Optimization of cultural conditions for pectinase production by Diaporthe isolate Z1-1N and its pathogenicity on kiwifruit
- Published
- Accepted
- Received
- Academic Editor
- Héctor Mora-Montes
- Subject Areas
- Agricultural Science, Microbiology
- Keywords
- Kiwifruit, Soft rot disease, Diaporthe, Pectinases, Optimization, Orthogonal design
- Copyright
- © 2025 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Optimization of cultural conditions for pectinase production by Diaporthe isolate Z1-1N and its pathogenicity on kiwifruit. PeerJ 13:e19207 https://doi.org/10.7717/peerj.19207
Abstract
Diaporthe Z1-1N, the primary causal agent of soft rot disease in kiwifruit, exhibited higher pectinase activity compared to cellulase activity in both in vitro and in vivo incubation models. To gain deeper insights into the role of pectinases in the pathogenicity of this fungus, we evaluated the effects of incubation temperature (ranging from 18 to 38 °C), duration (1 to 7 days), and medium pH (4.0 to 9.0) on the activities of two crucial pectinases: polygalacturonase (PG) and polymethylgalacturonase (PMG). Our single-factor experiments revealed that the optimal conditions for maximizing PMG yield were a pH of 7.5 and a temperature of 28 °C, with peak activity occurring after three days of incubation. Notably, PG activity peaked on the fourth day under the same pH and temperature conditions. Under the optimal conditions identified through an orthogonal experimental design, PMG exhibited higher activity than PG. Further analysis showed that temperature was the most influential factor on PMG activity, followed by incubation duration and pH. The lesion size caused by the purified pectinase extracts was 50% the lesion size that caused by the fungal mycelium of Diaporthe Z1-1N. These findings underscore the significance of PG and PMG as key virulence factors in the pathogenicity of Diaporthe Z1-1N, providing a solid scientific basis for future research into the functions of these enzymes.
Introduction
Kiwifruit (Actinidia spp.), commonly referred to as “mihoutao” (monkey peach) in China, represents an important genus within the family Actinidiaceae. The most recent taxonomic revision identifies 55 species and 20 varieties within this genus (Li, Li & Soejarto, 2007). Among these, A. chinensis and A. deliciosa are the predominant species cultivated, collectively accounting for nearly all kiwifruit in international trade (Nishiyama, 2007). Currently, A. arguta is cultivated on a smaller scale in Europe, New Zealand, and the United States, both commercially and by enthusiastic amateurs (Ferguson, 2013). Kiwifruit is renowned for its excellent flavor and is rich in vitamin C, minerals, dietary fiber, phenols, carotenoids, and other essential nutrients (Liu et al., 2019; Ma et al., 2017; Nishiyama et al., 2004; Sivakumaran et al., 2018). However, soft rot disease poses a significant threat to the quality and yield of kiwifruit (Li et al., 2016). Research indicates that fungi from the Diaporthe spp. are among the primary agents responsible for this soft rot disease (Díaz et al., 2014; Lei et al., 2019; Zhou et al., 2015), resulting in considerable annual economic losses.
The genus Diaporthe comprises over 1,300 taxa, with Phomopsis, its asexual states, also encompassing more than 1,000 species listed in MycoBank (http://www.mycobank.org, accessed in December 2024), which exhibit broad host ranges and a global distribution. According to the International Code of Nomenclature for algae, fungi, and plants (Turland et al., 2018), Diaporthe takes precedence for recommendations regarding generic names due to the existence of two or more genera typified by either a sexual or asexual morph (Rossman et al., 2015). The Diaporthe genus encompasses a diverse group of fungi that can exist in various forms, including as endophytes, saprotrophs, and plant pathogens. Research has shown that some of the most harmful fungal pathogens can be capable of causing various diseases, including stem canker, leaf spot blight, and fruit decay (Bai et al., 2016; Li et al., 2017; Díaz et al., 2017; Wan et al., 2022). Currently, more than 10 Diaporthe species have been reported to cause soft rot disease during both the growing season and post-harvest storage of kiwifruit (Ling et al., 2024b). These pathogens can secrete cell wall degrading enzymes (CWDEs) that interact with host cell wall constituents, thereby facilitating pathogen penetration through the loosened host cell walls and middle lamella matrices. The CWDEs synthesized during plant infection by Diaporthe spp. include cellulases, xylanases, and pectinases (Chen et al., 2018; Zhang, 2010).
Pectinases are pivotal in plant pathogenesis, marking the initial enzymes synthesized by specific fungal and bacterial pathogens that colonize plant cell walls, priming these cell wall components for subsequent degradation by other enzymes (Abbott & Boraston, 2008). Typically, pectin degradation is orchestrated by a suite of pectinases, which include pectate lyases (families 1, 2, 3, and 9), polygalacturonases and rhamnogalacturonases (glycoside hydrolase family 28), pectin methylesterases (carbohydrate esterase family 8), and pectin acetylesterases (carbohydrate esterase family 12) (CAZY Database; https://www.cazy.org/). These enzymes are hypothesized to contribute to soft rot infections by dismantling cell wall polysaccharides, leading to fruit softening and a decrease in disease resistance. Polygalacturonase (PG), pectinesterase (PE), and polymethylgalacturonase (PMG) are particularly noted for their roles in facilitating pectin disassembly in postharvest fresh fruits (Lin et al., 2018). Our recent research has shown that kiwifruit inoculated with Diaporthe Z1-1N displayed a higher disease index compared to non-inoculated harvested kiwifruit, with two pectinases (PG and PMG) showing increased activities in both in vitro and in vivo incubation models (Chen et al., 2024; Ling et al., 2024a). This finding indicates that these enzymes might have the significant role in the pathogenic process and warrant further study.
Previous studies have indicated that pectinase activity levels are influenced by factors such as Ca2+, pH, and the culture medium (Pagel & Heitefuss, 1990; Qi et al., 2010). To better understand the potential role of the enzymes as virulence factors under various conditions in the fungi-host plant interaction, it is essential to optimate the culture conditions of these two pectinase activity levels. Therefore, this study aims to examine the activities of two pectinases, PG and PMG, at the different incubation temperature, incubation time, and the initial pH of the culture medium through single-factor and orthogonal optimization experiments. Specifically, we investigated the effects of various inculcation factors on the production of PMG and PG. Additionally, the pathogenicity of pectinase produced by Diaporthe isolate Z1-1N under the optimal condition was assessed and compared with that of mycelial plugs from the Diaporthe isolate Z1-1N. This research will lay the foundation for further exploring the pathogenic role of these two pectinases in the soft rot of ‘Hongyang’ kiwifruit.
Materials and Methods
Plant material and pathogenic fungi
Kiwifruit (A. chinensis cv. Hongyang) of similar size and consistent maturity was purchased from the local market in Liupanshui, Guizhou Province, China. The pathogenic fungi Diaporthe spp. Z1-1N, isolated in our previous studies from rotten ‘Hongyang’ kiwifruit during cold storage (Ling et al., 2023; Ling et al., 2024b), was cultured on potato dextrose agar (PDA) medium at 25 °C in the dark for 6 days for the enzyme assays and pathogenicity trials.
Optimization of pectinase production in liquid culture
In this study, we employed a modified Marcus liquid medium, as described in our previous research, to investigate the production of two pectinases: PG and PMG (Ling et al., 2024a). Five millimeter mycelial discs from a 6-day-old PDA culture were inoculated into 100 ml of liquid medium contained in 250 ml flasks, which were then incubated on an orbital shaker. Optimization of pectinase production was conducted through a series of single factor experiments, assessing variables such as incubation periods (1, 2, 3, 4, 5, 6, and 7 days), temperature (18, 23, 28, 33, and 38 °C), and the initial pH of the medium (ranging from 4 to 9 in 0.5 intervals). The optimal incubation time was determined based on the experimental design. When investigating the other two factors, cultures were harvested on the third day. The contents were filtered using a vacuum to remove the fungal mycelia. The filtrates were subsequently centrifuged at 10,000 g for 15 min at 4 °C, and the supernatant was utilized to assess the activitiesof PMG and PG using methods described in our previous study (Ling et al., 2024a).
Based on the results of the single-factor experiment, a three-factor, three-level standard orthogonal table, L9 (33), was selected to optimize the production conditions for two pectinases (PG and PMG) as shown in Table 1. The incubation temperature (A) was set at 25 °C, 28 °C, and 31 °C; the initial pH of the medium (B) was set at 6.5, 7.0, and 7.5; and the incubation time was set at 2, 3, and 4 days. These factors and their corresponding levels were incorporated into the L9 (33) orthogonal table, resulting in a total of nine experimental groups with various combinations of the three parameters in this study.
| Levels | Factors | ||
|---|---|---|---|
| Inoculation temperature (°C) | Initial pH of the medium | Inoculation time (d) | |
| A | B | C | |
| 1 | 25 | 6.5 | 2 |
| 2 | 28 | 7.0 | 3 |
| 3 | 31 | 7.5 | 4 |
Measurement of pectinase activity
The activities of PMG and PG were determined using the 3,5-dinitrosalicylic acid (DNS) colorimetric method, following the detailed procedures outlined by our previous study (Ling et al., 2024a). Three biological trials were conducted.
Purification of crude enzyme extracts and incubation on kiwifruit fruit
Pectinase was induced for production from the liquid culture of the Diaporthe isolate Z1-1N using orange pectin (Shanghai Aladdin Industries, Shanghai, China) as the carbon source. The purification process adhered to the method described (Ling et al., 2024a). The pathogenicity of various treatments, including pectinase extract and mycelial plugs from the Diaporthe isolate Z1-1N, was assessed by incubating them at 28 °C in darkness. Sterile water and sterile plugs served as controls, respectively. Ten kiwifruit fruits were used in each treatment. The lesions are classified based on their diameters (Li et al., 2019): grade 0 (0 cm); grade 1 (0 cm−1.5cm); grade 3 (1.5–3 cm); grade 5 (3–4.5 cm); grade 7 (4.5–6 cm); grade 9 (more than six cm). The Disease Index (DI) is calculated as follows:
Data and statistical analysis
Each experiment of enzymic assay was conducted in triplicate, and lesion size measurements were taken from ten kiwifruit fruits per treatment group. From the gathered data, we calculated the mean and standard error to provide a comprehensive analysis. To discern significant differences among the means, we employed one-way analysis of variance (ANOVA) using SPSS version 19.0 for Windows. Following the ANOVA, we applied Duncan’s multiple range test to separate the means, establishing statistical significance at the P < 0.05 threshold. Additionally, we leveraged the Orthogonal Designing Assistant II V3.1 software for the rigorous evaluation of our statistical experimental design, ensuring the precision of our experimental outcomes.
Results
Effect of initial pH of medium on two pectinase production
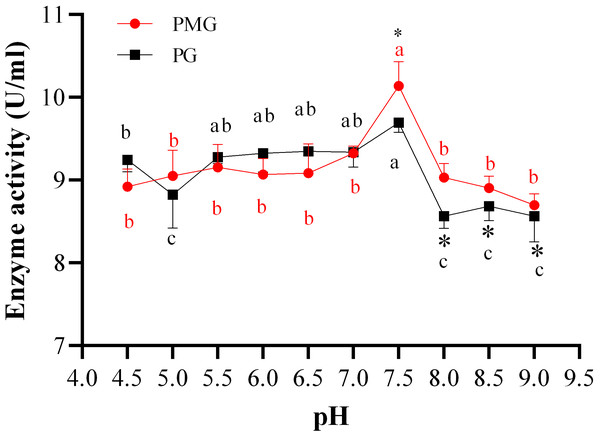
The initial pH of the medium is a crucial factor in the production of pectinases, as it influences both the type and quantity of enzymes produced by fungi. In this study, we found that the activities of pectin methylgalacturonase (PMG) and polygalacturonase (PG) produced by Diaporthe Z1-1N showed the similar trends but also exhibited some differences (Fig. 1). For example, significant differences in enzyme activities were only observed at pH levels above 7.5, specifically in the range of pH 7.5 to 8.0. Within this range (7.5–9.0), PG activity significantly decreased compared to that of PMG (Fig. 1). In addition, PMG activity remained relatively stable across different pH levels, with a significant peak at pH 7.5 (10.14 U/ml). Similarly, PG activity reached its highest level at pH 7.5 (9.70 U/ml) but it was lower than that of PMG. Therefore, these results indicate that the optimal production of both PMG and PG by Diaporthe Z1-1N occurs at pH 7.5, with PMG exhibiting a higher activity than PG.
Figure 1: Effect of pH on PMG and PG production.
Note: Different letters in each line indicate significant difference of each enzyme (P < 0.05); an asterisk (*) indicates the significant difference of two enzymes (P < 0.05).Effect of incubation temperature on two pectinase production
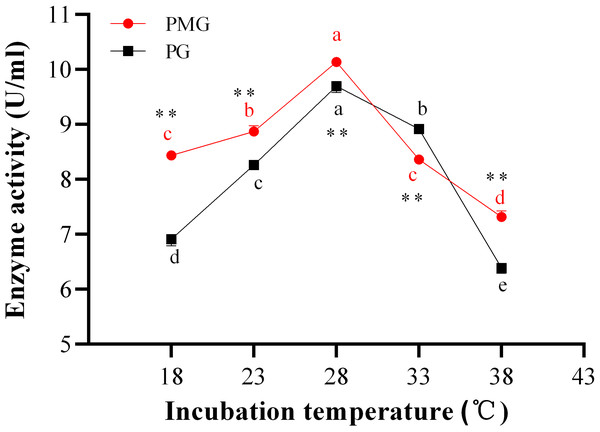
Temperature is directly related to the metabolic activities of microorganisms and significantly influences both growth and product formation. Figure 2 illustrates the effects of various temperatures on the production of two types of pectinase. The activity of PMG was consistently higher than that of PG across different temperatures, with the exception of 33 °C, where PG exhibited greater activity than PMG. Additionally, both PMG and PG displayed a similar trend, characterized by an initial increase in activity followed by a decline. Our results indicate that maximum pectinase production occurred at 28 °C, yielding 10.14 U/ml for PMG and 9.70 U/ml for PG. Subsequently, the second most favorable temperature for PMG production was 23 °C (8.87 U/ml), while for PG, it was 33 °C (8.92 U/ml). Conversely, the lowest production levels for both pectinases were observed at 38 °C, with values of 7.32 U/ml for PMG and 6.39 U/ml for PG. Therefore, these findings suggest that the optimal temperature for the growth and production of PMG and PG by Diaporthe Z1-1N is 28 °C.
Figure 2: Effect of incubation temperature on PMG and PG production.
Note: Different letters in each line indicate significant difference of each enzyme (P < 0.05); two asterisks (**) indicate the significant difference of two enzymes (P < 0.01).Effect of incubation periods on two pectinase production
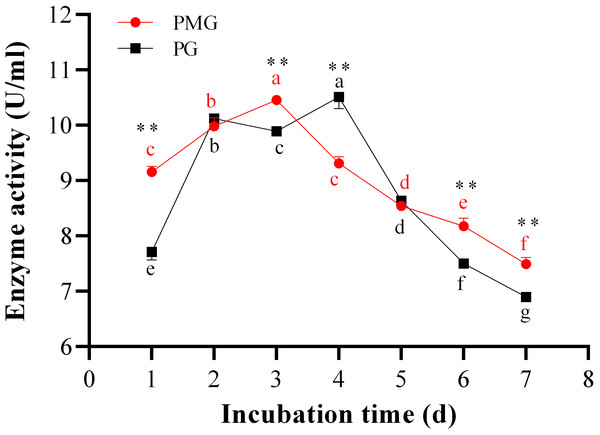
The incubation period is crucial for maximizing enzyme yield. Over the course of 1 to 7 days, we monitored the activities of PMG and PG, as illustrated in Fig. 3. Throughout this period, PMG generally exhibited a higher activity than PG, with the exception of the second and fifth days. Our data further indicated that both pectinases displayed significant fluctuations in activity and showed a similar trend with a slight variation. For PG, activity gradually increased from the start, peaking on the third day at a concentration of 10.52 U/ml, before gradually declining by the seventh day. PMG activity began to rise on the second day, dipped slightly on the third day, and then reached its maximum on the fourth day at 10.43 U/ml. Subsequently, PMG activity decreased, reaching its lowest point at the 7-day mark with a yield of 7.42 U/ml, as depicted in Fig. 3. These findings suggest that the optimal times for PMG and PG production are the third and fourth days of incubation, respectively.
Figure 3: Effect of incubation time on PMG and PG production.
Note: Different letters in each line indicate significant difference of each enzyme (P < 0.05); two asterisks (**) indicate the significant difference of two enzymes (P < 0.01).Optimization of cultural conditions for pectinase productivity
Culture conditions, such as pH levels, incubation temperatures, incubation duration, and the sources of carbon, nitrogen, and mineral salts, are pivotal in dictating the synthesis and secretion of extracellular enzymes by microorganisms (Jia et al., 2010; Koirala et al., 2014; Patidar et al., 2018). Hence, meticulous adjustment of these parameters can obtain the highest activity the major extracellular enzymes secreted by pathogenic fungi. An orthogonal experiment demonstrated that the highest activity of PMG, at 11.228 U/mL, was attained at a temperature of 28 °C, an initial pH of 7.5, and an incubation period of 2 days (Table 2). Under these same conditions, PG achieved its peak activity of 10.056 U/mL (Table 2). This finding demonstrated that under the optimal conditions identified through an orthogonal experimental design, PMG exhibited a higher activity than PG.
| Tested no. | Factors | Enzymatic activity (U/mL) | |||
|---|---|---|---|---|---|
| A | B | C | PMG | PG | |
| 1 | 25 | 6.5 | 2 | 9.702 | 9.027 |
| 2 | 25 | 7.0 | 3 | 9.755 | 9.213 |
| 3 | 25 | 7.5 | 4 | 9.306 | 7.864 |
| 4 | 28 | 6.5 | 3 | 10.949 | 9.999 |
| 5 | 28 | 7.0 | 4 | 10.002 | 9.415 |
| 6 | 28 | 7.5 | 2 | 11.228 | 10.056 |
| 7 | 31 | 6.5 | 4 | 8.730 | 6.141 |
| 8 | 31 | 7.0 | 2 | 10.661 | 8.536 |
| 9 | 31 | 7.5 | 3 | 9.467 | 8.384 |
Notes:
Factor A: Inoculation temperature (°C); Factor B: Initial pH of the medium; factor C: Inoculation time (d).
In this study, we examined the impact of three key factors—incubation temperature (A), initial medium pH (B), and incubation duration (C)—on PMG production by Diaporthe Z1-1N. A higher extreme R value for a factor indicates that it has a more significant effect on the outcome. By analyzing the magnitude of the extreme R values, we ranked the influence of these factors on PMG production activity as follows: A >C >B (Table 3). This ranking indicates that incubation temperature had the greatest impact, followed by incubation duration, and then initial pH value. As previously mentioned, the optimal conditions were determined to be an incubation time of 2 days, an incubation temperature of 28 °C, and an initial pH of 7.0. To confirm the effectiveness of the optimization approach based on the orthogonal experiment’s results, we conducted a verification experiment using the refined parameter combination. The findings revealed that PMG activity increased significantly to 11.50 U/mL (Table 3), surpassing the activity observed in all previous orthogonal experiments. This outcome validates the efficacy of the optimization strategy employed.
| PMG activity (U/mL) | |||
|---|---|---|---|
| A | B | C | |
| K1 | 9.59 | 9.79 | 10.53 |
| K2 | 10.73 | 10.14 | 10.06 |
| K3 | 9.62 | 10.00 | 9.35 |
| Range (R) | 1.14 | 0.35 | 0.58 |
| The factor importance | A>C>B | ||
| Optimal combination | A2B2C1 (11.50 U/mL) | ||
Notes:
Factor A: Inoculation temperature (°C); Factor B: Initial pH of the medium; factor C: Inoculation time (d).
Pathogenicity of the pectinase extract on kiwifruit
In this study, we investigated the effects of a purified pectinase extract on kiwifruit. The pectinase was prepared from a three-day shaken lipid culture using orange pectin as the sole carbon source, based on the optimal conditions for PMG production. Our results indicated that the pectinase extract induced significant necrotic lesions, with the DI value reaching 47.62% after seven days of treatment (Table 4). Additionally, we observed that the symptoms caused by the pectinase extract were similar to those resulting from infection with Diaporthe Z1-1N mycelium. However, the necrotic lesions induced by the pectinase extract were considerably less severe than those caused by the mycelium plug, which exhibited a DI of 94.81%. In contrast, the control fruits inoculated with sterile water or PDA plugs showed no lesions. Therefore, these results suggest that pectinase plays a role in lesion development associated with Diaporthe Z1-1N.
| Treatments | DI (%) |
|---|---|
| Sterile water | 0.00 |
| Pectinase extract | 47.62 |
| Sterile plug | 0.00 |
| Mycelial plug | 94.81 |
Notes:
The data was obtained after seven days of the different treatment.
Discussion
One of the primary barriers against phytopathogenic fungi is the plant cell wall, which is rich in polysaccharides. Most fungi must breach this barrier to access plant cells, necessitating the secretion of various enzymes capable of degrading cell wall polymers. Pectinases, a group of enzymes that decompose pectic substances, are classified into several categories: polygalacturonase (PG), polymethylgalacturonase (PMG), pectin lyase (PL), pectate lyase (PAL), and pectin methylesterase (PME) (Xue et al., 2018). Our recent study reported that the activities PG and PMG produced by Diaporthe Z1-1N exhibited the significant increase from 3 to 5 days when this fungi was cultivated in a kiwifruit infection model (Ling et al., 2024a). Furthermore, the production of these enzymes secreted by this fungus was biochemically assayed in an in vitro system (Ling et al., 2024a). To determine the optimal external conditions for enzyme production, this study aims to investigate the production of these two pectinases by Diaporthe Z1-1N under varying incubation temperatures (18–38 °C), incubation durations (1–7 days), and pH levels of the medium (4.0–9.0).
Our findings indicated that during the single-factor experiment, both PMG and PG achieved their maximum activities at the same incubation temperature of 28 °C and medium pH of 7.5. However, there was a slight variation in the timing of these peak activities over the incubation period. Specifically, PMG reached its peak on the third day, whereas PG attained its maximum on the fourth day. Previous research has established a sequence for the appearance of cell wall-degrading enzymes in phytopathogenic fungi (Lisker et al., 1975; Lisker, Katan & Henis, 1975). PG is widely recognized as a key virulence factor and is known to be induced in the early stages of infection (Lisker et al., 1975; Vyas, Shah & Jayawant, 2025). Indeed, our recent study has shown thatPMG and PG enzymes exhibited higher accumulation during the 3- to 5-day period of the infection of Diaporthe Z1-1N, which is associated with the development of kiwifruit soft rot. Furthermore, the levels of cell wall-degrading enzyme (CWDE) activity have been correlated with the severity of pathogenesis (Gawade et al., 2017; Zhang, Bruton & Biles, 2014; Zhou et al., 2016). Numerous studies have reported that PG activity is essential for full virulence across a range of host plants (Kubicek, Starr & Glass, 2014; Vyas, Shah & Jayawant, 2025). In this study, under the same incubation temperature and medium pH, PMG exhibited higher activities compared to PG. Similarly, during the infection of Diaporthe Z1-1N, PMG exhibited the larger activity levels than PG (Ling et al., 2024a). Therefore, we inferred that PMG enzyme is likely a significant virulence factor that should not be overlooked. The optimal conditions for PMG production by Diaporthe Z1-1N, as determined through an orthogonal experimental design, significantly enhanced its activity in an in vitro cultivated model. Additionally, the purified pectinase extracts resulted in a 50% lesion size of fungal mycelium. Both PG and PMG are crucial virulence determinants were observed in Rhizoctonia solani Kühn (Chen et al., 2006). Whether these two enzymes as crucial virulence determinants in the pathogenicity of Diaporthe Z1-1N requires further investigation. However, other pectinases, such as PME, PL, and β-galactosidase (β-gal), are also involved in disease development caused by phytopathogenic fungi (Gawade et al., 2017). Consequently, further investigation into the roles of these additional enzymes is warranted.
Moreover, varying cultural conditions have led to differences in the activities and types of cell wall-degrading enzymes produced. For instance, PMG exhibited a higher activity when soluble starch was used as a carbon source, while PG demonstrated a greater activity when utilizing pectin as a substrate during in vitro cultivation of Phomopsis longanae Chi (Chen et al., 2018). Additionally, some other cultivation factors, such as metal ion concentrations (Ca2+, Fe2+) and shaking conditions, also influence the production of pectinases (Chen et al., 2006; Pagel & Heitefuss, 1990). Consequently, further investigation is needed to elucidate the relationship between specific cultural conditions and the virulence of cell wall-degrading enzymes. Notably, our result of orthogonal experiments indicated incubation temperature was the most influential factor for PMG production by Diaporthe Z1-1N among the three tested factors. Previous research has indicated that temperature affects the mycelial growth of Sclerotium rolfsii and the severity of rot on potato tubers (Daami-Remadi et al., 2010). Given that Diaporthe Z1-1N was isolated from rotten kiwifruit during cold storage, it is essential to investigate the effects of low temperature on the activities of PG and PMG. Additionally, pathogenicity tests should be conducted to better understand the role of these enzymes under such conditions.
Conclusions
In this study, we optimized the production of two pectinase enzymes, PG and PMG, from Diaporthe Z1-1N, the microorganism responsible for soft rot disease in kiwifruit. This was achieved by evaluating three key factors—pH, temperature, and incubation duration—using an orthogonal experimental design. Our findings revealed that the highest production of PMG occurred at pH 7.0 and 28 °C on the third day, whereas maximum PG production was observed at the same pH and temperature but on the fourth day. Notably, PMG demonstrated the higher activity compared to PG when each factor was optimized individually. A more detailed analysis of PMG activity, based on the orthogonal design, indicated that incubation temperature was the most influential factor, followed by incubation time and pH. The purified pectinase produced by Diaporthe Z1-1N under the optimized culture conditions exhibited significant pathogenicity, as demonstrated by a 50% lesion size of the fungal mycelium of this fungi. These results provide a solid foundation for future research into the functions of PMG and PG.