A new species of Scutellaria (Scutellarioideae, Lamiaceae) from Sichuan Province in southwest China
- Published
- Accepted
- Received
- Academic Editor
- Charlotte Lindqvist
- Subject Areas
- Biodiversity, Plant Science, Taxonomy
- Keywords
- China, Endemisim, Morphology, Scutellaria, SEM observation, Taxonomy
- Copyright
- © 2017 Zhao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. A new species of Scutellaria (Scutellarioideae, Lamiaceae) from Sichuan Province in southwest China. PeerJ 5:e3624 https://doi.org/10.7717/peerj.3624
Abstract
Scutellaria wuana, a new species discovered from a xeric valley in Muli County of Sichuan Province in southwest China, is described and illustrated. Morphologically, the new species shares similarities with S. mairei, but can be readily distinguished by a suite of morphological characters including a white-pubescent erect stem, conspicuous leaf petioles, and a yellow corolla with a trapeziform lower-middle lip lobe. The habitat and distribution of S. wuana are also distinctive. The position of the new species within Scutellaria is examined in a phylogenetic context using the nuclear ribosomal internal and external transcribed spacers. Additionally, we examine leaf epidermal and pollen grain micromorphology of the new species and putative relatives.
Introduction
The subfamily Scutellarioideae, as presently circumscribed, consists of five genera and about 380 species: Holmskioldia Retz., Renschia Vatke, Scutellaria L., Tinnea Kotschy ex Hook. f., and Wenchengia C.Y. Wu & S. Chow (Harley et al., 2004; Li et al., 2012; Li et al., 2016). With approximately 360 species (Paton, Suddee & Bongcheewin, 2016), Scutellaria is one of the largest genera within Lamiaceae. Although Scutellaria is primarily distributed in temperate regions and on tropical mountains (Paton, 1990a; Paton, 1990b), the genus can be found in most regions of the world except the Amazon basin, lowland tropical Africa, and Pacific islands (Paton, Suddee & Bongcheewin, 2016). China is perhaps the center of diversity of Scutellaria, with 101 species and 25 varieties reported (Li & Hedge, 1994; Zhou & Guo, 2001; Xiao & Wu, 2003; Hsieh, 2013; Xiang, 2016). Forty-six species and 11 varieties occur in southwest China (Wu & Li, 1977), with 10 taxa endemic to Sichuan (Li, 1992).
Scutellaria species are annual or perennial herbs or subshrubs that have various growth forms and habitats (Epling, 1942). The stems are generally quadrangular and covered with glandular or non-glandular hairs. Leaves are typically simple and polytropic in shape, but taxa within sect. Lupulinaria possess pinnatifid leaves. Flowers are always solitary in the axils of +/−diminished leaves or bracts. The calyx is 2-lipped, and the posterior lip is usually folded to produce a scutellum, which is a unique character of the genus. Based on inflorescence and bract characters, Paton (1990a) divided the genus into two subgenera: subgen. Scutellaria Briquet and subgen. Apeltanthus (Nevski ex Juz.) Juz. The former is characterized by having a one-sided inflorescence and flowers subtended by leaves or leaf-like bracts, while the latter has a four-sided inflorescence, with decussate flowers subtended by cucullate bracts. The subgenus Scutellaria was further divided into five sections (i.e., sect. Scutellaria, sect. Anaspis (Rech.f.) Paton, sect. Perilomia (Kunth) Epling, sect. Salazaria (Torrey) Paton, and sect. Salviifoliae (Boiss.) J.R.Edm.), and the subgenus Apeltanthus consists of two sections, i.e., sect. Apeltanthus and sect. Lupulinaria A. Hamilton. This infrageneric classification proposed by Paton (1990a) is the most comprehensive taxonomic treatment of the genus Scutellaria, but only 13 species from China were included. Thus, it is not possible to employ his divisions for the classification of the genus in China. In the updated edition of Lamiaceae in Flora of China, Li & Hedge (1994) divided Scutellaria (98 spp.) into seven groups on the basis of inflorescence, bract, calyx, and nutlet morphology.
During field trips to Sichuan Province in southwest China in July of 2011 and August of 2015, we encountered a noteworthy Scutellaria population in Muli County. However, we were unable to key our collections according to the descriptions from Flora Sichuanica (Li, 1992), Flora Reipublicae Popularis Sinicae (Wu & Li, 1977) and Flora of China (Li & Hedge, 1994). After carefully examining the specimens, we concluded that this population represents a new species of Scutellaria and henceforth describe and illustrate the new species.
Materials & Methods
The morphological study is based on our field collections and herbarium specimens. Additionally, protologues of all published names in the genus Scutellaria from China and adjacent areasas well as related taxonomic literature (Wu & Li, 1977; Li & Hedge, 1994; Zhou & Guo, 2001; Xiao & Wu, 2003; Hsieh, 2013; Paton, Suddee & Bongcheewin, 2016) were assembled and reviewed. To verify morphological differences among the potential new species and other morphologically similar congeners, herbarium material (including types) from CDBI, E, IBK, IBSC, K, KUN, L, PE, SZ were examined (acronyms follow Thiers, 2016). After careful investigation of specimens, we deemed the potential new species to be most similar to S. mairei H. Lév., which guided us in further morphological comparisons.
Leaf materials for scanning electron microscopy (SEM) and light microcopy (LM) were taken from our collections (C.L. Xiang 1200 and/or E.D. Liu et al. 2902) of the new species and herbarium specimens of S. mairei H. Lév. (M.Fr. Ducloux 6438). Methods for examining leaf epidermal microfeatures of both the new and closely related species followed Xiang et al. (2010). Size measurements of 25 pollen grains were made under a Leica DM2500 light microscope (Leica Microsystems GmbH, Wetzlar, Germany). The pollen samples were prepared for the SEM as described by Xiang et al. (2013).
| Taxon | Voucher/Herbarium barcode | Location | Genbank accession number | |
|---|---|---|---|---|
| ITS | ETS | |||
| Scutellaria discolor | Xiang CL et al., 438 (KUN) | Yunnan, China | MF193504 | MF193550 |
| Scutellaria hainanensis | Jiang L et al., 398 (KUN) | Hainan, China | MF193505 | MF193551 |
| Scutellaria yunnanensis var. yunnanensis | Liu Ed et al., 3037 (KUN) | Yunnan, China | MF193506 | MF193552 |
| Scutellaria yunnanensis var. cuneata | Xiang CL et al., 574 (KUN) | Yunnan, China | MF193507 | MF193553 |
| Scutellaria obtusifolia | Chen YP et al., EM202 (KUN) | Sichuan, China | MF193508 | MF193554 |
| Scutellaria sichourensis | Xiang CL et al., 566 (KUN) | Yunnan, China | MF193509 | MF193555 |
| Scutellaria wenshanensis | Zhao F et al., 008 (KUN) | Yunnan, China | MF193510 | MF193556 |
| Scutellaria yangbiense | Liu ED et al., 2238 (KUN) | Yunnan, China | MF193511 | MF193557 |
| Scutellaria calcarata | Shui YM et al., Z-03343396 (KUN) | Yunnan, China | MF193512 | MF193558 |
| Scutellaria indica | Peng H, s.n (KUN) | Hongkong, China | MF193513 | MF193559 |
| Scutellaria indica fo. parvifolia | Anonymous,554 (KUN) | Shimoda, Japan | MF193514 | MF193560 |
| Scutellaria taiwanensis | Liao PC, s.n. (KUN) | Taiwan, China | MF193515 | MF193561 |
| Scutellaria mairei | Shui YM et al., 66205 (KUN) | Yunnan, China | MF193516 | MF193562 |
| Scutellaria tenax | Peng H et al., 2012-017 (KUN) | Guizhou, China | MF193517 | MF193563 |
| Scutellaria tapintzeensis _1 | Cai J et al., 15cs11358 (KUN) | Yunnan, China | MF193518 | MF193564 |
| Scutellaria tapintzeensis _2 | Cai J et al., 15cs11371 (KUN) | Yunnan, China | MF193519 | MF193565 |
| Scutellaria teniana | Xiang CL et al., 288 (KUN) | Yunnan, China | MF193520 | MF193566 |
| Scutellaria wuana sp. nov. | Xiang CL et al., 1200 (KUN) | Sichuan, China | MF193521 | MF193567 |
| Scutellaria tenera | Chen YP et al., EM187 (KUN) | Jiangxi, China | MF193522 | MF193568 |
| Scutellaria macrodonta | Zhao F et al., 2015-006 (KUN) | Beijing, China | MF193523 | MF193569 |
| Scutellaria likiangensis | Xiang CL et al., 373 (KUN) | Yunnan, China | MF193524 | MF193570 |
| Scutellaria baicalensis | Li DZ et al., 0513 (KUN) | Liaoning, China | MF193525 | MF193571 |
| Scutellaria viscidula | Zhao F, 2015-009 (KUN) | Hebei, China | MF193526 | MF193572 |
| Scutellaria orthocalyx | Xiang CL, 185 (KUN) | Yunnan, China | MF193527 | MF193573 |
| Scutellaria subintegra | Chen YP, EM223 (KUN) | Guangxi, China | MF193528 | MF193574 |
| Scutellaria axilliflora | Hu GX, H144 (KUN) | Fujian, China | MF193529 | MF193575 |
| Scutellaria shweliensis | Zhao F et al., ZF0068 (KUN) | Yunnan, China | MF193530 | MF193576 |
| Scutellaria hunanensis | Hu GX, H96 (KUN) | Hunan, China | MF193531 | MF193577 |
| Scutellaria franchetiana | Xiang CL, 287 (KUN) | Yunan, China | MF193532 | MF193578 |
| Scutellaria sessilifolia | Xiang CLi, 341 (KUN) | Chongqing, China | MF193533 | MF193579 |
| Scutellaria sessilifolia | Peng H et al., 117 (KUN) | Sichuan, China | MF193534 | MF193580 |
| Scutellaria galericulata | M-14212 | Iran | MF193535 | MF193581 |
| Scutellaria regeliana | Jiang L, 149 (KUN) | Neimenggu, China | MF193536 | MF193582 |
| Scutellaria dependens | Anonymous, 565 | Fujinomiya, Japan | MF193537 | MF193583 |
| Scutellaria dependens | Anonymous, 316 | Fujinomiya, Japan | MF193538 | MF193584 |
| Scutellaria barbata | Xiang CL, 282 (KUN) | Beijing, China | MF193539 | MF193585 |
| Scutellaria scordifolia | Yu WT et al., 2822 (KUN) | Qinhai, China | MF193540 | MF193586 |
| Scutellaria diffusa | Wang ZH, s.n(KUN) | Germany | MF193541 | MF193587 |
| Scutellaria kingiana | Zhang JW et al., ZJW-3890 (KUN) | Xizang, China | MF193542 | MF193588 |
| Scutellaria nuristanica | M-32142 | Iran | – | MF193589 |
| Scutellaria stocksii | M-30348 | Iran | MF193543 | MF193590 |
| Scutellaria alpina | Liao PC, s.n. | Europe alpine region | MF193544 | MF193591 |
| Scutellaria nepetifolia | TUH-27605 (THU) | Iran | MF193545 | MF193592 |
| Scutellaria platystegia | TUH-7697(THU) | Iran | MF193546 | MF193593 |
| Scutellaria supina | LiuB et al., CPG28095 (PE) | Xinjiang, China | MF193547 | MF193594 |
| Holmskioldia sanguinea | Anonymous, 209 | Guandong, China | MF193548 | MF193595 |
| Tinnea rhodesiana | Gary Stafford, GIS-359 (KUN) | Pietermaritzburg, South Africa | MF193549 | MF193596 |
For the molecular phylogenetic studies, the ingroup consisted of 45 samples (41 taxa) representing the two subgenera defined by Paton (1990a). Holmskioldia sanguinea Retz. and Tinnea rhodesiana S. Moore were used as the outgroup based on our previous studies (Li et al., 2016). Voucher information and GenBank numbers are provided in Table 1.
Total DNA was extracted from 0.3 g of silica-gel-dried leaves or from leaf fragments of herbarium specimens using a modified CTAB protocol by Soltis et al. (1991). The primer pair ITS4 and ITS5 (White et al., 1990) was used to amplify the entire nrITS region, and primers ETS-B from Beardsley & Olmstead (2002) and 18S-IGS from Baldwin & Markos (1998) were used to amplified the ETS region. The PCR reaction mixtures and program follow Chen et al. (2005).
Sequencing reactions were performed with the dideoxy chain termination method running on an ABI PRISM3730 automated sequencer. The same primers described above for PCR were used for the sequencing reactions. Sequences were assembled and edited with SeqMan (DNASTAR, Madison, Wisconsin, USA; Burland, 2000). Initial automated alignments of nrITS and ETS sequences were made using MEGA version7.0 (Kumar, Stecher & Tamura, 2016) and subsequently adjusted manually based on the similarity criterion in PhyDE v.0.997 (http://www.phyde.de/index.html). Gaps were treated as missing data. Finally, the matrices were concatenated in Phyutility v.2.2 (Smith & Dunn, 2008) and phylogenetic analyses conducted using Maximum likelihood (ML) and Bayesian inference (BI) methods. ML analyses were performed using RAxML HPC2 v.8.2.10 (Stamatakis, 2014), on the CIPRES Science Gateway v.3.3 (Miller, Pfeiffer & Schwartz, 2010). A partitioned model (-q) was selected, and 1,000 bootstrap iterations (-# — -N) were conducted, with other parameters using the default settings. BI analyses were implemented using the program MrBayes v.3.2.2 (Ronquist et al., 2012). The best-fit nucleotide substitution model (GTR +I +G) selected by Akaike information criterion (AIC) in Modeltest v.3.7 (Posada & Crandall, 1998). The Markov chain Monte Carlo (MCMC) algorithm was run for 10,000,000 generations with four chains, and trees were sampled every 100 generations. The first 25% of sampled generations were discarded as burnin, and a 50% majority-rule consensus tree was obtained using the remaining trees.
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants (ICN), and hence the new name contained in the electronic version are effectively published under that Code from the electronic edition alone. Additionally, the new name contained in this work has been issued with identifiers by IPNIand will eventually be made available to the Global Name Index. The IPNI LSIDs can be resolved and the associated information viewed through any standard web browser by appending the LSID contained in this publication to the prefix “http://ipni.org/.” The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Results
Morphological studies
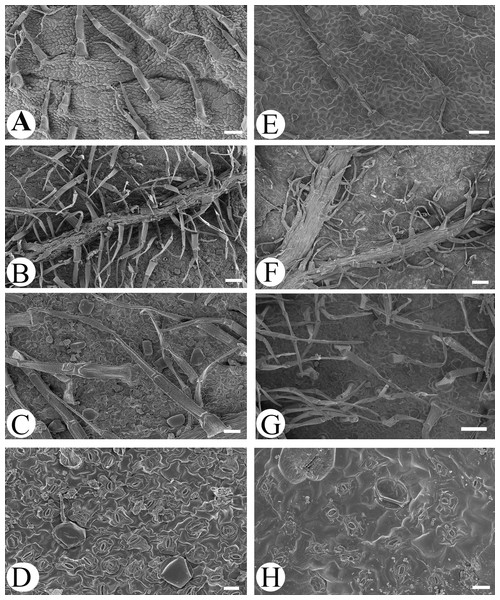
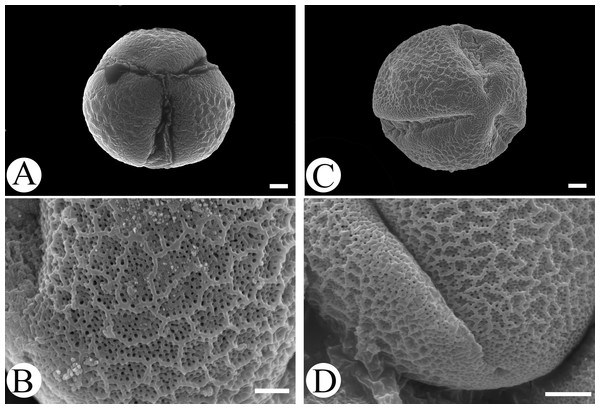
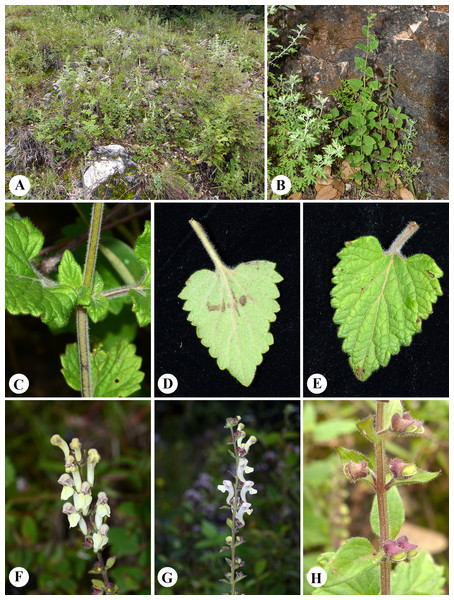
Microfeatures of the leaf epidermis of the new species (Figs. 1A–1D) and Scutellaria mairei (Figs. 1E–1H) are shown in Fig. 1. Epidermal cells for both species were polygonal to slightly irregular in shape (Figs. 1A, 1E). Leaf surfaces were all hypostomatic, and anomocytic stomata were present in the abaxial epidermis (Figs. 1D, 1H). Glandular and non-glandular trichomes were found. The capitate glandular trichomes can only be found on the abaxial surface in S. wuana (Figs. 1C, 1D), but on both surfaces in S. mairei (Figs. 1E, 1G). The multicellular non-glandular trichomes were present on both surfaces (Figs. 1A, 1C, 1E, 1G), and densely distributed along the midrib and veins on the abaxial surface (Figs. 1B, 1F). The pollen grains of S. wuana (Figs. 2A–2B) and S. mairei (Figs. 2C–2D) are tricolpate, with a circular outline in polar view (Figs. 2A, 2C). The exine sculpturing in both species possess bireticulate perforations (Figs. 2B–2D).
Molecular systematics
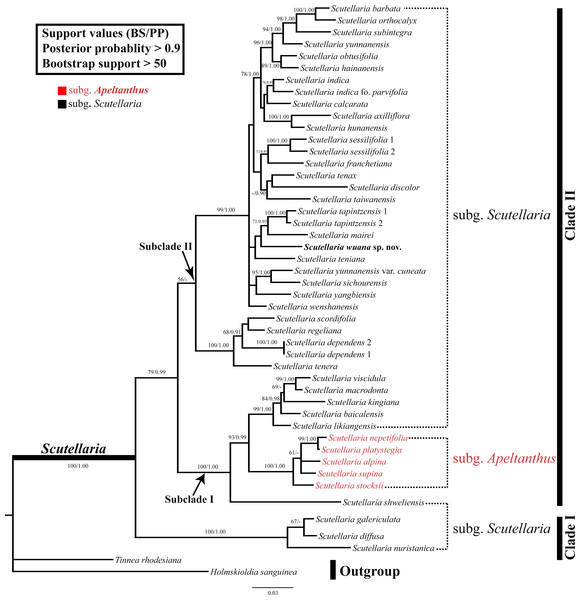
The combined nrDNA dataset contained 1,110 positions, of which 332 characters were parsimony-informative characters. The topologies were congruent between the trees obtained in the ML and BI analyses, and thus only the BI topology tree is provided (Fig. 3). In both analyses, the monophyly of the Scutellaria is strongly supported (ML-BS = 100%, BI-PP = 1.00; all values follow this order hereafter) and consists of two main clades (Fig. 3). Clade I (100%, 1.00) comprises three species, Scutellaria galericulata L., S. diffusa Benth., and S. nuristanica Rech.f. Clade II (78%, 0.99) consists of the remaining species and two subclades can be identified. Subclade I (100%, 1.00) contains S. shweliensis W.W. Sm., S. stocksii Boiss., S. alpina L., S. supina L., S. nepetifolia Benth., S. platystegia Juz., S. likiangensis Diels, S. baicalensis Georgi, S. kingiana Prain, S. viscidula Bunge, and S. macrodonta Hand.-Mazz. It is noteworthy that within this subclade, five species from subgen. Apeltanthus formed a clade with moderate support values (65%, 0.91). Subclade II (57%, −) contains the remainder of Scutellaria and is shown as a polytomy. The new species we described here is a member of this subclade, and grouped with S. teniana Hand.-Mazz. S. mairei, and S. tapintzensis C.Y. Wu & H.W. Li., but with weak support.
Figure 1: Scanning electron micrographs of leaf epidermis of Scutellaria wuana (A–D), S. mairei (E–H).
(A) Non-glandular trichomes on adaxial surface in S. wuana. (I) Capitate glandular trichomes mixed with non-glandular on adaxial surface in S. mairei. (B, F) Non-glandular trichomes and capitate glandular trichomes distribute along the veins. (C, G) Non-glandular trichomes and capitate glandular trichomes on abaxial surface. (D, H) Stomata on abaxial surface. Scale bars = 100 μm (A, B, E, F, G), 40 μm (C), 20 μm (D, H).Figure 2: Scanning electron micrographs of pollen grains for Scutellaria wuana (A, B) and S. mairei (C, D).
(A, C) Pollen grain (polar view), (B, D) exine surface. Scale bars = 2 μm (A–C), 1.5 μm (D).Figure 3: BI tree inferred from the combined dataset of nrITS and ETS sequences.
With support values (BS/PP) indicated at branches. The outgroup and recognized clades summarized by the right bars.Taxonomic treatment
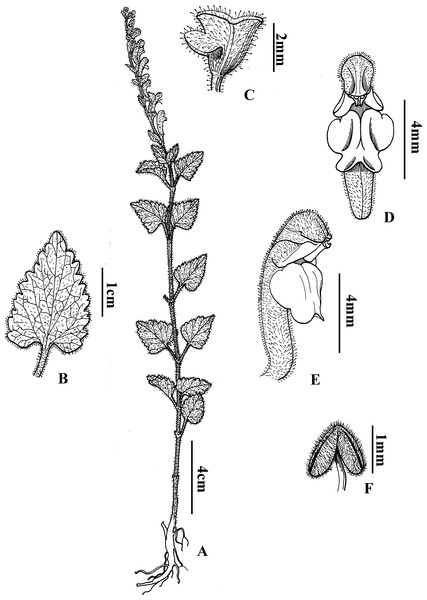
Scutellaria wuana C.L. Xiang & F. Zhao, sp. nov. (Figs. 4 and 5)
Figure 4: Diagnostic morphologcial features of Scutellaria wuana C. L. Xiang & F. Zhao (C.L. Xiang 1200) (Holotype).
(A) Habit. (B) Leaf blade. (C) Calyx. (D) Flower in frontal view. (E) Flower in lateral view. (F) Anther. Drawing by Lin Wang.Figure 5: Photographs of Scutellaria wuana in the wild.
(A) Plant habitat. (B) Habitat. (C) Stem. (D) Abaxial surface of leaf. (E) Adaxial surface of leaf. (F) Inflorescence in frontal view. (G) Inflorescence in lateral view. (H) Fruiting calyces. Photographs by CL Xiang.Type
CHINA. Sichuan Province: Muli County, Xiamaidi Village, in rock-crevice in dry and warm valley, elevation 3,080 m, 27°43′11.5″N, 101°14′07.4″E, 25 August 2015, C.L. Xiang 1200 (Holotype KUN!, isotypes PE!, KUN!).
Diagnosis
Scutellaria wuana is similar to S. mairei H. Lév. (Léveillé, 1912) but differs by having an erect stem with white pubescent hairs (vs. suberect stem with gray to yellowish hirsute hairs in S. mairei), a leaf petiole 1–1.5 cm long (vs. 1.5–3 mm in S. mairei), a yellow corolla tube with a pink spot on the galea (vs. limp rose corolla in S. mairei), the median lobe of the lower lip trapeziform (vs. broadly ovate in S. mairei), and an arcuate corolla tube base (vs. slightly saccate in S. mairei).
Description
Perennial herb. Rhizome slender and densely fibrous. Stems 20–60 cm tall, erect, densely white pilose, glandular pubsecent on angles, apically few-branched. Leaves papery, lamina triangular to triangular-cordate, 15–38 mm long, 10–15 mm wide, base broadly cuneate to cordate, margin crenate-serrate, apex obtuse, adaxially sparsely pilose (Fig. 1A), abaxially densely white hirtellous; midrib and veins pubescent (Fig. 1B); petiole 1–1.5 cm. One sided racemes terminal or terminal in axillary branches, 8–14 cm long; bracts sessile, rhombic-ovate, 2–5 mm, leaf-like basally, margin glandular puberulent. Pedicel ca. 2.5 mm, densely white glandular puberulent. Calyx ca. 2 mm, elongate to 5 mm in fruit, glandular puberulent outside; scutellum ca. 1 mm, elongate to 3 mm in fruit. Corolla tube yellow with pink spot on galea, 12–15 mm, densely white glandular puberulent outside, glabrous inside; tube zigzag, ca. 1 cm long, base arcuate; throat ca. 6 mm wide; upper lip galeate, lower lip 3-lobed, median lobe trapeziform, apex emarginate, lateral lobes oblong-ovate, ca. 4 mm wide. Nutlets unseen. Fl. Jul–Aug.
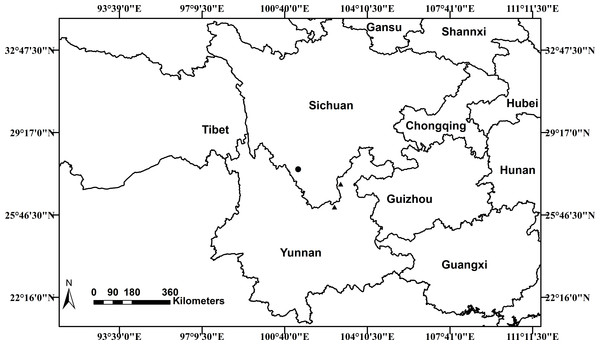
Distribution and Habitat
Scutellaria wuana is currently only known from Muli County, Sichuan Province, in southwest China. Based on the information from our own collections and herbarium specimens, a distribution map of S. wuana (solid circle) and S. mairei (triangle) is shown in (Fig. 6). Scutellaria wuana is found in vegetation mainly composed of herbaceous and shrub by plants such as Boenninghausenia albiflora (Hook.) Meisn., Campanula colorata Wall., Hypericum monogynum L., Origanum vulgare L., Salvia castanea Diels and Artemisia sp., etc. More fieldwork is needed to further elucidate its habitat and distribution area.
Figure 6: Distribution map of Scutellaria wuana (•) S. mairei (▴) in China.
Etymology
The epithet of the new species is named in honor of Professor Cheng-Yi Wu (Zheng-Yi Wu; 1916–2013), a prominent botanist from Kunming Institute of Botany, Chinese Academy of Sciences, to honor his great contributions to the knowledge of the family Lamiaceae in China and his significant work on the flora and vegetation of China for over 70 years.
Additional specimens examined
Scutellaria wuana C.L. Xiang & F. Zhao CHINA. Sichuan: Muli Couunty, Xiamaidi Villlage, 26 July 2011, E.D. Liu et al. 2902. (KUN!) (Paratype).
Scutellaria mairei H. Lév. CHINA. Yunnan: Qiaojia County, 1913, E.E. Marie. 510 (K!); 22 July 1909, F. Ducloux 6438 (KUN!); 2 July 2004, H. Wang et al. 03-1469 (KUN!); Luquan County, 4 August 2008, H. Peng 9620 (KUN!).
Discussion
The monophyly of the genus Scutellaria was confirmed in the present study (Fig. 3), as reported by previous molecular phylogenetic studies (Li et al., 2012; Li et al., 2016; Xiang et al., 2013; Chen et al., 2014; Chen et al., 2016). However, the subgenus Scutellaria defined by Paton (1990a) is non-monophyletic with members of the subgenus scattered in various clades. In addition, all the sections and series defined by Wu & Li (1977) and all groups defined by Li & Hedge (1994) are also not monophyletic. The subgenus Apeltanthus circumscribed by Paton (1990a) formed a small subclade and it is probably monophyletic (subclade ‘Apeltanthus’), but only five species were selected in this study, and future studies including more species from different regions are needed to test the monophyly of this subgenus. The taxonomic level and/or circumscription of the subgen. Apeltanthus should be reconsidered, because subclade Apeltanthus is embedded within subgenus Scutellaria. Future studies of morphological characters in concert with molecular data may provide better evidence for relationships among Scutellaria species, and could clarify the infrageneric classification of the genus.
As shown in (Fig. 3), Scutellaria wuana is a member of subgenus Scutellaria. Since Scutellaria wuana typically has a one-sided inflorescence that is composed of secund flowers and leaf-like bracts, it should be placed in the sect. Scutellaria. However, because all the sections are not monophyletic based on our molecular studies, it is unreasonable to place the new species based on this artificial infrageneric classification. Because S. wuana has a zigzag corolla tube and the leaf margin is denticulate to dentate, we speculate that it has a close relationship with the group defined by Li & Hedge (1994) that includes S. mairei, S. tenax W.W. Sm., S. teniana, and S. tapintzenisis. After careful comparison of the specimens of those species in combination with the results from our molecular phylogenetic study, we conclude that S. wuana is most closely related to S. mairei. Morphologically, both species are perennial herbs with slender rhizomes, triangular leaves with denticulate to dentate margins, and a zigzag corolla tube. However, S. wuana can be readily distinguished from S. mariei by several characters. Additionally, the distribution and habitat are different in the two species. Scutellaria wuana is currently only known from Muli County, Sichuan Province, and grow in rock-crevices within a xeric valley, while S. mairei is only found on dry limestone mountains in northeast Yunnan.The external morphology of S. wuana is more or less comparable with S. tenax; both species are perennial herbs and have triangular leaves and a zigzag corolla tube. However, there are several characters that distinguish S. wuana from S. tenax. Detailed comparisons of the morphology, habitat, and distribution of S. wuana, S. tenax and S. mairei are given in Table 2.
| Characters | S. mairei | S. wuana | S. tenax |
|---|---|---|---|
| Stem | suberect, 16–22 cm tall, with dense yellow long-hirsute hairs | erect, 20–80 cm,tall, densely with white pubescence | erect, ca 60 cm tall, with white pubescence |
| Lamina | papery, petiole 1.5–3 mm | papery, petiole 1–1.5 cm | membranous, petiole 0.5–1.8 cm |
| Corolla color | tube white, corolla limb rose | tube yellow, with pink spot in galea | tube base yellow, galea blue |
| Corolla tube | zigzag, base slightly dilated | zigzag, base arcuate | zigzag, base bent |
| Middle lobe of lower lip shape | broadly ovate | trapeziform | broadly oblong-ovate |
| Habitat | dry limestone mountains | valley | streamside, grasslands, thickets, forests |
| Distribution area | northeast Yunnan | Muli, Southwest Sichuan | North Yunnan & West Sichuan |
Relationships between Scutellaria wuana and its allies (i.e., S. teniana, S. mairei and S. tapintzensis) were not fully resolved. These species formed a clade in our analyses (Fig. 3), but received meager support values. Part of the reason is that only two nuclear DNA regions were used for analyses in this study, and we predict that the use of more chloroplast DNA markers and broad phylogenomic sampling will improve the resolution of the phylogeny. Currently, it is difficult to resolve the species relationships for such a large genus. To this end a multi-disciplinary method including molecular systematics, phylogenomics, morphological anatomy, and taxonomy is necessary for future studies.