Morphological and molecular data confirm the transfer of homostylous species in the typically distylous genus Galianthe (Rubiaceae), and the description of the new species Galianthe vasquezii from Peru and Colombia
- Published
- Accepted
- Received
- Academic Editor
- Marcial Escudero
- Subject Areas
- Conservation Biology, Plant Science, Taxonomy
- Keywords
- Homostyly, Neotropic region, Pollen, Spermacoceae, Taxonomy
- Copyright
- © 2017 Florentín et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. Morphological and molecular data confirm the transfer of homostylous species in the typically distylous genus Galianthe (Rubiaceae), and the description of the new species Galianthe vasquezii from Peru and Colombia. PeerJ 5:e4012 https://doi.org/10.7717/peerj.4012
Abstract
Galianthe (Rubiaceae) is a neotropical genus comprising 50 species divided into two subgenera, Galianthe subgen. Galianthe, with 39 species and Galianthe subgen. Ebelia, with 11 species. The diagnostic features of the genus are: usually erect habit with xylopodium, distylous flowers arranged in lax thyrsoid inflorescences, bifid stigmas, 2-carpellate and longitudinally dehiscent fruits, with dehiscent valves or indehiscent mericarps, plump seeds or complanate with a wing-like strophiole, and pollen with double reticulum, rarely with a simple reticulum. This study focused on two species that were originally described under Diodia due to the occurrence of fruits indehiscent mericarps: Diodia palustris and D. spicata. In the present study, classical taxonomy is combined with molecular analyses. As a result, we propose that both Diodia species belong to Galianthe subgen. Ebelia. The molecular position within Galianthe, based on ITS and ETS sequences, has been supported by the following morphological characters: thyrsoid, spiciform or cymoidal inflorescences, bifid stigmas, pollen grains with a double reticulum, and indehiscent mericarps. However, both species, unlike the remainder of the genus Galianthe, have homostylous flowers, so the presence of this type of flower significantly modifies the generic concept. In this framework, a third homostylous species, Galianthe vasquezii, from the Andean region is also described. Until now, this species remained cryptic under specimens of Galianthe palustris It differs however from the latter by having longer calyx lobes, the presence of dispersed trichomes inside the corolla lobes (vs. glabrous), fruits that are acropetally dehiscent (vs. basipetally dehiscent), and its Andean geographical distribution (vs. Paranaense). Additionally, a lectotype has been chosen for Diodia palustris, Borreria pterophora has been placed under synonymy of Galianthe palustris, and Galianthe boliviana is reported for the first time from Peru. A key of all Galianthe species with indehiscent mericarps is also provided.
Introduction
Galianthe Griseb. is a neotropical genus belonging to tribe Spermacoceae (Groeninckx et al., 2009). The genus was revised by Cabral (2002) and divided into two subgenera (Cabral & Bacigalupo, 1997): Galianthe subgen. Galianthe, from South America with 39 species, and Galianthe subgen. Ebelia (Rchb.) E.L. Cabral & Bacigalupo, with 11 Mesoamerican and South American species. Historically, Galianthe was associated with Diodia L., which has been described based on only D. virginiana L. The type species has a palustrine habit, pauciflorous axillary inflorescences, filiform corolla tube, bifid style with two long filiform stigmatic lobes, and indehiscent fruits. Later, others authors (i.e., Swartz, 1788; Walter, 1788; Candolle, 1830; Small, 1913) added other species into this genus with diverse kinds of habits and inflorescences, different floral morphology (e.g., distyly or homostyly, infundibuliform or campanulate corollas, bifid or bilobate stigma), and 2-carpellate schizocarpic fruits, being currently comprised by ca. 180 names (called Diodia s. lat.). Later, Bacigalupo & Cabral (1999) revised the genus Diodia and maintained only five species that were morphologically similar to the type species D. virginiana L. (description as above, and constituting Diodia s. str.). Species that did not match with these diagnostic features, were transferred to other genera as follows: eight species to Borreria subgen. Dasycephala (DC.) Bacigalupo & E.L. Cabral (Bacigalupo & Cabral, 1996), 12 species to Hexasepalum Bartl. ex DC. (Kirkbride, 2014; Kirkbride & Delprete, 2015; Cabaña Fader et al., 2016), and four species to Galianthe subgen. Ebelia (Cabral & Bacigalupo, 1997). The four Galianthe species are distylous, Diodia bogotensis (Kunth) Cham. & Schltdl. [=Galianthe bogotensis (Kunth) E.L. Cabral & Bacigalupo]; D. brasiliensis Spreng. [=G. brasiliensis (Spreng.) E.L. Cabral & Bacigalupo]; D. cymosa Cham. [=G. cymosa (Cham.) E.L. Cabral & Bacigalupo], and D. hispidula A. Rich. ex DC. [=G. hispidula (A. Rich. ex DC.) E.L. Cabral & Bacigalupo]. The remaining species with an uncertain position (ca. 150 names) are currently under revision (A Cabaña Fader, pers. comm., 2017). In this sense, (Bacigalupo & Cabral, 1996; Bacigalupo & Cabral, 1998) transferred these species to Borreria subgen. Dasycephala because of their homostylous flowers and indehiscent mericarps, while Delprete, Smith & Klein (2005) and Delprete (2007), alluding to a broad concept, transferred the two species to Spermacoce mainly based on fruit characters. Dessein (2003) informally proposed to consider Diodia spicata as part of Galianthe based on molecular data (ITS intron), palynological data (double reticulum), and fruit morphology. The aim of this work is to confirm the taxonomic position of D. palustris and D. spicata based on morphological and molecular data, and perform their formal combination in Galianthe. In addition, a third homostylous species (Galianthe vasquezii R.M Salas & J. Florentín) is described and illustrated based on specimens from Colombia and Peru (previously identified as D. palustris). Additionally, a lectotype has been chosen for Diodia palustris whereas Borreria pterophora has been placed under synonymy of Galianthe palustris. Moreover, Galianthe boliviana E.L. Cabral is for the first time recorded in Peru. Finally, we provided a distribution map for the species investigated in this study, as well as a dichotomous key for all taxa with indehiscent mericarps.
Materials and Methods
Morphological study
This study is based on classical taxonomy techniques. Collections deposited at the BA, BHCB, CEPEC, CTES, ESA, FUEL, FPS, FURB, HAS, HOXA, HUT, IAC, IAN, IFFSC, IPA, K, LIL, MBM, MO, NY, P, PR, SI, SP, UB, UFRN, USB, US, USM and UEC herbaria were analysed. Furthermore, the databases of the ‘Catálogo de plantas e fungos do Brazil’ and ‘Missouri Botanical Garden’ were examined. In order to carry out scanning electron microscope (SEM) analyses, flowers were dehydrated using a graded series of ethanol solutions and afterwards critically point dried and sputter-coated with gold-palladium. SEM images were obtained with a JEOL 5800 LV scanning electron microscope. Pollen grains were acetolyzed according to Erdtman (1966) and mounted in glycerine jelly for analysis by light microscopy (LM). Conventional parameters (P = polar axis, E = equatorial axis) of at least 20 grains were measured under LM, and the exine was analyzed using SEM. Pollen terminology follows Punt et al. (2007). Species distribution maps were generated from distribution data that was present on the herbarium labels for each specimen and subsequently georeferenced using Google Earth (www.google.earth.com.ar) and Hijmans (2013).
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants (ICN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. In addition, new names contained in this work which have been issued with identifiers by IPNI will eventually be made available to the Global Names Index. The IPNI LSIDs can be resolved and the associated information viewed through any standard web browser by appending the LSID contained in this publication to the prefix “http://ipni.org/”. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central, and CLOCKSS.
Molecular study
In total, 45 species (47 accessions) were included to infer the phylogenetic relationship of Diodia palustris and D. spicata. The ingroup contains species from the Borreria, Carajasia R.M. Salas, E.L. Cabral & Dessein, Crusea Cham. & Schltdl., Diodia, Emmeorhiza Pohl ex Endl., Ernodea Sw., Galianthe, Hexasepalum, Mitracarpus Zucc., Psyllocarpus Mart. & Zucc., Richardia L., Schwendenera K. Schum., Spermacoce, and Staelia Cham. & Schldtl. genera, and Bouvardia ternifolia (Cav.) Schltdl. as the outgroup. Leaf samples of these studies were obtained from silica gel-dried material or herbarium materials. Forty-three species (44 accessions) were previously used by Salas et al. (2015). Four accessions belonging to D. palustris has been added. All studied species with geographical information, collector, herbarium and GenBank accession numbers are provided in the Appendix.
Molecular protocols
Total genomic DNA was isolated from silica-dried leaf material using a modified CTAB protocol (Doyle & Doyle, 1987). Nuclear ribosomal ETS and ITS fragments were amplified following Baldwin & Markos (1998) and Negrón-Ortiz & Watson (2002), and White et al. (1990), respectively. PCR reactions for both gene markers investigated in this study consisted of 2 min initial denaturation at 94 °C and 30 cycles of 30 s denaturation at 94 °C, 30 s primer annealing at primer specific temperature and 1 min extension at 72 °C. Primer annealing for ETS and ITS were at 47 °C and 48 °C respectively. Amplification reactions were carried out on a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA, USA). Purified amplification products were sent to Macrogen, Inc. (Seoul, South Korea) for sequencing. Sequences obtained in this study were deposited at GenBank [Diodia palustris, Verdi et al. 1905, ETS (MF166824), ITS (MF166826); Miguel et al. 19, ETS (MF166825), ITS (MF166827).
Phylogenetic analyses
Contiguous sequences were assembled using Geneious v7.0.6 (Biomatters, Auckland, New Zealand). Automatic alignments were carried out with MAFFT (Katoh et al., 2002) Subsequent manual finetuning of the aligned dataset was done in Geneious v7.0.6. Congruency between the different datasets was inferred using different methods. First, a series of incongruence length difference tests (ILD; Farris et al., 1995) were carried out with PAUP* v.4. 0b10 (Swofford, 2003) using the following parameters: simple taxon addition, TBR branch swapping and heuristic searches of 1,000 repartitions of the data. Despite the well-known sensitivity of the ILD test (Barker & Lutzoni, 2002), the results of this test were compared in light of the resolution and support values of the obtained nuclear and nuclear ribosomal topologies. As a result, possible conflict between data matrices was visually inspected, searching for conflicting relationships within each topology that are strongly supported (hard vs. soft incongruence; Johnson & Soltis, 1998. Model selection for the Bayesian inference analysis was conducted with ModelTest 3.06 (Posada & Crandall, 1998) under the Akaike Information Criterium (AIC). The GTR+G model was selected for both ITS and ETS. Bayesian analyses of the concatenated dataset were carried out with MrBayes 3.1 (Huelsenbeck & Ronquist, 2001; Ronquist & Huelsenbeck, 2003). Four chains (one cold, three heated), initiated from a random starting tree were run simultaneously for 10 million generations. Every 1,000 generations, a tree was sampled from the chain for a total of 10,000 trees. Due to the burn-in, 50% of the sample points were discarded. Convergence of the chains was examined with TRACER 1.4 (Rambaut & Drummond, 2007). This resulted in an effective sampling size (ESS) parameter exceeding 100, which assumes a sufficient sampling and acceptable mixing.
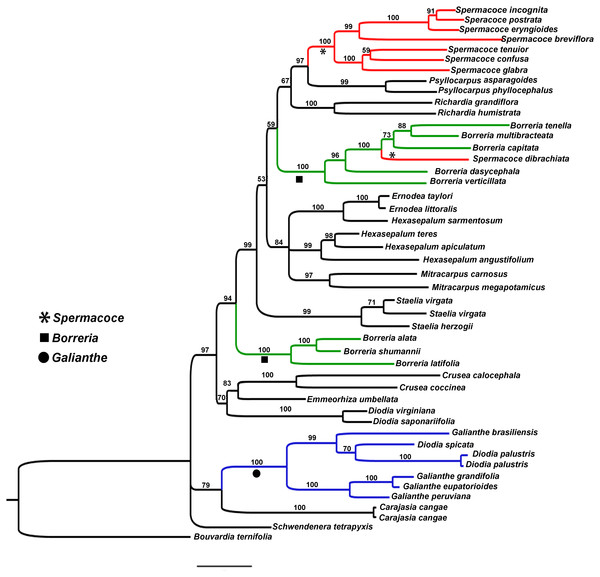
Figure 1: Bayesian tree.
Showing the relationship of Galianthe with the remaining genera of Spermacoce clade.Results
Phylogenetic results
The ingroup contains 14 genera represented by 45 species of the Spermacoce clade. Of these, Diodia spicata and D. palustris are analysed for the first time in this context. ITS and ETS datasets were analysed both separately and combined. Because topology of each gene marker is very similar, we only present the results of the combined analysis (Fig. 1). Current results indicate that most clades coincide with most currently accepted genera (e.g., Crusea, Emmeorhiza, Ernodea, Diodia s.s. (sensu Bacigalupo & Cabral, 1999), Mitracarpus, Psyllocarpus, Richardia and Staelia). Spermacoce, Borreria and Hexasepalum however, appear unresolved or as polyphyletic genera being present in several different parts of the tree. In regard of the species studied, we found that G. spicata and G. palustris fall intermingled among the Galianthe species. The Galianthe clade, including both former Diodia species, is strongly supported (Bayesian Posterior Probability (BBP): 100). The genus is divided into two strongly supported subclades, one subclade consists of G. grandifolia E.L. Cabral, G. eupatorioides (Cham. & Schltdl.) E.L. Cabral, and G. peruviana (Pers.) E.L. Cabral (BPP: 100), all from G. subgen. Galianthe. The other subclade (BPP: 99) comprises G. spicata, G. palustris, and G. brasiliensis (type species of Galianthe subgen. Ebelia). The clade of Galianthe and Carajasia is only moderately supported (BBP: 79). The genera Galianthe and Carajasia share the occurrence of pollen grains with a double reticulum, mostly associated with the distyly and bifid stigma. The Galianthe-Carajasia clade forms an unsupported trichotomy with Schwendenera (also distylous) and the remaining genera of the Spermacoce clade (all homostylous species never associated to double reticulum pollen grains). Interestingly, all clades that coincide with generic concepts are strongly supported (e.g., Psyllocarpus (BBP:99), Spermacoce s.s. (BBP:100), Richardia (BBP:100), Borreria s.s. (BBP:100), Mitracarpus (BBP:97), Hexasepalum s.s., Staelia (BBP:99), Diodia s.s. (BBP:100), Borreria latifolia group (BBP:100), and Crusea (BBP:100)). The species assigned to Borreria (sensu Bacigalupo & Cabral, 1996) are divided into two clades that are intermingled with other morphologically well-defined genera. One of these clades, further referred to as the Borreria latifolia group, comprises Borreria alata, B. schumannii, and B. latifolia (BBP:100). The other clade comprises five Borreria species from North and South America (B. capitata, B. multibracteata, B. tenella, B. dasycephala, and B. verticillata), as well as the African Spermacoce dibrachiata (BBP: 100). Spermacoce is divided into two unrelated branches, of which one clade comprises the type species S. tenuior, other American species with similar flower morphology (S. eryngioides, S. prostrata, S. incognita, S. confusa, and S. glabra, all with stamens and style included), and the Australian S. breviflora (support 100). As mentioned above, the other species of Spermacoce (S. dibrachiata) falls among the species of Borreria. Hexasepalum species are also divided into two clades, one of them is well supported (BBP: 99) and contain H. angustifolium Bart. ex DC. (type species), H. apiculatum and H. teres. The other, only represented by H. sarmentosum appears as sister species of the Ernodea (BBP: 100). The genus Ernodea, represented by E. taylori and E. littoralis, constitutes a strongly supported clade (BBP: 100). The results explained above allow us to support the following taxonomic changes.
Taxonomic Treatment
Description of the new species
Galianthe vasquezii R. M. Salas & J. Florentín, sp. nov. TYPE. PERU: Pasco, Oxapampa, Parque Nacional Yanachaga-Chemillen, Quebrada Yanachaga, 2,250 m, 10°24′S, 75°28′W, 14 Jun 2003, R. Vásquez M. 28284 (holotype: HOXA!; isotypes MO!, HUT, USM).
Description
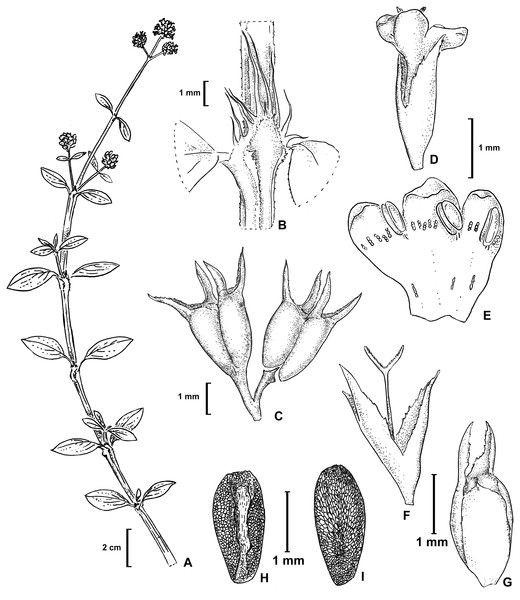
Herb decumbent or prostrate, stems quadrangular, angle strongly alate, with scabridous papillae, more densely disposed near nodes. Leaves sessile or pseudopetiolate, pseudopetiole up to 4 mm long, blades elliptic or obovate, apex acute, base attenuate, 12–32 × 5–17 mm, plicate-nervose, adaxially glabrous or puberulous, abaxially scabridous on nerves, margin scabridous, with 3–5 secondary nerves; stipular sheath 3.2–5.6 mm long, with 7–9 linear fimbriae, glabrous, fimbriae 3.5–6.8 mm long. Inflorescences thyrsoid, partial inflorescences subglomeriform, multiflorous. Flower pedicellate; pedicel 1–2 mm long; calyx (3-) 4-lobed, hypanthium 1.1–1.3 mm long, glabrous or glabrescent, lobes narrowly triangular, 1–1.4 mm long, glabrous, apex acute; corolla infundibuliform, 3-lobed, 1.75–2.1 mm long, white; lobes ovate, internally with hairs scattered at base, tube internally with some dispersed hairs near its base and externally glabrous, straight; stamens subincluded, anther 0.4–0.6 mm long, oblong, filament fixed immediately below interlobular sinuses; pollen grains 7–8 zonocolpate, oblate-spheroidal to prolate spheroidal, small, P = 31 µm, E = 29 µm, colpi long, endoaperture an endocingulum, exine semitectate, reticulate, muri nanospinose, 0,18–0,3 µm long; style bifid, 1.5–1.8 mm long, stigmatic branches ca. 0.2 mm long, with conspicuous papillae. Fruit a capsule, cordate or deltoid in outline, 1.8–2 × 1.6–1.9 mm, glabrous, with two indehiscent mericarps which split from the base upwards, each valve remains temporary attached in upper half, at maturity caduceus, seed 1.8–2 × 0.8–1 mm, ovoid, ventral face longitudinally furrowed, partially covered by the strophiole; exotesta reticulate-foveate. Figures 2 and 3. LSID: 77166460-1—Galianthe vasquezii.
Figure 2: Galianthe vasquezii.
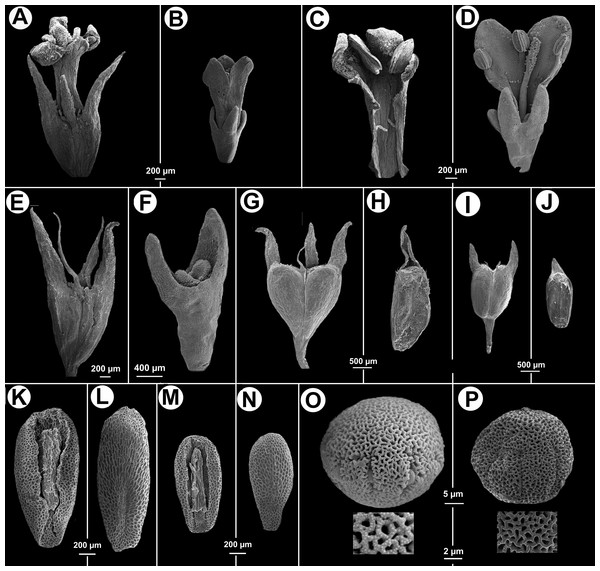
(A) Apical part of flowering branch. (B) Stipular sheath. (C) Fruit. (D–F) Flower. (E) Inside of corolla. (F) Style, stigma and calyx. (G) Ventral view of indehiscent valve, calyx tube and lobes. (H–I) Seeds. (H) Ventral view. (I) Dorsal. All from isotype (MO).Figure 3: Morphological characters distinguishing.
Galianthe vasquezii (pictures A, C, E, G, H, K, L, O from the isotype at MO) and G. palustris (pictures B, D, F, I, J, M, N, P from A. A. Cabaña 19 at CTES). (A) Flower. (C) Inside the corolla with two stamens. (E) Hypanthium and dimorphic calyx lobes. (G) Entire fruit. (H) indehiscent valve. (K) Ventral face of seed. (L) Dorsal face of seed. (O) Equatorial view of pollen grains, with simple, below a detail of exine. Galianthe palustris. (B) Flower. (D) Opened flower showing inside of corolla and three stamens, style and stigma. (F) Hypanthium, nectariferous disc, and calyx lobes. (I) Entire fruit. (J) Indehiscent valve. (M) Ventral face of seed. (N) Dorsal face of seed. (P) Pollen with double reticulum, below a detail of exine showing the suprareticulum incomplete.Distribution—Andes of Peru and Colombia
Observations—All specimens of the G. vasquezii were previously identified as Galianthe palustris. However, it differs from G. palustris, in having calyx lobes 1–1.4 mm long, with acute apex (vs. calyx lobes 0.4–0.6 mm long, obtuse), corolla 1.75–2.1 mm long, lobes internally with hairs scattered at base, tube internally with some dispersed hairs near its base (vs. corolla 1–1.5 mm long, internally glabrous), pollen grains with reticulate exine, muri nanospinose (vs. pollen grains with bireticulate exine, suprareticulum psilate and incomplete, infrareticulum nanospinose), fruit 1.8–2 mm long, deltoid in outline, acropetally dehiscent (vs. fruit 1.1–1.5 mm long, oblong or obovate in outline, basipetally dehiscent), and seeds 1.8–2 mm long (vs. seeds 1–1.42 mm long).
Ecology—Galianthe vasquezii grows in Montane Forest of Peru and Colombia, which represents a severely fragmented type of vegetation. It grows between 1,800 and 2,500 m altitude.
Conservation status—The extent of occurrence (EOO) was calculated to be 397 km2 (cell sized 2 km). Following the IUCN criteria (IUCN, 2014), this species should be considered endangered [EN B1 ab (ii, iii)] due to the continuing decline in area and quality of its habitat.
Additional Specimens Examined—COLOMBIA: Antioquia, Monte del Diablo, 21 Jul. 1944, Bro. Daniel 3303 (US); Rio Negro, 16 Dec. 1933, Bro. Daniel 165 (US). PERU: Distrito Huancabamba, sector Grapanazú, límite Parque Nacional, Yanachaga-Chemillen, 10°26′S, 75°23′W, 15 Oct. 2003, R. Rojas et al. 1892 (MO); idem, sector Tunqui, camino hacia María Puñis, 1,895 m, 10°16′31″S, 75°30′59″W, M. Cueva 193 (HOXA, HUT, MO, USM); Luispicanchi, Cuzco, Quincemil, 13°14′S, 70°45′W, Oct. 1950, F. Marín 2731 (CTES, LIL); Pasco, Oxapampa, carretera de Cochabamba, 10°33′42″S, 75°27′23″W, 11 Nov. 2004, A. Monteagudo et al. 7587 (CTES, MO).
New combinations
Galianthe palustris (Cham. & Schltdl.) Cabaña Fader & E. L. Cabral, comb. nov. Diodia palustris Cham. & Schltdl., Linnaea 3: 347. 1828. Borreria palustris (Cham. & Schltdl.) Bacigalupo & E. L. Cabral, Hickenia 2: 264. 1998. Spermacoce palustris (Cham. & Schltdl.) Delprete, Fl. Il. Catarin. (2): 740. 2005. TYPE: BRAZIL, Santa Catarina, “Ad fretum St. Catharinae Brasiliae ipsi legimus, in palustribus Brasiliae aequinoctiales”, s. d., F. Sellow s.n. (holotype: B destroyed, lectotype here designed PR!).
Borreria gymnocephala DC., Prodr. 4: 549. 1830. Diodia gymnocephala (DC.) K. Schum., in Martius, Fl. Bras. 6(6): 16. 1888. TYPE: BRAZIL. s. d., J.P. Pohl s. n. (holotype: G-DC!).
Borreria pterophora C. Presl., Abh. Königl. Böhm. Ges. Wiss. V, 3: 516. 1845. nov. syn. TYPE: BRAZIL, Rio Janeiro, s.l., s.d., Beske s.n. (holotype: PR!).
Diodia alata Nees & Mart., Nova Acta Acad. Caes. Leop. Carol., Wied-Neuwied 12: 12. 1824. Dasycephala alata (Nees & Mart.) Benth. & Hook. f. ex B.D. Jacks, Index Kew. 2: 719. 1893. TYPE: BRAZIL, s.l., s.d., M. Wied s.n. (holotype BR!; isotypes: LD, LE, W!).
Diodia microcarpa K. Schum. ex Glaz., Bull. Soc. Bot. France 56 (Mém. 3d): 361. 1909. TYPE: BRAZIL, “Brasília”, A.F.M. Glaziou 18283 (holotype B destroyed, photo F 867!).
Description
Herb stoloniferous, sometimes with ascendant stems. Stems quadrangular, angle strongly winged, wing with long and slightly recurved fimbriae, or with scabridous and retrorse papillae, especially near foliar nodes. Leaves subsessile or pseudopetiolate; pseudopetiole 1–4 mm long; blades elliptic or obovate, rarely orbicular, 22–30 mm × 10–18.6 mm, apex obtuse, acute or acuminate, base attenuate, plicate-nervose, with 3–4 secondary nerves, adaxially glabrous or scaberulous, margin scaberulous, abaxially scabridous only on nerves; stipular sheath 3–4 mm long, basally alate, 7–9 fimbriate, fimbriae linear, glabrous, 5–8 mm long. Inflorescences thyrsoid, partial inflorescences congested and multiflorous, sometimes arranged on pleiochasium. Flowers shortly pedicellate; pedicel 0.5–1 mm long; calyx 2(-3)-lobed, hypanthium glabrous, lobes triangular, apex obtuse, succulent, 0.4–0.6 mm long; corolla 2-3-lobed, infundibuliform, white, 1–1.5 mm long, tube internally and externally glabrous, scarcely papillate on apex of the dorsal face of lobes; stamens 2–3, anthers 0.2–0.34 mm long, oblong, dorsal surface with a bullate connective, immediately above the insertion of the filament; pollen grains 6–7(-8) zonocolporate, oblate-spheroidal, small, P = 24 µm, E = 25.3 µm, long colpi, endoaperture an endocingulum, exine bireticulate, suprareticulum incomplete, muri psilate, infrareticulum complete, muri nanospinose, 0,15–0,28 µm long; style bifid, 1–1.5 mm long, stigmatic branches 0.2–0.46 mm long, notoriously papillate. Fruit a capsule, which separates from the apex downwards into two indehiscent mericarps, both mericarps remain basally united to the pedicel, tardily deciduous, oblong or ovate in outline, glabrous; seeds 1–1.42 × 0.7–0.8 mm, ovate or obpiriforme in outline, ventral surface with a longitudinal furrow covered by a persistent strophiole; exotesta reticulate-foveate. Figure 3. LSID: 77166461-1—Galianthe palustris.
Distribution—Brazil (Bahia, Minas Gerais, Paraná, Rio de Janeiro, Rio Grande do Sul, Santa Catarina, and São Paulo), and Argentina, Misiones province.
Ecology—Galianthe palustris is a heliophilous plant that inhabits in swampy areas near lotic water bodies, especially along main rivers and their tributaries.
Additional Specimens Examined—ARGENTINA. Misiones: San Pedro, Parque Provincial Moconá, embarcadero, 7 Mar. 2013, M. D. Judkevich et al. 46 (CTES); idem, borde de arroyo, 11 Dec. 2011, L. M. Miguel et al. 19 (CTES). BRAZIL. Bahia: Belmonte, 23 Nov. 1970, T. S. Santos 1124 (CEPEC); Minas Gerais: Camanducaia, Monte Verde, Estrada Camanducaia, 27 Apr. 2013, J. A. M. Carmo 125 (UEC); ídem, Monte Verde, 24 Jan. 2013, J. A. M. Carmo 111 (UEC); ídem, Mata dos Vargas, 22 Mar. 2000, R. B Torres et al. 1176 (FUEL); Santos Dumont, s. d., H.L.M. Barreto 11339 (BHCB); São João do Manhuaçu, 19 km S of the intersection of Highway BR-116 & BR-262, just N of the village of São João do Manhuaçu, 27 Mar. 1976, G. Davidse & W. G. D’Arcy 11434 (SP). Paraná: Mun. Bocaiúva do Sul, Serra da Bocaína, 31 Mar. 2001, E. Barbosa et al. 654 (CTES, ESA, MBM); Serra de São Luís, BR 277, 19 Jan. 1985, M.S. Ferrucci et al. 284 (CTES); Fazenda Reserva, 85 Km SW of Guarapuava, on bank of brook near Barbaquá, 17 Mar. 1967, J. C. Lindeman et al. 4959 (CTES, MBM, NY, UB); Mun. Morretes, Serra Morumbi, picada ao Olimpo, 19 Jan. 1995, O. S. Ribas et al. 761 (CTES); Mun. Piraquara, Floresta, 9 Mar. 1947, G. Hatschbach 640 (CTES, LIL); BR-476, 7 Km E de Contendas, 26 Jan. 1985, A. Krapovickas & C. L. Cristóbal 39632 (CTES); Fazenda de J. Rickli near Turvo, 40 km N of Guarapuava, forest, 9 May. 1967, J. C. Lindeman et al. 5280 (CTES); Curitiba, 17 May. 2002, J. Cordeiro 2233 (ESA). Rio Grande do Sul: Barracão, Parque Estadual de Espigão Alto, 1 Mar. 2001, M. Sobral & J. Larocca s.n. (FURB); Capivari, Viamão, 15 Mar. 1975, Porto et al. 1389 (CTES); Esteio, 23 Mar. 1949, B. Rambo 40638 (LIL); Leopoldo, on Monte Jacaré, 7 Dec. 1948, B. Rambo 38588 (LIL); Pareci, Prope Montenegro, 31 Mar. 1950, B. Rambo 46536 (CTES); Porto Alegre, 17 Dec. 1932, B. Rambo s.n. (P04541549); idem, Morro da Gloria, 16 Dec. 1931, B. Rambo 577 (LIL); San Salvador, 14 Mar. 1947, A. Sehnem 2676 (SI); ídem, 16 Dec. 1933, B. Rambo 577 (SP); idem, Montenegro, 1 Mar. 1950, A. Sehnem 4426 (SI); Santana, 6 Apr. 1974, M.C. Sidia 27 (HAS, CTES). Rio de Janeiro: 17 Km from praça da Parati on road from Parati to Cunha, 26 Apr. 1972, J. H. kirkbride 1729 (US); Nova Friburgo, 12 Nov. 1890, A. Glaziou 18283 (P02088844); Petrópolis, vale Bonsucesso, 13 Apr. 1968, B.D. Sucre 2738 (US); Serra da Mantiqueira, Maciço do Itatiaia, Parque Nacional do Itatiaia, 16 Apr. 1971, I. Gottsberger et al. 110 (CTES); idem, I. Gottsberger 110-16471 (CTES); Serra dos Orgãos, 11 Jan. 1905, G. Gardner 445 (US). Santa Catarina: 6.5 KM NW de Aguas Mornas, caminho a Lourdes, 6 Feb. 1994, A. Krapovickas et al. 44793 (CTES); Am Wege in del Velha bei Blumenau, Oct.1888, E. H. G. Ule 1062 (US); Fazenda Farofa, trilha da estrada do meio, 6 Apr. 2007, R. P. M., Souza 103 (ESA); Pilões, Palhoça, 6 Apr. 1956, R. Reitz et al. 2997 (US, NY); Santa Terezinha, Urubici, 7 Apr. 2009, M. Verdi et al. 1905 (IFFSC); São Bento do Sul, Rio Natal. Estrada rumo ao Xikavitska (Salto Seco), 19 Feb. 2011, F. S. Meyer 982 (UFRN); São Bento do Sul, Trilha do Parque 23 de September, 14 Dec. 2014, P. Schwirkowski 732 (FPS); Taió, Fazenda Tarumã, 18 Feb. 2010, A. Korte & A. Kniess 1821 (FURB); Três Barras, Guruvá, San Francisco do Sul, 7 Nov. 1957, R. Reitz et al. 5621 (NY, US); Urubici, Santa Terezinha, 7 Apr. 2009, M. Verdi et al. 1905 (CTES); São Paulo: Barra do Turvo, 24 Mar. 2005, M. Carboni, 110 (ESA); 10 km de Barra do Turvo em direção a Pariquera-Açu, 14 Feb. 1995, J. P. Souza, et al. 96 (SP); Boracéia, 26 Mar. 1940, N.G. Blanco s. n. (SP); Campinas, Lago próximo ao parque ecológico da UNICAMP, 1 Jun. 1995, L. Y. S Aona & A. D. Faria 95/50 (SP); Campos das Sete Lagôas, Fazenda Campininha, just north of Rio Mogi-Guaçu 1,8 km NW of Pádua Sales, Mogi Guaçu, 4 Dec. 1961, G. Eiten 3517 (SP); Cananéia, Serra do Tambor, Vale do Ribeira, sul do Estado de São Paulo, 20 Nov. 2006, M. A Pinho-Ferreira et al. 673 (UEC); Cunha, Trilha do Rio Bonito, Parque Estadual da Serra do Mar, 19 Mar. 1996, A. Rapini et al. 73 (UEC); Cunha, Parque Estadual da Serra do Mar, Núcleo Cunha, 19 Mar. 1996, A. Rapini, et al. 73 (SP); Estação Biológica, Alto da Serra, 800–900 m, 6 Mar. 1929, A. Smith 2076 (BA, NY); Estação Experimental, área nativa, Pariquera-Açu, 2 Apr. 1997, R. B. Torres et al. 182 (IAC); Eldorado, May. 2012, A. Oriani, et al. 450 (ESA); Eldorado Paulista, P.E. Jacupiranga, Núcleo, Caverna do Diabo, Ilha da Caverna, 24°38′91″S, 48°23′31″W, 9 Feb. 1995, Leitão Filho et al. 32980 (UEC); Estação Visconde do Rio Claro, 12 Dec. 1888, A.C.G.G. Loefgren 1220 (SP); Ilha do Cardoso, Jacareu, forest and mangrove swamp, 8 Sep. 1976, P.H. Davis et al. 60747 (UEC); Itapetininga, 9 Feb. 1976, H. F. Leitão Filho et al. 1630 (UEC); Itirapina, Ipiranga, 23 Mar. 1906, A. Usteri s.n. (SP); Juquiá Sitio Areia Dourada, 29 Nov. 1994, K. D. Barreto et al. 3290 (CTES); Paranapiacaba, 16 Jun. 1966, T.M. Pedersen 7795 (CTES, SI); Parque Estadual da Serra do Mar, Núcleo Curucutu, 13 Apr. 2001, L. D. Meireles et al. 151 (UEC); Pinheiros, 8 Jan. 193, A. Gehrt s.n. (IAC); Pindamonhangaba, Fazenda São Sebastião do Ribeirão Grande, noroeste do talhão 10, 22 Feb. 1996, S. A. Nicolau et al. 1051 (SP); Ponta da Praia, 22 Dec. 1938, E. Guimarães 5 (SP); Rio Claro, 12 Dec 1988, A.C.G.G. Loefgren 11782 (NY); Santo Amaro, Seminário do Espírito Santo, 20 Mar. 1943, L. Roth 10317 (IPA); São Francisco Xavier, Caminho para Cachoeira das Couves, 14 Apr. 1995, J.Y. Tamashiro et al. 902 ( UEC); São José dos Campos, Distrito de São Francisco Xavier, 14 Apr. 1995, J.Y. Tamashiro et al. 902 (SP); São Miguel Arcanjo, Parque Estadual Carlos Botelho, 20 Mar. 2002, S. Bortoleto et al. 31 (UEC); São Sebastião, 22 Apr. 2000, J. P. Souza et al. 3398 (UEC); Tapiraí, Reserva Particular da Votorantim, 26 Mar. 2013, C. B. Virillo et al. 8 (UEC).
Taxonomic notes—Until the present, Borreria pterophora has been considered as an imperfectly known but valid name, which is at present day also registered as an endemism of Rio de Janeiro state, Brazil (BFG, 2015), however the examination of the holotype deposited at PR revealed us that is a new synonym of Galianthe palustris.
Galianthe spicata (Miq.) Cabaña Fader & Dessein, comb. nov. Diodia spicata Miq., Stirp. Surinam. Select. 179–180, t. 52. 1850. Dasycephala spicata (Miq.) Benth. & Hook. f. ex B.D. Jacks., Gen. Pl. 2: 144. 1873. Borreria spicata (Miq.) Bacigalupo & E.L. Cabral, Opera Bot. Belg. 7: 307. 1996. Spermacoce spicata (Miq.) Delprete, J. Bot. Res. Inst. Texas 1(2): 1028. 2007. TYPE: SURINAM: Sylvarum prope Bergendaal, H.C. Focke s.n. (holotype U!, isotypes HAL0113849!, K000265575!).
Diodia denudata Standl., J. Wash. Acad. Sci. 15(5): 105. 1925. Type: PANAMÁ, on wet stream bank along the Río Tapia, near sea level, 24 Dec 1923, P. C. Standley 28123 (holotype: US01154022!).
Description
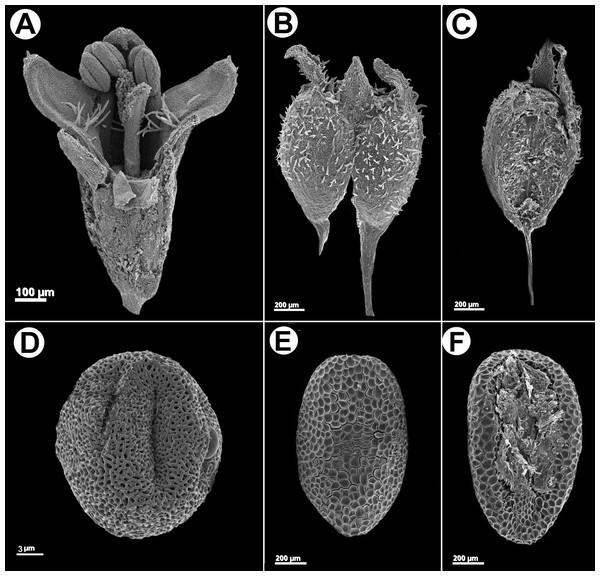
Herb or subshrub 80–140 cm alt., erect, stems simple to much branched. Stems quadrangular, fistulose, glabrous, angle weakly alate or without wings, glabrous. Leaves pseudopetiolate, pseudopetiole 0.5–2 mm long, blades elliptic or narrowly elliptic, papery or subcoriaceous when dry, adaxially glabrescent or scabridous, abaxially glabrous, only scabridous on nerves, base acute or cuneate, apex acute or acuminate, 30–110 × 10–33 mm; 5–7 secondary nerves, visible on both faces; stipular sheath 1.5–3 mm long, margin truncate or scarcely triangular, pilose, with 5–7 fimbriae, fimbriae 2–7 mm long, with some antrorse hairs. Inflorescences spiciform, partial inflorescences glomeriform, axillary, (5)10–25 per flowering branch, with 3–20 flowers, bracts foliaceous, decreasing in size towards the apex, sometimes up to the same size than the partial inflorescence. Flowers homostylous, calyx 4-lobed, hypanthium obconic, 0.55–6 mm long, puberulous, lobes 0.2–0.3 mm long, unequal, subtriangular, apex acute, margin ciliate; corolla subtubular, slightly expanded to the apex, 1–1.2 mm long, white or greenish white, sometimes with apex of lobes lilac, glabrescent outside, with a ring of moniliform hairs near insertion of the filaments, tube 0.5–0.7 mm long, lobes ovate, apex acute, internally with some scattered hairs, externally pilose and papillose, especially at the apex, 0.2–0.5 mm long, anthers 0.2–0.25 mm long, oblong, sometimes with a theca visibly smaller than the other, filament 0.15 mm long; pollen grains 7-zonocolpate, prolato-spheroidal, small, P = 30.3 µm, E = 28 µm, colpi long, endoaperture an endocingulum, tectum bireticulate, microreticulate, suprareticulum psilate, infrareticulum with muri nanospinose or psilate; stigma bifid, divided up to the half of its length, with papillae only in the internal face of the stigmatic branches, scarcely exerted. Fruit a capsule, 1.2–1.4 × 1–1.2 mm, longitudinally separated from the pedicel upwards up to median portion of the fruit, both mericarps remain attached to each other at the upper part, mericarps indehiscent, subglobose, ventral face flat, slightly laterally compressed, dorsal face pubescent, hispidulous or glabrescent; seeds 0.8–1 × 0.35–0.45 mm, oblong or ovate in outline, plane-convex, ventral face with a ample groove, dark brown or nigrescent; exotesta reticulate-foveate, cells polygonal, almost isodiametric. Figures 4 and 5. LSID: 77166462-1—Galianthe spicata.
Figure 4: Galianthe spicata.
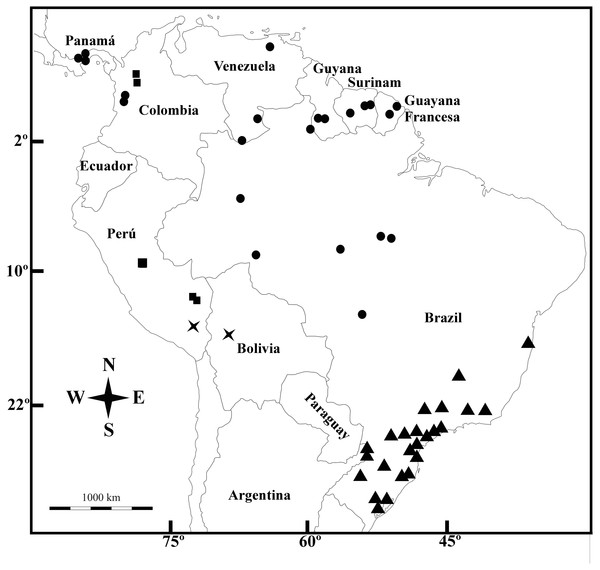
(A) Longitudinal section of flower. (B) Fruit with acropetal dehiscence. (C) Indehiscent valve. (D) Equatorial view of pollen grains showing exine with double reticulum. (E) Dorsal face of seed. (F) Ventral face of seed A: From P. G. Delprete 11876 (CAY), (B–D): from M. Sobral et al. 10020 (BHCB).Figure 5: Geographic distribution.
Galianthe boliviana (X), G. palustris (triangle), G. spicata (dots) and G. vasquezii (square).Distribution—Brazil (Amazonas, Roraima, Rondônia, Para, Mato Grosso), Colombia (Chocó), French Guaina, Panamá, Suriname, and Venezuela (Amazonas y Anzoátegui).
Ecology—Galianthe spicata grows inside or edges of humid forests.
Additional Specimens Examined—BRAZIL: Amazonas, vicinity of Camp Tucano, Rio Tucano, 3 Dec. 1965, B. Maguire et al. 60319 (IAN, MO); Rondonia, Porto Velho, along hwy 364 92 km, by road NE of junction with, 09°22′S 064°40′W, 20 Apr. 1987, H. M. Nee 34960 (MO); Roraima, Dormida, Serra do Lua, foothills of Serra da Lua, 13 Jan. 1969, G. T. Prance 9271 (MO); Pará, Conceição do Araguaia, near Corrego São João and Troncamento Santa Teresa, 8 Feb. 1980, T. C. Plowman 8524 (MO, NY); Altamira Gleba Curuaé, Jul. 2005. M. Sobral et al. 10020 (BHCB). COLOMBIA: Chocó, near Madurex Logging Campn above Teresita and below the rapids on Rio Truando, Feb. 1967, J. A. Duke 9977 (MO); ídem, logging road ca. 2–4 km NW of Teresita, 100 m, 18 May. 1967, J. A. Duke 11055 (MO). FRENCH GUIANA: Kamakusa, upper Mazaruni River, 23–29 Nov. 1922, J. S. de la Cruz 2808 (MO); Route de l’Est (N2), Montagne Maripa, c. 31 km S of the Comte bridge, c. selectively logged forest, 04°26′N, 52°20′W, 3 Dec. 1994, L. Andersson 1961 (MO).
GUYANA: Rupununi, Kanuku Mts., Crabwood Cr. Camp 2 forest, on brown loamy sand, 3°07′N, 59°06′W, 260 m, 2 Apr. 1994, M. J. Jansen-Jacobs et al. 3564 (MO); idem, E Kanuku Mts, NE of Warimure, in forest, 03°05′N 059°20′W, 200–500 m, 23 Jan. 1991, M. J. Jansen-Jacobs et al. 2189 (MO). PANAMA: Canal Area, Barro Colorado Island, 10–100 m, 9°09′17″N, 79°50′53″W, 16 Dec. 1967, T. B. Croat 4373 (MO); idem, 9°9′17″N, 79°50′53″W, 18 Mar. 1969, T. B. Croat 8738 (MO); idem, 9°9′0″N, 79°51′0″W, 1931, S. Aviles s.n. (MO); Cerro Azul, 700 m, 9°10′2″N, 79°24′59″W, 29 Jul. 1972, W.G. D’Arcy 6199 (MO). idem, 700 m, 9°10′13″N, 79°25′13″W, 7 Jun. 1970, A. Kant 46 (MO); Cerro Jefe, 1,000 m, 9°14′0″N, 79°22′0″W, 12 Sep. 1994, C. Galdames et al. 1604 (MO); Colón: Santa Rita, 9°20′0″N, 79°47′0″W, 6 Apr. 1969, W. H. Lewis et al. 5238 (MO); idem, 9°20′0″N, 79°47′0″W, 6 Apr. 1969, W. H. Lewis et al. 5238 (MO); idem, 9°20′0″N, 79°47′0″W, 6 Apr. 1969, W. H. Lewis et al. 5238 (MO); idem, 9°20′13″N 079°46′04″W, 31 Jan. 1971, T. B. Croat 13191 (MO); idem, 9°19′42″N, 79°47′27″W, 9 Jul. 1971, T. B. Croat & M. P. Duncan 15339 (MO); Gamboa Pipeline Road, 90 m, 9°9′36″N, 79°44′44″W, 9 Feb. 1974, M. H. Nee 9577 (MO); Pipeline Road, 50–100 m, 9°10′0″N, 79°46′0″W, 23 May. 1969, R. L. Lazor 3464 (MO). SURINAME: 1850, F.A.W. Miquel s.n. (K000265073). Brokopondo: Brownsberg Nature Park, Trail to Mazaroni Val. Primary forest, 04°56′N, 55°11′W, 400–450 m, 24 Jan. 1999, P.G. Delprete 7083 (MO); Marowijne: Nassau Mts, Plataeu C, lateritic rocky soil, 4°49′N, 54°36′W, 500–550 m, 26 Jan. 2003, M.J. Jansen-Jacobs et al. 6254 (MO). VENEZUELA: Amazonas, Atabapo, 5 km al Norte de la desembocadura del Rio Orinoco, 2°24′N, 64°24′W, 400 m, Oct. 1991, E. Marín 1678 (MO); Anzoátegui, Cabeceras del Morichas Largo, entre Santa Elena y San Pedro a unos 30 km Sur de la Viuda, 9 Nov. 1984, R. A. Montes 2524 (MO).
First record from Peru
Galianthe boliviana E.L. Cabral, Brittonia 57(2): 142, f. 1. 2005. TYPE. BOLIVIA: La Paz: Inqusivi, Cerro Aguada, 2,500–2,800 m, 22 Nov 1991, M. Lewis 40645 (holotype: LPB0000936!; isotype MO!).
Distribution— Sub-Andean foothills of Bolivia and Peru. Galianthe boliviana grows mainly on eroded slopes between 1,800 and 3,150 m of altitude, especially in open forest of Alnus acuminata Kunth. of Yungas at 2,800–3,000 m of altitude.
Taxonomic notes—Galianthe boliviana is similar to Galianthe dichasia and G. cymosa in having cymoidal inflorescences with subglomeriform partial inflorescence, but differs from these in possessing stems 20–30 cm tall, with smooth, glabrous, and narrowly winged angles.
Additional Specimens Examined—PERU: Cusco, Quispicanchis, Marcapata, 176 km from Cusco on road to Maldonado, Marcapata to Cocha, 8 Mar. 1991, 13°25′S 070°54′W, 3,150 m, Percy Núñez V. & C. Paycarmayta 13140 (MO).
Key to Galianthe species with indehiscent mericarps (modified from Cabral & Bacigalupo, 1997)
| 1. | Stipular sheath tubular, prolonged above the insertion of the corresponding pairs of leaves | 2 |
| 1′. | Stipular sheath truncate, never surpassing the insertion of the corresponding pairs of leaves | 3 |
| 2. | Stipular sheath pilose; stems with strongly alate angles; Brazil | G. vaginata E.L. Cabral & Bacigalupo |
| 2′. | Stipular sheath glabrous; stems without wings. Brazil | G. polygonoides E.L. Cabral & Bacigalupo |
| 3. | Leaves only with one nerve visible on abaxial face | 4 |
| 3′. | Leaves plicate nervose | 7 |
| 4. | Inflorescences pauciflorous, in lax cymoid, partial inflorescences 1-florous. Ecuador, Perù | G. dichotoma |
| 4′. | Inflorescences multiflorous, partial inflorescences multiflorous, in fascicles or glomeruli | 5 |
| 5. | Inflorescences cymoid, partial inflorescences glomeriform, calyx 4-lobed, pollen with reticulate exine. Colombia. | G. bogotensis (Kunth) E. L. Cabral & Bacigalupo |
| 5′. | Inflorescences thyrsoid, spiciform, or cymoid, partial inflorescences fasciculate, calyx 2-4-lobado, pollen grains with bireticulate exine | 6 |
| 6. | Inflorescences thyrsoid-spiciform or cymoid, primary axis shorter than the laterals, calyx 2 (-4) lobed, corolla of long-styled flowers with a fringe of hairs from apex of anthers to base of tube; pollen grains 6–7 zonocolpate, both reticula complete, fruit 2–3 times wider than long. Argentina, SE, and S Brazil, Paraguay, and Uruguay | G. brasiliensis (Spreng.) E.L. Cabral & Bacigalupo |
| 6′. | Inflorescences thyrsoid-spiciform, primary axis longer than the laterals, calyx always 4-lobed, corolla of the long-styled flowers with ring of hairs, pollen grains 8–10 zonocolpate, suprareticulum incomplete, fruit as long as wide, , Mesoamerica | G. angulata (Benth.) Borhidi |
| 7. | Partial inflorescences congested, glomeriform or subglomeriform | 8 |
| 7′. | Partial inflorescences pauciflorous, fasciculate | 13 |
| 8. | Inflorescences spiciform, con 5–20 partial inflorescences per flowering branch. Brazil, Colombia, French Guiana, Panama, Surinam, and Venezuela | G. spicata |
| 8′. | Inflorescences thyrsoid or cymoid, with 3–5 partial inflorescences per flowering | 9 |
| 9. | Stems notoriously alate | 10 |
| 9′. | Stems obscurely alate | 12 |
| 10. | Inflorescences thyrsoid or with a simple axis, partial inflorescences glomeriform, flowers homostylous, calyx 2–3 lobed, corolla 2–3 lobed | 11 |
| 10′. | Inflorescences cymoid, partial inflorescences subglomeriform, flowers distylous, calyx 4-lobed, corolla 4-lobed, Argentina, Brazil, Paraguay, and Uruguay | G. dichasia (Sucre & C.G. Costa) E.L. Cabral |
| 11. | Calyx lobes 1–1.4 mm long, with acute apex, corolla 1.75–2.1 mm long, corolla lobes internally with hairs scattered at base, tube internally with some dispersed hairs near its base, pollen grains with reticulate exine, muri nanospinose, fruit 1.8–2. mm long, deltoid in outline, acropetally dehiscent, seeds 1.8–2 mm long | G. vasquezii R.M. Salas & J. Florentín |
| 11′. | Calyx lobes 0.4–0.6 mm long, obtuse, corolla 1–1.5 mm long, internally glabrous, pollen grains with bireticulate exine, suprareticulum psilate and incomplete, infrareticulum nanospinose, fruit 1.1–1.5 mm long, oblong or obovate in outline, basipetally dehiscent, seeds 1–1.42 mm long | G. palustris (Cham. & Schltdl.) Cabaña Fader & E. L. Cabral |
| 12. | Stems retrorse-scabridous on angles, leaves 1–7 mm lat. Brazil | G. cymosa (Cham.) E.L. Cabral & Bacigalupo |
| 12′. | Stems glabrous, leaves 7–12 mm lat. Bolivia and Perú | G. boliviana E.L. Cabral |
| 13. | Stems scarcely branched; fruit sub-hemispherical, 1.6–2 mm long; Brazil, Paraguay, and Argentina | G. hispidula (A. Rich. ex DC.) E.L. Cabral & Bacigalupo |
| 13′. | Stems much branched; fruit turbinate, 5 mm long; Brazil | G. humilis E.L. Cabral & Bacigalupo |
Discussion
Galianthe palustris and G. spicata share the same taxonomic and nomenclatural history. First, they were described under Diodia, later they were added to genus Borreria (Bacigalupo & Cabral, 1996; Bacigalupo & Cabral, 1998) due to the presence of homostylous flowers and type of fruit. Later they were, transferred to the genus Spermacoce (Delprete, Smith & Klein, 2005; Delprete, 2007). In 1998, Bacigalupo & Cabral (1998) observed that G. palustris (then still Borreria palustris) is characterized by a thyrsoid inflorescence that is similar to that of Galianthe. Despite this remarkable observation, the authors decided to transfer the species to genus Borreria. Nearly a decade later, Delprete, Smith & Klein (2005) and Delprete (2007) transferred both species to Spermacoce in an attempt to create a broad genus concept for Spermacoce.
Despite overall molecular evidence, Galianthe spicata and G. palustris also share similar morphological characteristics with the other Galianthe species (e.g., spiciform and thyrsoid inflorescences, a bifid stigma and pollen grains with a double reticulum). This last character appears in most species of Galianthe, except for G. bogotensis (Kunth) E.L. Cabral & Bacigalupo, G. dichotoma (Willd. ex Roem. & Schult.) E. L. Cabral & Bacigalupo, and the new species G. vasquezii, which have simple reticulum. Pire (1997) hypothesized that in a genus mainly represented by species with double reticulum pollen grains, the simple reticulum is the result of the absence of an infrareticulum persisting only a suprareticulum.
Current molecular data indicates that the phylogenetic position of Diodia palustris (Galianthe palustris) and D. spicata (G. spicata) make Galianthe paraphyletic. The Galianthe clade, including both former Diodia species, is strongly supported and has two molecularly well-defined clades. The (Diodia palustris + D. spicata) + G. brasiliensis clade is composed only by species with capsules separating into two indehiscent mericarps and which is a diagnostic character of Galianthe subgen. Ebelia. The sister clade, [G. eupatorioides + G. grandifolia ] + G. peruviana, includes species of Galianthe subgen. Galianthe, and is characterized by fruits with dehiscent valves. Both morphological and molecular data support the transfer of two former Diodia species to Galianthe, and more specifically in subgen. Ebelia. Additionally, and according to present sampling, the two subgenera described by Cabral & Bacigalupo (1997) seem to be monophyletic. The transfer of Diodia spicata to Galianthe was originally proposed by Dessein (2003), based on fruit, polynological and molecular features.
Even though morphological and molecular data show that three species share several characteristics with Galianthe subgen. Ebelia, there is a significant difference with the other species of the subgenus. The three species, unlike the remainder, have homostylous flowers. As a result, these results demonstrate the presence of a new floral trait in Galianthe and therefore strongly modify the generic concept of the genus.
According to Groeninckx et al. (2009), distyly is often related with double reticulum pollen grains in the tribe Spermacoce. Nevertheless, in the genus Galianthe there are some exceptions to this generalization (e.g., G. bogotensis (distyly and simple reticulum), G. spicata and G. vasquezii (homostyly and double reticulum), and G. palustris (homostyly and simple reticulum). Cabral & Bacigalupo (1997) mentioned that G. dichotoma presents an intermediate state between distyly/homostyly and pollen with simple reticulum. The authors defined this phenomenon as an “unclear dimorphism” (in Spanish “dimorfismo poco manifiesto”). Future studies are necessary in order to clearly define the floral morphs that are present in these species.