Phenolic compounds profiling of nine dogwood species (Cornus L.) leaves
- Published
- Accepted
- Received
- Academic Editor
- Ganesh Nikalje
- Subject Areas
- Biochemistry, Plant Science
- Keywords
- Cornus L., Flavonoids, Quercetin, Kaempferol, HPLC
- Copyright
- © 2025 Forman et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Phenolic compounds profiling of nine dogwood species (Cornus L.) leaves. PeerJ 13:e19457 https://doi.org/10.7717/peerj.19457
Abstract
This study analysed the phenolic compound profile in the leaves of nine Cornus species (C. alba, C. amomum, C. sericea var. baileyi, C. florida, C. kousa, C. mas, C. officinalis, C. coreana, and C. racemosa) to evaluate their potential as stable sources of bioactive compounds. The main phenolic acids (gallic, ellagic and chlorogenic acids) and flavonoids were quantified by high-performance liquid chromatography with diode-array detection (HPLC-DAD). Among the phenolic acids, C. mas and C. officinalis contained the highest levels of chlorogenic acid, while C. coreana totally lacked this compound. Notably, the chlorogenic acid concentration in C. mas also exceeded previously reported values for other plant parts. The highest ellagic acid content was found in C. sericea var. baileyi, while the lowest was observed in C. racemosa. C. coreana showed the highest concentration of gallic acid. Flavonoid analysis revealed that quercetin-3-O-galactoside was present in all species studied, with the highest levels in C. racemosa and the lowest in C. florida. Quercetin-3-O-glucoside was abundant in C. kousa but absent in C. mas. Quercetin-3-O-rhamnoside was detected in significant amounts only in C. racemosa and C. amomum. Among the kaempferol derivatives, kaempferol-3-O-glucoside was the most abundant, with the highest concentration in C. coreana. Furthermore, C. racemosa and C. amomum were the richest sources of quercetin, while C. coreana was particularly rich in kaempferol. These results highlight the diverse phenolic profiles of Cornus species and their potential as valuable sources of bioactive compounds.
Introduction
The dogwood genus (Cornus L., Cornaceae) comprises 59 subordinate taxa distributed throughout Asia, North and South America, Europe, and Africa, mainly in boreal and temperate climate regions, as well as at higher altitudes in subtropical and tropical zones. The greatest diversity of Cornus L. species is found in East Asia and North America (Eyde, 1988; Xiang et al., 2006; WFO Plant List, 2024). In Slovakia, the richest collection of Cornus L. taxa was established in the Mlyňany Arboretum of the Slovak Academy of Sciences (SAS). It includes C. mas L., C. officinalis Siebold & Zucc., C. florida L., C. alba L., C. amomum Mill., C. sericea var. baileyi (JM Coult. & WH Evans) Mohlenbr., C. coreana Wangerin, C. racemosa Lam., and C. kousa Bürger ex Hance (Hot’ka & Barta, 2012; WFO Plant List, 2024). Cornus species are mostly deciduous with oppositely arranged leaves and hermaphrodite flowers arranged in more or less conspicuous inflorescences, with stone fruits (Ball, 1968).
Historically, various dogwood parts have been used for teas, balms, and creams to treat many ailments (Ercisli et al., 2008). In Slovak folk medicine or ethnomedicine, C. mas is used to treat fever, digestive disorders, and inflammation (Bertová, 1984), while in Iran, it represents an important medicinal plant for malaria, diarrhoea, inflammatory bowel disease, fever, kidney stones, urinary tract infections, and cancer treatment (Asadov, Ibrahimov & Sadigova, 1990; Celik, Bakirci & Sat, 2006). In Korea and China, C. controversa is used as an astringent and tonic (Jang et al., 1998), and in traditional Korean medicine C. kousa is used to treat diarrhoea, and as a haemostatic agent (Lee et al., 2010). In addition, Cornus leaves have been studied for their antiproliferative and immunomodulatory properties (Forman, Bukovský & Grancai, 2016). The fruits possess astringent (Kucharska et al., 2015), anti-inflammatory (Asgary et al., 2013), antioxidant (Gulcin et al., 2005; Pantelidis et al., 2007), and anti-diabetic effects (Yamabe et al., 2007). Several species, such as C. canadensis, C. alba, C. florida, and C. kousa, are valued for horticultural purposes (Dirr, 1998), while others, such as C. mas, C. officinalis, C. controversa, and C. kousa, produce edible fruits that are consumed and processed in many parts of Europe and Asia (Seeram et al., 2002).
In general flavonoids are secondary metabolites that are abundant in leaves, fruits or seeds, contributing to their colour, aroma, and flavour. With regard to their pharmacological properties, a large group of beneficial activities can be identified, including antioxidant (Al-Rimawi et al., 2024; Gallia et al., 2024; Hagaggi, Abdul-Raouf & Radwan, 2024), anti-inflammatory (Al-Rimawi et al., 2024; Bhavikatti et al., 2024; Duangiad et al., 2024), antiviral (Zhang et al., 2018; Jackman et al., 2024), antimicrobial (Bhavikatti et al., 2024; Hagaggi, Abdul-Raouf & Radwan, 2024), immunomodulatory (Das et al., 2024; Wu et al., 2024), anticancer (Al-Rimawi et al., 2024; Limam et al., 2021). They may also play an important role in the prevention and treatment of lifestyle diseases such as metabolic syndrome and associated diabetes mellitus (Salah et al., 2024), cardiovascular problems (Duangiad et al., 2024) as well as, certain types of tumour lesions, neurodegenerative diseases such as Alzheimer’s and Parkinson’s (Ibrahim et al., 2024). There is considerable interest in Cornus species because of their flavonoid content. However, research has mainly focused on the edible fruits, especially the berries of C. mas. Other species that have been studied include C. officinalis, C. controversa, C. kousa, C. alba, and C. canadensis. Despite many differences among them, scientific studies consistently highlight Cornus fruits as excellent sources of quercetin, kaempferol glycosides (Pawlowska, Camangi & Braca, 2010), and various glycosides of cyanidin (Seeram et al., 2002), delphinidin (Vareed et al., 2006), and pelargonidin (Du & Wang, 1974). On the other hand, leaves can also be a very important source of these substances (Milenkovic-Andjelkovic et al., 2014).
The current trend to find new ways to influence biological functions and contribute to the protection and prevention of various human health complications promotes the “plugging in” of natural resources. It is the plant kingdom that represents a source of diverse substances that are already or have the potential to become candidates for the treatment of various health problems, either alone or as adjuvant therapies. In terms of efficacy, there is also a significant difference between pure isolated substances and extracts. Plant extracts allow us to benefit from the synergy of the different molecules they contain (Khedkar & Khan, 2024).
So far, there is insufficient information on the distribution of flavonoids and generally phenolic substances in the dogwood leaves. Therefore, our main goal was to identify and quantify this type of secondary metabolites in selected Cornus taxa originating from the Mlyňany Arboretum SAS. The results of this study could contribute to widespread use of Cornus species beyond horticulture to the pharmaceutical and food industries.
Materials and Methods
Chemicals
Methanol, acetonitrile with 0.1% formic acid and water with 0.1% formic acid were purchased from VWR International (Radnor, PA, USA). Hydrochloric acid was obtained from Centralchem (Bratislava, SK). Standards of gallic acid (purity ≥ 99%), and flavonoids—quercetin-3-O-rutinoside (purity ≥ 95%), quercetin-3-O-glucoside (purity ≥ 98%), quercetin-3-O-galactoside (purity ≥ 97%), kaempferol-3-O-rutinoside (purity ≥ 98%), kaempferol-3-O-glucoside (purity ≥ 97%), quercetin (purity ≥ 98%), kaempferol (purity ≥ 98%) were purchased from Sigma Chemicals (St. Louis, MO, USA). Ellagic acid (purity ≥ 95%), quercetin-3-O-rhamnoside (purity ≥ 98%), quercetin-3-O-glucuronide (purity ≥ 98%), and kaempferol-3-O-galactoside (purity ≥ 98%) were purchased from Extrasynthese (Genay, FR), while chlorogenic acid (purity ≥ 94%) was obtained from HWI Group (Ruelzheim, DE). All chemicals used were of per analysis and HPLC-Gradient Grade, as applicable.
Plant material
Plant material (leaves) was collected in the Mlyňany Arboretum SAS, Vieska nad Žitavou, Slovakia 48°19′11″S 18°22′08″V) in September 2023 (Fig. 1). GPS coordinates of the mother plants are included as (Supplement S1). The plant material was identified by dendrologist Ing. Hot’ka & Barta (2012) and listed in the collection inventory. For each species, a single tree was selected and twenty leaves were collected from the sunlit part of the crown. The trees were of different ages. Analyses of the bioactive compounds were carried out on four East Asian taxa (Cornus alba L., Cornus coreana Wangerin, Cornus kousa Bürger ex Hance, and Cornus officinalis Siebold & Zucc.), four North American taxa (Cornus racemosa Lam., Cornus sericea var. baileyi (JM Coult. & WH Evans) Mohlenbr., Cornus amomum Mill., and Cornus florida L.), and one European taxon (Cornus mas L.). Leaves were dried at room temperature and stored in the dark until analysed.
Figure 1: Images of the examined Cornus species leaves.
C. alba L. (A), C. amomum Mill. (B), Cornus sericea var. baileyi (JM Coult & WH Evans) Mohlenbr. (C), C. coreana Wangerin (D), C. florida L. (E), C. kousa Bürger ex. Hance (F), C. mas L. (G), C. officinalis Siebold & Zucc. (H), C. racemosa Lam. (I).Extracts preparation
A total of 50 mg of dry plant tissue were extracted with two mL of 50% (v/v) methanol and used for the analysis of flavonoid glycosides and phenolic acids. The extracts were centrifuged (high-speed micro centrifuge Frontier 5515R, OHAUS Europe GmbH, Nänikon, CH) at 10,000 rpm for 5 min and filtered using a 0.2 µm nylon membrane (Pall Corp., Ann Arbor, MI, USA). Samples were analysed using Agilent 1260 Infinity Quarternary LC system with 1260 Infinity DAD detector (Agilent Technologies, Inc., Santa Clara, CA, USA) and a reversed phase column ACE 5 C18, 5 µm, 250 × 4.6 mm (Avantor, Inc., Radnor, PA, USA) with column temperature: 35 °C, using gradient elution. Filtered extracts (one mL) were hydrolysed with 10% (v/v) hydrochloric acid (HCl) (0.5 mL) for 1 h at 95 °C in a thermo-block (analog dry block heater; VWR International, Radnor, PA, USA) and used for aglycones quantification. Prior to HPLC analysis, each sample was filtered again through a 0.45 µm pore size nylon membrane. The mobile phase flow rate was at a 0.850 mL/min. Injection volume was 10 µL. Mobile phase comprised two solvents: (A) water with 0.1% formic acid (v/v), and (B) 80% (v/v) acetonitrile with 0.1% formic acid. Gradient program for determination of flavonoids, phenolic acids, was as follows: 0 min A/B (95:5); 35 min A/B (78:22); 50 min A/B (74:26); 55 min A/B (5:95); 60 min A/B (95:5). The gradient program for determination of flavonoid aglycones was as follows: 0 min A/B (80:20); 5 min A/B (70:30); 10 min A/B (50:50); 20 min (0:100); 25 min (80:20). Gallic and ellagic acids were detected at 280 nm, phenylpropanoids (chlorogenic acid) at 325 nm, flavonoid glycosides at 350 nm, and flavonoid aglycones at 370 nm. The evaluation was performed using ChemStation (Rev. C. 01.02) software.
Statistical analysis
All the experiments were performed in triplicates—three extracts were prepared from the leaves of each plant species in the same way. Those extracts were then analysed.
The results are expressed as means ± standard error (SE). Data analysis was conducted using SigmaPlot v.10 software (Systat Software GmbH, Erkrath, DE). Comparison of means was conducted using Duncan’s multiple range test, where different letters indicate statistically significant differences between species at a 95% confidence level.
Results
We identified and quantified phenolic acids, flavonol-type aglycones (quercetin and kaempferol), and their mono- and diglycosides in leaves of nine dogwood species (Cornus officinalis, C. florida, C. racemosa, C. alba, C. mas, C. sericea var. baileyi, C. amomum, C. coreana, C. kousa) using the HPLC-DAD method (Table 1).
| Retention time (min) | Cornus officinalis (mg/g) | Cornus florida (mg/g) | Cornus racemosa (mg/g) | Cornusalba (mg/g) | Cornusmas (mg/g) | C. sericea var. baileyi (mg/g) | Cornus amomum (mg/g) | Cornus coreana (mg/g) | Cornuskousa (mg/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | ||||||||||
| Gallic acid | 7.31 | 0.26a ± 0.01 | 0.36b ± 0.02 | 0.39b ± 0.01 | 0.95e ± 0.04 | 0.60d ± 0.03 | 0.56d ± 0.02 | 0.48c ± 0.01 | 1.63f ± 0.04 | 0.34b ± 0.02 |
| Ellagic acid | 38.51 | 0.48d ± 0.03 | 0.21b ± 0.05 | 0.03b ± 0.03 | 0.68b ± 0.08 | 0.53d ± 0.03 | 1.17a ± 0.27 | 0.19b ± 0.007 | 0.70a ± 0.07 | 0.85c ± 0.04 |
| Chlorogenic acid | 20.492 | 18.32bc ± 1.28 | 3.80ab ± 0.20 | 3.43a ± 0.20 | 2.76cd ± 0.21 | 18.41c ± 1.01 | 0.43e ± 0.03 | 2.27ab ± 0.09 | n.d.cd | 7.16d ± 0.40 |
| Flavonol glycosides | ||||||||||
| Quercetin-3-O-rutinoside | 39.183 | 0.39b ± 0.02 | n.d.a | 0.82c ± 0.03 | 0.28ab ± 0.01 | 0.31ab ± 0.01 | 1.19d ± 0.09 | 0.36b ± 0.02 | 4.18e ± 0.28 | 1.08cd ± 0.17 |
| Quercetin-3-O- glucoside | 40.83 | 1.49b ± 0.10 | 3.88a ± 0.29 | 9.01c ± 0.41 | 1.26b ± 0.07 | n.d.a | 2.93b ± 0.29 | 4.14b ± 0.22 | 6.27b ± 0.22 | 10.69b ± 0.70 |
| Quercetin-3-O-galactoside | 39.83 | 4.25f ± 0.29 | 0.58a ± 0.19 | 27.99a ± 1.33 | 4.27b ± 0.32 | 0.62e ± 0.06 | 4.39c ± 0.43 | 3.34a ± 0.27 | 2.84d ± 0.09 | 4.21a ± 0.66 |
| Quercetin-3-O-rhamnoside | 47.13 | n.d.b | n.d.cd | 7.73f ± 0.32 | n.d.b | n.d.a | n.d.c | 4.42d ± 0.37 | n.d.e | n.d.g |
| Quercetin-3-O-glucuronide | 40.343 | 6.98a ± 0.61 | n.d.a | n.d.c | 1.51a ± 0.12 | 5.87a ± 0.52 | 2.91a ± 0.23 | n.d.b | 3.95a ± 0.26 | n.d.a |
| Kaempferol-3-O-rutinoside | 44.83 | n.d.b | n.d.a | 0.54a ± 0.01 | n.d.a | n.d.a | n.d.a | n.d.a | 1.31c ± 0.06 | n.d.a |
| Kaempferol-3-O-glucoside | 46.83 | 0.38a ± 0.02 | 0.63a ± 0.06 | n.d.b | n.d.a | 0.67a ± 0.05 | 0.2a ± 0.01 | n.d.a | 3.74c ± 0.19 | 0.11a ± 0.01 |
| Kaempferol-3-O-galactoside | 44.63 | 0.16b ± 0.01 | n.d.c | n.d.a | n.d.a | n.d.c | n.d.ab | n.d.a | 0.35d ± 0.01 | n.d.a |
| Aglycones (after the acidic hydrolysis reaction) | ||||||||||
| Quercetin | 15.544 | 2.33a ± 0.21 | 5.52a ± 0.19 | 61.61e ± 3.81 | 11.20b ± 0.35 | 18.72c ± 2.14 | 23.69c ± 1.49 | 31.71d ± 1.01 | 22.18c ± 0.46 | 22.46c ± 2.73 |
| Kaempferol | 17.34 | 0.09ab ± 0.02 | 0.23c ± 0.01 | 0.032a ± 0.003 | 0.08ab ± 0.003 | 0.11b ± 0.008 | 0.08ab ± 0.002 | 0.09ab ± 0.007 | 0.91d ± 0.05 | 0.07ab ± 0.009 |
Notes:
Different letters indicate statistically significant differences in flavonoid content among various Cornus species, as determined by Duncan’s multivariate test at P < 0.05.
Measurements were taken at wavelengths: 1 = 280 nm, 2 = 320 nm, 3 = 350 nm, and 4 = 370 nm. Data are presented as mean ± SE (n = 3).
“n.d.” denotes values that were not detected. All results are expressed in mg/g of leaf dry weight (DW).
Our analysis confirmed the presence of three phenolic acids: gallic, ellagic, and chlorogenic acid. Gallic and ellagic acids were present in all samples analysed. The highest gallic acid content was found in C. coreana (1.63 ± 0.04 mg/g dry weight (DW)). For ellagic acid, it was C. sericea var. baileyi (1.17 ± 0.27 mg/g DW). Chlorogenic acid was present in eight out of nine samples, except C. coreana. Its highest abundance was found in C. mas (18.41 ± 1.01 mg/g DW).
We further identified two diglycosides (quercetin-3-O-rutinoside, and kaempferol-3-O-rutinoside), and six monoglycosides (quercetin-3-O-glucoside, quercetin-3-O-galactoside, quercetin-3-O-rhamnoside, quercetin-3-O-glucuronide, kaempferol-3-O-glucoside, and kaempferol-3-O-galactoside). The two basic structures of flavonoid aglycones, quercetin and kaempferol, were also determined after acid hydrolysis.
Our results show that among the quercetin derivatives, quercetin-3-O-galactoside was the predominant glycoside. Its presence was detected in all the species studied. Its highest content was found in the leaves of C. racemosa (27.99 ± 1.33 mg/g DW). The next two most abundant glycosides were quercetin-3-O-rutinoside and quercetin-3-O-glucoside. Quercetin-3-O-rutinoside was present in all samples except C. florida, quercetin-3-O-glucoside was absent only in C. mas. The highest content of quercetin-3-O-rutinoside was found in C. coreana (4.18 ± 0.28 mg/g DW), for quercetin-3-O-glucoside it was C. kousa (10.69 ± 0.70 mg/g DW). According to our results, quercetin-3-O-glucuronide, which was found in only five species, and quercetin-3-O-rhamnoside, confirmed in only two species studied, were significantly less abundant molecules. The highest content of quercetin-3-O-glucuronide was determined in C. officinalis (6.98 ± 0.61 mg/g DW), while for quercetin-3-O-rhamnoside it was in C. racemosa (7.73 ± 0.32 mg/g DW).
In the group of kaempferol derivatives, kaempferol-3-O-glucoside was the most dominant, present in six out of nine species. It was most abundant in C. coreana (3.74 ± 0.19 mg/g DW) (Fig. 2). The other two molecules, kaempferol-3-O-rutinoside and kaempferol-3-O-galactoside, were only confirmed in two species studied (Table 1). The highest levels of both kaempferol-3-O-rutinoside (1.31 ± 0.07 mg/g DW) and kaempferol-3-O-galactoside (0.35 ± 0.01 mg/g DW) were consistently found in C. coreana.
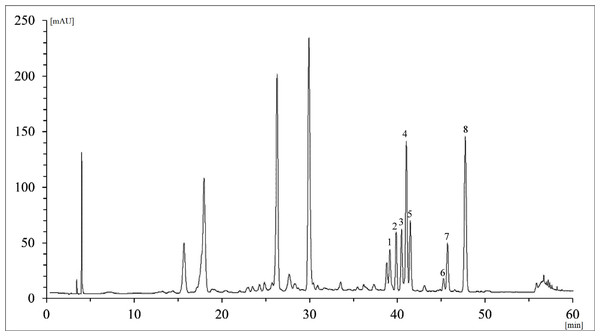
Figure 2: The representative HPLC chromatogram of methanolic extracts from Cornus coreana leaves (Mlyňany Arboretum SAS, Slovakia) acquired at 350 nm.
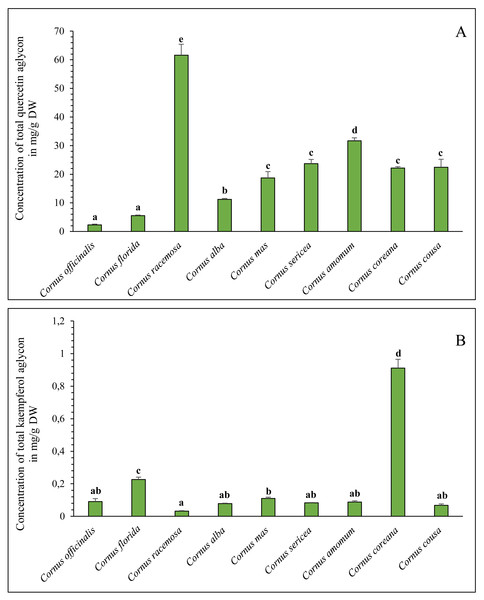
1, ellagic acid; 2, quercetin-3-O-rutinoside; 3, quercetin-3-O-galactoside; 4, quercetin-3-O-glucuronide; 5, quercetin-3-O-glucoside; 6, kaempferol-3-O-galactoside; 7, kaempferol-3-O-rutinoside; 8, kaempferol-3-O-glucoside.From the results of the aglycones assay alone, it is evident that quercetin exceeds kaempferol in abundance by several fold (Fig. 3). The highest quercetin content was found in C. racemosa (61.61 ± 3.81 mg/g DW). For kaempferol, it was C. coreana (0.91 ± 0.05 mg/g DW).
Figure 3: (A–B) The concentration of total quercetin and kaempferol aglycones in Cornus species leaf extracts determined by HPLC analysis.
Different letters indicate statistically significant differences in the content of total kaempferol and quercetin among different Cornus species, determined by Duncan’s multivariate test at P < 0.05.The obtained results highlight the significant variability in flavonoid and phenolic acid content among analysed dogwood species. C. racemosa, and C. coreana stand out as having the highest concentrations of the quercetin and kaempferol aglycones suggesting potential for selective use in nutraceutical and pharmaceutical applications.
Discussion
Dogwood species can generally be considered as a rich source of phenolic compounds, especially flavonoids (Pawlowska, Camangi & Braca, 2010) phenolic acids (Tenuta et al., 2022), and anthocyanins (Vareed et al., 2006; Pawlowska, Camangi & Braca, 2010). Within individual plant organs, fruits are probably among the most studied. The intention of our study was to investigate leaves, for several reasons. One of them is their almost year-round availability, even in large quantities. Another practical factor is the effect of stress (biotic or abiotic), which may result in one or more seasons in which the plant has no or very few fruits. Post-harvest leaf adjustment is also usually a less demanding process (Belmin et al., 2021).
Based on our results (Table 1), the leaves of C. coreana (1.63 mg/g DW) seem to be the most promising in terms of gallic acid content. Ellagic acid, which is a very important substance as it serves as a marker for the presence of hydrolysable tannins-ellagitannins - within the genus Cornus L., was present in the highest amount in the leaves of C. sericea var. baileyi (1.17 mg/g DW) (Jourdes et al., 2013). The highest chlorogenic acid content was observed in the leaves of C. mas (18.41 mg/g DW). The presence of these acids and their derivatives in different dogwood species was confirmed by numerous authors. Badalica-Petrescu et al. (2014) described presence of the chlorogenic acid and caffeic acid derivatives in fresh leaves of C. mas collected in the mountainous region of Romania. Efenberger-Szmechtyk et al. (2020) identified gallic acid, ellagic acid and derivatives of ellagic and caffeic acids in leaves of the same species, coming from Polish village of Zadzim. The content of the above-mentioned acids can be ideally compared with a study of Milenkovic-Andjelkovic et al. (2014), where the authors also examined leaves of the C. mas. The tested material came from the area of northeastern Serbia and was collected in two consecutive growing seasons. The gallic acid content ranged from 0.37 to 0.41 mg/g DW, the ellagic acid content from 2.55 to 2.62 mg/g DW, and the chlorogenic acid content from 0.28 to 0.33 mg/g DW. In all cases these were free acids. If we compare the same species with our research, we can see that they obtained comparable values for gallic acid (0.60 mg/g DW). On the other hand, the content of ellagic acid (0.53 mg/g DW) was about five-fold lower. However, the greatest difference was observed in chlorogenic acid content (18.41 mg/g DW), which is more than 50 times higher in the leaves of our C. mas. If we consider all the species tested in our study, we can observe that the levels found by Milenkovic-Andjelkovic et al. (2014) are within our determined range (Table 1) only in the case of gallic acid. For ellagic acid, on the other hand, our values are about 2 times lower. Chlorogenic acid is the most abundant in our species.
The comparison with other plant organs is also interesting. Moldovan et al. (2016) tested fresh fruits of C. mas in which they identified ellagic, chlorogenic, and caffeic acids. They determined ellagic and chlorogenic acids at 1.88 mg/g FW and 0.33 mg/g FW, respectively. Also in this comparison, we can observe a similar trend to that of the results reported by Milenkovic-Andjelkovic et al. (2014). The highest content of ellagic acid (1.17 mg/g DW) in our case is about 1.6 times lower, and for chlorogenic acid (18.41 mg/g DW) it is again an order of magnitude higher. Similar comparison can be made with another paper by Zhang, Li & Jiang (2022), in which dried seeds, pericarp, and bark of C. officinalis were analysed. From our point of view, the ellagic acid was found to be the highest in the seeds (0.25–0.94 mg/g DW) in this study. We can say that these values correspond to our results in the leaf drug (Table 1). With regard to the last two studies mentioned above, the quantitative comparison itself is early illustrative, since the authors of these papers dealt with different plant organs. An interesting feature that emerges from the qualitative and quantitative comparison of the abundance of phenolic acids with the above studies is the complete absence of caffeic acid or its derivatives, in addition to the strikingly higher content of chlorogenic acid in our species.
Comparison with the recent studies shows that the abundance profiles of the individual flavonols are very similar. Efenberger-Szmechtyk et al. (2020) determined the content of quercetin-3-O-rutinoside (0.008 mg/g FW), quercetin-3-O-glucoside (0.006 mg/g FW), and quercetin-3-O-glucuronide (0.061 mg/g FW) in fresh leaves of C. mas coming from the region of central Poland. Interestingly, quercetin-3-O-glucoside was not detected in our leaf sample of the same species. Regarding the abundance of the other two, we can speak of a much higher content in our case (Table 1). On the other hand, the above-mentioned study of C. mas did not show the presence of quercetin-3-O-rhamnoside or kaempferol-3-O-rutinoside, which is also in agreement with our results. On the contrary, Milenkovic-Andjelkovic et al. (2014) reported the highest content (9.28–9.37 mg/g DW) in the dried leaves of C. mas in the case of quercetin-3-O-glucoside only. The authors of this study also reported significantly higher levels for two other molecules, specifically quercetin-3-O-rutinoside (6.09–6.11 mg/g DW), and kaempferol-3-O-glucoside (4.27–4.37 mg/g DW), compared to our results. The presence of quercetin-3-O-galactoside was not confirmed in the leaves of C. mas from Serbia, in contrast to our results.
Our research focused on leaves, especially because there is much less information on their chemical profile and biological activities. However, the fruits of Cornus L. species have been the subject of various experiments worldwide. As far as the identification of flavonoid molecules is concerned, we can speak of very similar profiles as in our leaf samples. Among the group of kaempferol derivatives, kaempferol-3-O-glucoside (Vareed et al., 2006; Lee et al., 2007), kaempferol-3-O-galactoside (Pawlowska, Camangi & Braca, 2010; Klymenko et al., 2021), and the aglycone itself (Lee et al., 2007) were the most frequently reported. In the case of quercetin derivatives, all have been described in fruits and we have identified them in leaf samples as well. These are quercetin-3-O-galactoside, quercetin-3-O-rutinoside, quercetin-3-O-glucoside, quercetin-3-O-rhamnoside, and quercetin-3-O-glucuronide (Lee et al., 2007; Pawlowska, Camangi & Braca, 2010; Bajic-Ljubicic et al., 2018; De Biaggi et al., 2018; Schmitzer et al., 2020; Klymenko et al., 2021).
The situation is different when it comes to the quantification of single molecules. The authors of the above-mentioned studies mainly focused on the fruits of C. mas, C. kousa, and C. officinalis. A closer comparison of these species in the light of our results shows that in the case of C. mas the highest content was attributed to quercetin-3-O-glucuronide (5.87 mg/g DW). This result is in agreement with the studies of Pawlowska, Camangi & Braca (2010) and Bajic-Ljubicic et al. (2018). The authors of the first study worked with fresh C. mas fruits from Tuscany (Italy) and determined the quercetin-3-O-glucuronide content to be 6.99 mg/g FW. In the second study, they quantified this secondary metabolite again in fresh fruits, this time from Serbia, with a result of 0.15 mg/g FW. For C. kousa leaves, the most abundant compound was quercetin-3-O-glucoside (10.69 mg/g DW). Schmitzer et al. (2020) reported the content of this derivative in fruits from the Arboretum Volcji Potok (Slovenia) in the range of 0.15–0.16 mg/g DW. Klymenko et al. (2021), who studied fresh fruits of C. mas, and C. officinalis collected in the National Botanical Garden of Ukraine, found that in the latter species the highest content was attributed to quercetin-3-O-glucuronide (0.08–0.11 mg/g FW). This fact is in agreement with our results, since in this species we also found that the highest content (6.98 mg/g DW) belonged to the same compound.
The comparison of our results with those of the authors of experiments dealing with other plant organs is more important from the point of view of qualitative analysis. There are some interesting differences or similarities in the content determinations, but a fully adequate comparison was complicated for several reasons. However, on the basis of the results obtained (Table 1), we can conclude that most leaves of the tested Cornus L. species have the potential to be used, either as a source of relevant secondary metabolites or for their biological activities that bind to the molecules in question. However, this requires further research (Ferreyra, Serra & Casati, 2021).
Conclusion
To the best of our knowledge, this study is the first to comprehensively analyse the phytochemical profile of leaf extracts from nine Cornus species, revealing significant variability in their phenolic acids and flavonoid composition. Our results highlight C. mas and C. officinalis as the richest sources of chlorogenic acid, C. sericea var. baileyi as having the highest ellagic acid content, and C. coreana as the most abundant in gallic acid. Furthermore, C. racemosa and C. amomum exhibited remarkably high concentrations of total quercetin, suggesting their potential as valuable sources of natural antioxidants. Given the well-documented antioxidant and anti-inflammatory properties of flavonoids, these Cornus species represent promising candidates for nutraceutical and pharmaceutical applications. The diversity in their phytochemical composition underlines their potential for further research, particularly in exploring their bioactivity, bioavailability and therapeutic benefits. Future studies should focus on evaluating their functional properties and potential applications in health-promoting formulations.
Supplemental Information
GPS coordinates for Cornus L. mother plant locations
The vouchers are archived at the Department of Dendrobiology, Institute of Forest Ecology of the Slovak Academy of Sciences, Mlyňany Arboretum, Slovakia.