), the values of KT and To were varied for germination time courses at T >To until a fit was obtained that resulted in values of θHT, Ψb(50) and σΨb matching those obtained at or below To.
Statistical analyses
A two-way analysis of variance (ANOVA) was carried out using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA) to evaluate the influence of T, Ψ, and their interactions on seed germination variables of A. teniussimum. The results showed with mean and SE value of four replicates. All probit analyses of HT and HTT models were fitted in SAS 8.2 statistical package (SAS Institute, Cary, NC, USA) using the PROC PROBIT routine, which employs a maximum-likelihood weighted regression method (Bradford, 1990; Dahal & Bradford, 1994).
Results
Seed germination in response to temperature and water potential
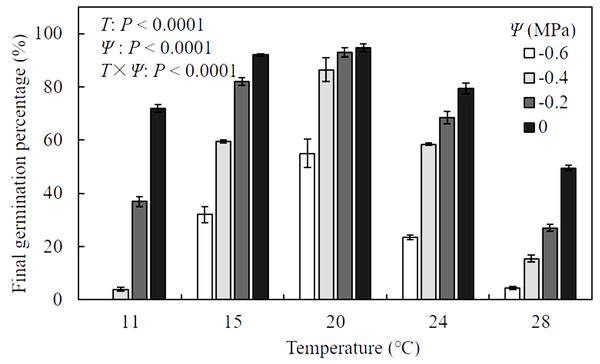
Results of analysis of variance indicated that the final germination percentage (FGP) of A. tenuissimum seeds was significantly influenced by T (F = 587.9, P <0.0001), Ψ (F = 643.0, P < 0.0001), and their interactions (F = 24.2, P < 0.0001) (Fig. 1, Table S1). When values of Ψ remained constant, FGP increased as T increased within sub-optimal ranges (11 to 20 °C), while it declined within supra-optimal ranges (24 to 28 °C). In distilled water (i.e., Ψ = 0 MPa), FGP changed from 72.0 ± 1.4 to 94.8 ± 1.4% over various T conditions. Maximum FGP was observed at 20 °C under all levels of Ψ and ranged from 55.0 ± 5.3 to 94.8 ± 1.4%. For all T settings, FGP decreased with decreasing Ψ levels. Few seeds germinated under the combination of −0.6 MPa and 11 °C. In contrast, FGP reached 94.8 ± 1.4% when seeds were incubated in water at 20 °C, indicating that the seeds in this analysis were non-dormant.
Figure 1: Mean (± standard error) final germination percentage of A. tenuissimum seeds under variable temperature (T = 11, 15, 20, 24, and 28 °C) and water potential (Ψ = 0, −0.2, −0.4, and −0.6 MPa) levels.
Hydrotime analysis
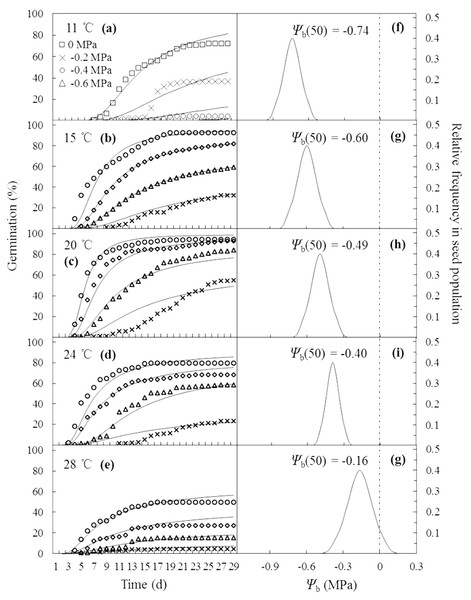
Parameters generated by the HT model are presented in Table 1. Values of θH decreased from 6.4 MPa d at 11 °C to 4.4, 4.2, 3.0 and 3.0 MPa d at 15, 20, 24 and 28 °C, respectively. This suggests that the time required for germination declined as T increased within sub-optimal ranges, while it remained constant at supra-optimal ranges. Values of Ψb(50) increased as T increased from 11 to 28 °C. Notably, estimated values of Ψb(50) increased more positively at supra-optimal temperatures, rising from −0.40 MPa at 24 °C to −0.16 MPa at 28 °C. Values of σΨb varied from 0.20 to 0.37 MPa across all regimes of T. Specifically, values of σΨb were nearly constant within the range of 15 to 24 °C, indicating that the variation in Ψb among all seeds in this A. tenuissimum population was small.
| Temperature (°C) | θH (MPa d) | Ψb(50) (MPa) | σΨb (MPa) | R2 |
|---|---|---|---|---|
| 11 | 6.4 | −0.74 | 0.20 | 0.95 |
| 15 | 4.4 | −0.60 | 0.29 | 0.97 |
| 20 | 4.2 | −0.49 | 0.27 | 0.92 |
| 24 | 3.0 | −0.40 | 0.28 | 0.90 |
| 28 | 3.0 | −0.16 | 0.37 | 0.94 |
Notes:
- θH
-
hydrotime constant
- Ψb(50)
-
base water potential for 50% seed germination
- σΨb
-
standard deviation for Ψb(g)
- R2
-
coefficient of determination
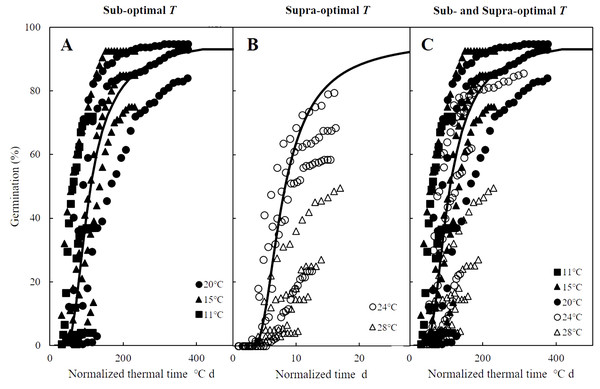
The curves in Figs. 2A–2E are germination time courses predicted by HT model based on the Ψb(g) threshold distributions (Figs. 2F–2G) and the estimated parameters (Table 1). At each T setting, the predicted curves closely matched actual seed germination data under the various Ψ levels (Figs. 2A–2E). Normalization of germination time courses across various Ψ levels at sub- and supra-optimal T levels incorporated into a common curve are shown in Fig. 3. At sub-optimal T, the difference between groupings of normalized observations and common curve is indistinct (Fig. 3A). However, the grouping of normalized observations at 28 °C did not resemble the profile from the common curve and fell into a distinct group (Fig. 3B). This indicates that the estimates of HT model interacted with T. Furthermore, these HT estimates consistently showed the largest shift in Ψb with increasing T (Figs. 2F–2G) and the grouping of normalized observations accurately predicted seed germination in this population.
Figure 2: Germination time courses of A. tenuissimum seeds across a range of water potential and temperature (A–E) and normal distributions of the relative frequencies of base water potential at each temperature (11, 15, 20, 24 and 28 °C) (F–G).
Symbols represent actual data and lines indicate values predicted by probit analysis using the parameters presented in Table 1.Figure 3: Normalized time courses of the sub- and supra-optimal hydrotime models.
(A) Germination data from Figs. 2A–2C is plotted on a normalized thermal time scale showing the predicted time courses in water according to the parameters of sub-optimal T shown in Table 2. (B) Germination data from Figs. 2D–2E are plotted on a normalized time scale showing the predicted time courses in water according to the parameters of supra-optimal T shown in Table 2. (C) Germination time courses across all T and Ψ shown in Figs. 2A–2E merged into a single normalized thermal time scale.Hydrothermal time analysis
Parameters for the HTT model, estimated in the sub- and supra-optimal T ranges, are shown in Table 2. The hydrothermal time requirement (θHT) for seed germination was 43.9 MPa °C d. Values Tb and Ψb(50) were estimated to be 7.0 °C and −0.67 MPa, respectively. FGP increased until 20.5 °C (To), then it declined towards ceiling temperatures (Tc(g)), at which germination theoretically ceased when T exceeded To. In addition, Tc(50), the ceiling temperature for germination of 50%, was 27.2 °C. The estimates for KT was 0.1 MPa °C−1, indicating that Ψ declined by 0.1 MPa for every degree that T exceeds To. Applying the HTT models using the A. tenuissimum germination data resulted in high R2 values at both sub-optimal (R2 = 0.89) and supra-optimal (R2 = 0.81) T ranges, indicating a high degree of congruency between predicted and observed germination responses.
| Hydrothermal time model parameters | Sub-optimal T | Supra-optimal T |
|---|---|---|
| Tb (°C) | 7.0 | 7.0 |
| θHT (MPa °C d) | 43.9 | 43.9 |
| Ψb(50) (MPa) | −0.67 | −0.67 |
| σΨb (MPa) | 0.28 | 0.32 |
| R2 | 0.89 | 0.81 |
| To(°C) | – | 20.5 |
| Tc(50) (°C) | – | 27.2 |
| KT(MPa °C−1) | – | 0.1 |
Notes:
- Tb
-
base temperature
- To
-
optimal temperature
- θHT
-
hydrothermal time constant
- Ψb(50)
-
base water potential for 50% seed germination
- σΨb
-
standard deviation for Ψb(g)
- R2
-
coefficient of determination
- KT
-
the slope of the relationship between Ψb(50) and temperatures when T exceeds To
- Tc(50)
-
ceiling temperature to germination of 50%. The value of Tc(50) was calculated by parameters found after fitting the HTT model at supra-optimal T (Eq. (6))
Discussion
Seed germination of A. tenuissimum in response to various temperature and water availability conditions
Successful establishment of cultivated plants depends on rapid and uniform seed germination (Fakhfakh, Anjum & Chaieb, 2018; Bidgolya et al., 2018); however, suitable water availability and temperature conditions for seed germination are only available during a short period in most arid and semi-arid regions (Fakhfakh, Anjum & Chaieb, 2018; Belo et al., 2014; Watt & Bloomberg, 2012). Here, we presented a comprehensive study of seed germination responses to different temperatures and water potential levels in A. tenuissimum, an important wild onion species from temperate-desert steppes of north central Asia (He, 2008; Li & Zhang, 2011). Our results showed that final germination percentage of A. tenuissimum strongly depended on the interaction of these two factors. Furthermore, applying HTT models to our dataset provided insights into suitable conditions to enhance consistent germination responses of this valuable native species. Later, we discuss in detail the nuances of our results that underpin this methodological approach and expedite industrialization of wild species under stressful environmental conditions.
Numerous studies have illustrated how temperature-dependent seed germination is related to the geographical and ecological distribution of a particular species such as common vetch (Vicia sativa) (Liu, 2010), Stipa species (Ronnenberg, Wesche & Hensen, 2008) and temperate sedges (Carex) (Schütz & Rave, 1999). For A. tenuissimum, the final germination percentage was quite high (i.e., 92.5 to 94.8%) in the temperature range of 15 to 20 °C (Fig. 1). In some mediterranean grassland species, 15 °C and 20 °C also were found to be the best temperature for seed germination (Herranz, Ferrandis & Martínez-Sánchez, 1998; Marques & Draper, 2012). In contrast, the lower temperatures for preferable germination of A. tenuissimum seeds was consistent with a prior study of wild species of Allium distributed in temperate desert steppe (Zhao, Li & Badema, 2011). In any constant temperatures, final germination percentage declined when seeds were incubated at reduced water potential levels (Fig. 1). This response is likely associated with enzyme activity and oxygen availability of seeds, which are known to decrease when germinated at unfavorable temperatures and limiting moisture conditions (Bewley & Black, 1994). Furthermore, higher incubation temperatures and more negative values of water potential during germination likely induced secondary dormancy of seeds, leading to prolonged seed germination over non-optimal temperatures and more negative water potential levels (Fig. 2).
Predicting seed germination with mathematical models
Mathematical models to predict and quantify the influence of environmental factors on seed germination are essential when little information is known about the ideal conditions for potential agronomic species such as zucchini (Cucurbita pepo) (Atashi et al., 2015), red brome (Bromus rubens) and cheatgrass (Bromus tectorum) (Horn, Nettles & Clair, 2015), and safflowers (Carthamus tinctorius) (Bidgolya et al., 2018). While accounting for the influence of only temperature, TT models can successfully predict germination time courses in sub-optimal temperature ranges, but they become less effective at predicting germination in supra-optimal temperature ranges (Bradford, 2002; Bewley et al., 2013), which spurred the development of hydrothermal models combine the influences of temperature and water availability on seed germination (Windauer et al., 2012). Such HT and HTT models have been widely applied and accurately describe seed germination across variable temperatures and water potential levels (Alvarado & Bradford, 2002; Bakhshandeh et al., 2015; Bakhshandeh et al., 2017). In addition, normalizing thermal time scales, as we did for the germination time courses of A. tenuissimum (Bradford, 1990; Bradford, 2002), accurately described the influences of temperature and water availability (Fig. 3A). However, the grouping of normalized observations at 28 °C fell into a distinct group instead of resembling the profile from the common curve (Fig. 3B). Similarly, previous studies documented that HT analysis was unable to predict the germination time courses at supra-optimal temperatures (Bakhshandeh et al., 2017; Rowse & Finch-Savage, 2010), suggesting that HT estimates were not consistent across variable temperatures. In other words, grouping of normalized observations resulted in large shifts in the theoretical threshold or base water potential (Ψb(g)) that can prevent the fraction of germination as temperature increases (Rong, Li & Johnson, 2015). Furthermore, when the distribution of Ψb(g) overlaps with 0 MPa at a given temperature, germination of some seeds may be inhibited (Alvarado & Bradford, 2002; Larsen et al., 2004; Watt, Bloomberg & Finch-Savage, 2011). For this reason, Alvarado & Bradford (2002) developed the HTT model to account for the linear increase of Ψb(g) value as temperature increased above To in order to eliminate the positive shift in Ψb(g) values at supra-optimal temperatures. This modified model can quantify and predict both final germination percentage and germination rate across different temperatures and water potential levels at which seed germination occurs (Bakhshandeh et al., 2015; Rowse & Finch-Savage, 2010) as we observed for germination time course of A. tenuissimum seeds at supra-optimal temperatures, which were well described by HTT model (i.e., R2 = 0.81).
Model parameters and their biological roles
Because changes in dormancy state are related to Ψb (Meyer, Debaenegill & Allen, 2000) and high temperatures often induce secondary dormancy, soil water availability is critical for seed germination at supra-optimal temperatures (Hills, Staden & Thomas, 2003). Likewise, values of Ψb(50) (−0.74 to −0.16 MPa) determined in our analysis revealed that seeds of A. tenuissimum are relatively sensitive to water restrictions at temperatures above To. Thus, seeds of A. tenuissimum should be germinated at sub-optimal temperatures to enhance crop establishment in arid and semi-arid regions. Results from HT analysis also indicated that the minimum value of Ψb(50) was observed at 11 °C and then increased, particularly in supra-optimal temperature ranges. Similar to our results, the linear increase of Ψb(50) has been observed in zucchini (Atashi et al., 2015), watermelon (Bakhshandeh et al., 2015), and sesame (Bakhshandeh et al., 2017). In general, the Ψb(50) value of a seed lot gives an indication of its tolerance to water stress. If water potential levels are more negative than Ψb(50), germination times will be extended and germination rates will be reduced (Bradford, 2002). This could be caused by a decrease in both enzyme activity and oxygen availability during the seed germination period, particularly when germinated at supra-optimal temperatures (Bewley et al., 2013). When soil temperature approaches To, less negative water potential levels will cause an increase in the activity of enzymes and water uptake rate (Kebreab & Murdoch, 1999).
Base water potential coefficient (σΨb) is related to life history strategy of various species (Bradford, 1990) and indicates the uniformity of seed germination among individual seeds within a seed lot. A smaller value of σΨb represents an increasingly uniform germination among seed population (Bradford & Still, 2004; Bidgolya et al., 2018). The values of σΨb we estimated for A. tenuissimum seeds varied from 0.20 to 0.37 MPa, indicating that there was less variation in Ψb among individual seeds and that uniform germination was observed. This pattern is likely a reflection of survival adaptations to harsh environments. Under initial favorable soil temperature and water availability, faster and uniform germination will allow plants to possibly dominate a plant community in space and time (Wang et al., 2009). In contrast, most plant populations must adopt a different germination strategy to mitigate the harsh environment-induced damage on germination by allowing a few seeds to rapidly germinate, and then delaying germination until consistent suitable environment conditions are reached before the remaining seeds germinate (Bradford, 1990; Batlla et al., 2009; Watt, Bloomberg & Finch-Savage, 2011; Rong, Li & Johnson, 2015). Consistent with our results, the variation of Ψb was small and σΨb varied from 0.16 to 0.31 MPa among Vicia sativa seeds (Liu, 2010), indicating that the long-term cultivation and domestication can result in uniform germination.
The hydrotime (MPa d) constant of the seeds required for germination (θH) represents the inherent speed of seed germination in a seed lot (Bradford & Still, 2004). Our results showed that θH declined from 6.4 to 3.0 MPa d as temperature increased from 11 °C to 24 °C, indicating that germination rate was faster at higher temperatures (Fig. 2, Table 1). Similarly, other studies reported that θH was constant in supra-optimal temperature ranges, but it increased as temperature declined in the range of sub-optimal temperatures (Bakhshandeh et al., 2015; Dahal & Bradford, 1994). Thus, seed germination was greatly altered with decreasing temperature.
Seed germination responses to temperature are commonly characterized with three cardinal temperatures ( Tb, To and Tc) estimated according to the HTT model (Bewley et al., 2013). These cardinal temperatures of A. tenuissimum seeds were Tb = 7.0 °C, To = 20.5 °C, and Tc(50) = 27.2 °C, respectively. In practical terms, these values suggest A. tenuissimum seeds should not be sown in soils where temperature do not exceed 7.0 °C. Maximum final germination percentage would be observed as soil temperature approaches 20.5 °C, a value when some seeds within the population will probably germinate quickly if they are not exposed to water stress or dormancy induction within optimal ranges (Rong, Li & Johnson, 2015; Windauer et al., 2012). In addition, because seeds of A. tenuissimum germinated in a relatively narrow range of temperature according to estimations of Tc(50), seeds of A. tenuissimum are likely unable to tolerate high soil temperature. From a crop production standpoint, our results document that seed germination of A. tenuissimum occurs over a soil temperature range of 7.0 to 27.2 °C, but 20.5 °C is the optimal temperature. These estimated cardinal temperatures are consistent with wild Allium species (Zhao, Li & Badema, 2011).
Conclusions
The mathematical models or the estimated value of parameters can be used to quantitatively predict the seed germination of A. tenuissimum under various T and Ψ conditions. Soil temperature for seed germination of A. tenuissimum should be at the range of 7.0 °C to 27.2 °C, and the optimum temperature is 20.5 °C. The water potential should be less negative than −0.67 MPa. The relatively lower Tb and more negative Ψb(50) indicate that this wild Allium species might be established in arid and semi-arid regions, while relatively narrow threshold in response to T and Ψ variations might sufficiently delay or even prevent seed germination in extreme arid or harsh desert regions.
Supplemental Information
ANOVA analysis for the final germination percentage of A. tenuissimum seeds incubated at various temperatures and water potential conditions
T, temperature; Ψ, water potential; T ×Ψ, the interaction of temperature and water potential; df, degree of freedom