Cordyceps cateniannulata, a new potential strain for controlling Allantus luctifer from China
- Published
- Accepted
- Received
- Academic Editor
- Viktor Brygadyrenko
- Subject Areas
- Agricultural Science, Entomology, Mycology, Toxicology, Zoology
- Keywords
- Cordyceps cateniannulata, Mortality, Biological control, Histopathology, Allantus luctifer
- Copyright
- © 2024 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Cordyceps cateniannulata, a new potential strain for controlling Allantus luctifer from China. PeerJ 12:e18345 https://doi.org/10.7717/peerj.18345
Abstract
Allantus luctifer is one of the most serious pests of buckwheat, with its larvae mainly damaging leaves during the seedling and flowering stages. Entomogenous fungi play a crucial role as biological regulators of arthropod populations in nature. In this paper, a newly isolated strain 19GZAl-1, was identified as Cordyceps. cateniannulata through the observetions of macroscopic and microscopic morphological features, and the results of rDNA- ITS sequence alignment and phylogenetic analysis. By comparing the efficacy of ten entomopathogenic fungal strains, including six strains of C. cateniannulata and four other Cordyceps species, against A. luctifer larvae, the new strain 19GZAl-1 exhibited the highest mortality rate. Mortality rates of A. luctifer larvae treated by spraying with the strain 19GZAl-1 increased as larval stage decreased, with first instar larvae showing the highest mortality rate of 85%. The appressorium from conidia invaded the larval body through areas with thin cuticle, such as larva pod base and internode folds, after which the hyphae grew rapidly, filling the haemocoel, and finally protruding from the integument upon melanization and decomposition of the intestinal wall cells. The results suggest that the strain 19GZAL-1 of C. cateniannulata has potential against A. luctifer larvae, which is significant for further study on the infection mechanism of C. cateniannulata on insects.
Introduction
Buckwheat (Fagopyrum esculentum Moench, Polygonaceae: Fagopyrum) stands as the only pseudograin crop of Polygonaceae in the world (Ren et al., 2022). It is a dicotyledonous plant belonging to the Polygonaceae family, known for its cold resistance, drought tolerance and short growth period. Widely cultivated, it is rich in active compounds, including balanced amino acids, trace elements, and dietary fiber. These components contribute to its ability to improve blood circulation, alleviate gastrointestinal stagnation and chronic diarrhea, and reduce blood sugar, cholesterol, and triglycerides (Bai et al., 2015). According to the data of the Food and Agriculture Organization of the United Nations (FAO, http://www.fao.org/faostat), as of 2020, buckwheat cultivation has been primarily concentrated in Eastern Europe, Asia and the Americas, with China boasting a planting area exceeding 620,000 hectares, accounting for more than thirty percent of the global planting area and ranking first in the world. However, buckwheat, as a catch crop, becomes highly susceptible to various pests due to its rapid growth, long flowering period, and frequent rotation or intercropping with other crops. In addition, the frequent use of chemical pesticides has further exacerbated pest issues (Razze, Liburd & McSorley, 2016). Among these pests, A. luctifer (F. Smith 1874) (Hymenoptera: Tenthredinidae) has emerged as a significant threat to buckwheat at the seedling stage (Zhang et al., 2020), seriously affecting the yield of buckwheat.
A. luctifer is mainly distributed in China, including Jiangsu, Guizhou, Taiwan, etc., as well as Korea, Japan, etc., (Zhou, He & Di, 1996; Zhang et al., 2020; Wei & Niu, 2013; Park et al., 2008). A. luctifer larvae primarily damage leaf tissue of the family Polygonaceae (Park et al., 2008; Wu et al., 2022), favoring plants within the genera Fagopyrum and Polygonum (Chen & Wei, 2018). At present, chemical pesticides such as organophosphorus pesticides (Zhou, He & Di, 1996), pyrethroids and others are commonly employed for A. luctifer control. However, the prolonged use of these chemicals can lead to severe environmental and food safety issues, disrupting ecosystem balance and posing risks to human health (Sharma et al., 2020). The urgency to develop environmentally friendly alternatives, such as biopesticides, is apparent.
Entomopathogenic fungi (EPF) constitute the largest group of Entomopathogenic microorganisms, with nearly 1,000 species reported worldwide (Wen et al., 2016; Roberts & Humber, 1981). EPF penetrate the host cuticle, colonize the host until its death, then emerge from the integument to produce a large number of conidia, facilitating continuous pest control and the formation of epidemics, particularly among pests with piercing-sucking mouthparts, eggs and pupae during the non-feeding period (Li, 2015; Srinivasan et al., 2019). In addition, with safety to the environment, plants and human (Litwin, Nowak & Róalska, 2020; Moraga, 2020; Sharma et al., 2020), EPF have been recognized as an important natural regulation factor for pest populations in agriculture and forest ecosystems and potential resources for biological control of insect pests (Wang et al., 2010).
From a buckwheat field in Guizhou, China, diseased A. luctifer with white hyphae were collected and preliminarily suggesting infection by entomogenous fungus. This study aims to isolate and identify the fungal species infecting the larvae, comparing with the observed EPF strains in the laboratory, and to assess their pathogenicity and infection processes. This marks the first report of EPF pathogenicity against A. luctifer, along with the identification of a newly isolated strain with potential aganist A. luctifer as a biological control agent.
Materials and Methods
Insect pest rearing
A. luctifer individuals were collected from buckwheat (F. esculentum Moench) fields in Baiyi Town (26°52′N, 106°53′E, 1,345 m a.s.l.), Guizhou, China. The insects were reared on buckwheat leaves in an artificial climate chamber maintained at 25 ± 1 °C, with a RH of 80 ± 5% and a photoperiod of L:D 16:8 in the Laboratory at the Research Center of Buckwheat Industry Technology, Guizhou normal University, China.
Different instar larvae of A. luctifer were cultured and collected from the A. luctifer breeding room at the Research Center of Buckwheat Industry Technology, Guizhou Normal University (26°35′31″N, 106°43′10″E, 1,090 m a.s.l.).
Isolation of a new EPF strain from A. luctifer and preparation
Dead A. luctifer larvae covered with the hyphae were collected from Baiyi Town, Guiyang Guizhou, China. The larvae were disinfected by immersing their cuticles in 75% alcohol and then incubated at 25 °C in the dark for several days in Petri dishes with layers of moist cotton and filter paper until consistent fungal sporulation was obvserved. The cadavers showed white sporulation and fungal growth and were subsequently transferred to potato dextrose agar (PDA) medium (adding penicillin and streptomycin). The newly isolated strain was designated as 19GZAl-1, with the identification number No. 10, and stored at 4 °C in the Research Center of Buckwheat Industry Technology, Guizhou Normal University, China.
Strain 19GZAl-1, along with nine other strains, including five strains of C. cateniannulata and four other Cordyceps species (C. farinosa, C. fumosorosea, C. hepiali), are listed in Table 1. All strains were obtained from the Buckwheat Industry Technology Research Center, Guizhou Normal University, and maintained on potato dextrose broth (PDB) medium. They were cultured under constant condition at 150 r/min and 25 °C for 6 days. The submerged conidia were filtered through two layers of lens paper and harvested. The final conidia concentrations were adjusted to 1 × 108 conidia ml−1 using a hemocytometer.
| Serial number in paper | Species | Strain codes | Locations and hosts |
|---|---|---|---|
| 1 | Cordyceps cateniannulata | GNU-1 | Guizhou/Lepidoptera Pupae |
| 2 | C. farinosa | GNU-2 | Guizhou/Lepidoptera Pupae |
| 3 | C. fumosorosea | GNU-3 | Guizhou/Lepidoptera Pupae |
| 4 | C. fumosorosea | GNU-4 | Guizhou/Lepidoptera Pupae |
| 5 | C. cateniannulata | GNU-5 | Guizhou/Lepidoptera Pupae |
| 6 | C. cateniannulata | GNU-6 | Guizhou/Lepidoptera Pupae |
| 7 | C. cateniannulata | GNU-7 | Guizhou/Lepidoptera Pupae |
| 8 | C. hepiali | GNU-8 | Guizhou/Lepidoptera Pupae |
| 9 | C. cateniannulata | GNU-9 | Guizhou/Lepidoptera Pupae |
| 10 | C. cateniannulata | 19GZAl-1 | Guizhou/Hymenoptera Allantoides luctifer (F. Smith) larvae |
Morphological observation of the newly isolated strain 19GZAl-1
The morphological observation followed the method described by Zhou et al. (2020). The 19GZAl-1 isolate was incubated on PDA medium to examine its morphological characteristics. A total of 1-week-old cultures were inoculated in the centre of ten Petri dishes containing PDA medium and incubated at 25 °C. After 7 d, macroscopic characteristics such as colony growth and appearance, and micromorphological characteristics including the size and shape of conidia, as well as the sizes and arrangements of phialides, were recorded (n = 30). The microscopic features of the fungus were observed and photographed using an OLYMPUS CX41 microscope (Olympus, Tokyo, Japan) and processed with Photoshop CS6.
DNA extraction and ITS1/ITS4 sequencing of the strain 19GZAl-1
DNA extraction was conducted following the method of Wang et al. (2014) using fungal genomic DNA extraction reagent (solarbio, D2300). PCR amplification reactions were performed in 50 µL volumes containing template 2, 2 µL of each primer 1, 2 µL of each primer 4, 2 × Master Mix 25 µL, ddH2O 19 µL. The internal transcribed spacer was amplified using the ITS1/ITS4 primer pair, namely, ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). Amplification conditions were as follows: an initial denaturation at 95 °C denature for 6 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealed at 53 °C for 30 s, and extension at 72 °C for 50 s. A final extension step was performed at 72 °C for 10 min. Sequencing was carried out by Sangon Biotech (Shanghai, China) using the aforementioned primers. The obtained sequences were stored in GenBank and aligned using ClustalW for multiple sequence alignment and manual correction. The phylogenetic tree was constructed using the neighbor-joining method (NJ) in MEGA 7. Confidence values for respective branches were determined by bootstrap analysis (1,000 replications).
Bioassay of the Cordyceps strains
Screening of the strains of entomopathogenic fungi with high mortality
Twenty 3rd instar larvae of A. luctifer were immersed in the conidial suspensions of the ten fungal strains listed in Table 1 for 5 s. After immersion, each larva was transferred onto a Petri dish (9 cm diameter) with a rearing platform made of two pieces of wet filter paper. The larvae were provided with detached buckwheat leaves, with the petioles wrapped in degreased cotton to maintain moisture. The Petri dishes were sealed with plastic wrap perforated with uniform-sized holes to allow ventilation, and then placed in an incubator (25 ± 1 °C, 80 ± 5%, 16:8 L:D). The control group was treated with sterile water. All treatments were repeated three times.
The infection and mortality of the A. luctifer larvae were observed for 10 d, and observations lasted until the infection or mortality rates stabilized for 3 d. Infected and dead larvae were then observed for 2–3 days to confirm the infection through the presence of sporogenous structures and conidia of the fungi under an optical microscope.
Infection comparison of the screened strain under different treatments
Considering the differences between laboratory and field application methods, both spraying and impregnation methods were employed for comparison in this experiment. The 3rd instar larvae of A. luctifer were either immersed for 5 s or sprayed with the conidial suspension of the strain exhibiting the highest mortality rate (as shown in Table 1). Approximately 1.7 × 104 conidia/larva were applied to the larval cuticle using both immersion and spraying methods, while sterile water was used as the control. The treated larvae were then transferred to sterilised Petri dishes (9 cm in diameter) and placed in an incubator (25 ± 1 °C, 80 ± 5%, 16:8 L:D). The experiment was repeated three times, with 20 larvae used in each replication.
The dead larvae were recorded daily and observed for 2 d to confirm fungal infection through the presence of sporogenous structures and conidia under an optical microscope.
Pathogenicity tests of the screened strain against different larval instars of the A. luctifer
To assess the pathogenicity of the strain with the highest mortality rate against different larval instars of the A. Luctifer, larvae at different developmental stages were infected using the methods previously described. The infected larvae were then transferred to sterilized Petri dishes. The experiment was repeated three times, with sterile water as the control, and each replication consisted of 20 larvae. All other conditions and the observations remained consistent with those described above.
Infection process of the fungus on different instar larvae of A. luctifer
For the observation of the infection process, larvae at each instar were cultivated and sampled at 0, 2, 4, 8, 12, 24, 48 and 72 h after treatment. The larvae were then sectioned into 6 μm thick slices using a full-automatic microtome (LEiCA RM2016; Shanghai Leica Instruments, Shanghai, China), following dewaxing and water washing. The sections were stained with PASM reagent (Leagene, DG0090) and the histopathological changes in the A. luctifer larvae infected with C. cateniannulata were observed and photographed using an OLYMPUS CX41 microscope (Olympus, Tokyo, Japan), with images processed in Photoshop CS6.
Statistical analysis
All statistical analyses were conducted using SPSS v.18.0. The Tukey test was used for multiple sets of data analysis (examining homogeneity of variance between trials and then combining the results from the three tests). All the data were represented as ME ± SE. All the graphs were generated with Excel 2022.
Results
Morphological characteristics of of the strain 19GZAl-1
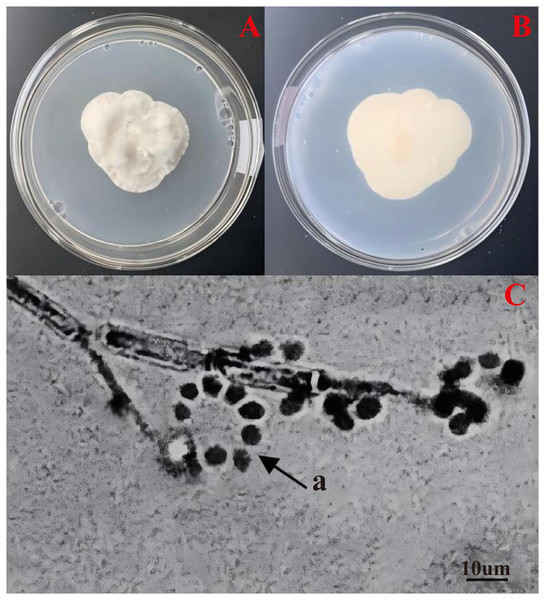
The colonies displayed velvety, protuberant, dense white conidia on the upper surface (Fig. 1A), and pale yellow to orange-brown undersides (Fig. 1B). The colonies gradually reached 4.68 × 4.01 cm in area after cultured 1 week at 25 °C. The hyphae of strain No. 10 were 1–3 µm wide, septate, diaphanous and smooth. The 2–4 phialides (12–19 × 2–3 µm) were typically found at the base of conidiophore structures (16–29 × 2–5 µm), mostly inflated at the base and tapering from basal and abruptly upwards halfway (Fig. 1C). The conidia (4–6 × 2–5 µm) were hyaline, smooth, ovoid or sub-globular, often forming imbricate oblique chains or rings (Fig. 1C-a). The colony colour, conidial stem and conidial morphology of this strain were all consistent with those of C. cateniannulata. Strain No. 10, designated as 19GZAl-1, was preliminarily identified as C. cateniannulata.
Figure 1: Morphological characteristics of C. cateniannula strain No. 10, 19GZAl-1.
(A and B) Culture plate shows the top and bottom of the colony, which is cultured on PDA medium; (C) conidiogenous structure and conidia of the No. 10.Taxonomic state of the strain 19GZAl-1 based on ITS1/ITS4 sequence and phylogenetic analysis
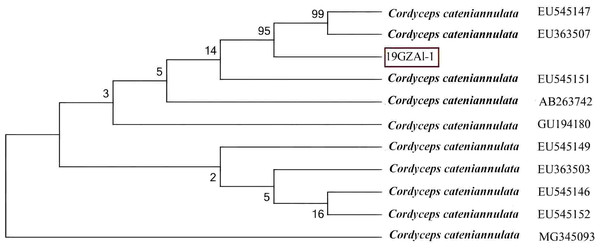
The ITS data for isolate 19GZAl-1 were deposited in GenBank with the accession number MW750414. The phylogenetic analysis revealed that the sequence identity values were 99% (Fig. 2), identical to sequences of C. cateniannulata, indicating that molecular data supported the morphological identity of our isolate ITS1/ITS4 as C. cateniannulata.
Figure 2: Phylogenetic analysis of the partial rDNA-ITS sequences of the isolated strain (19GZAL-1) and related species (obtained from GenBank).
Virulence of the entomopathogenic fungal strains against A. luctifer larvae in 3rd instar
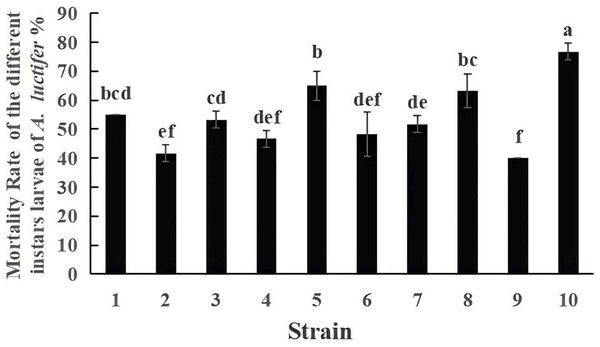
The mortality of A. luctifer 3rd instar larvae infected by the ten EPF strains were different (Fig. 3). Mortality of the larvae by strain No. 10, 19GZAl-1, reached 76.7%, higher than other fungal strains. The mortality of the larvae by strain No. 9 was 40%, which was the lowest. Among the six strains (No. 1, No. 5, No. 6, No. 7, No. 9 and No. 10) of the same species, C. cateniannulata, mortality rates of A. luctifer 3rd instar larvae infected varied significantly: strain No. 10, 19GZAl-1, demonstrated the highest mortality while strain No. 9 showed the lowest mortality, suggesting that strain 19GZAl-1 of C. cateniannulata may be a potential candidate agent for the control of A. luctifer larvae.
Figure 3: Mortality rate (%±SE) of A. luctifer larvae after inoculation with different EPFs within 10 days.
All data in the figure are mean ± standard error. The same lowercase letters indicated that the same difference was not significant (P > 0.05), and different lowercase letters indicated that the difference was not significant (P < 0.05).Mortality of A. luctifer larvae by the 19GZAl-1 of C. cateniannulata
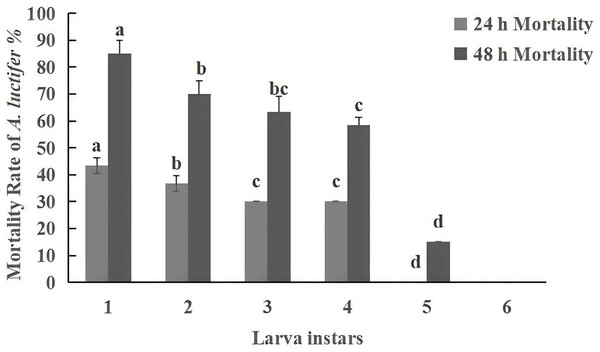
The experimental results in spray treatment revealed that the mortality of infected larvae reduced with the increase of larval instar (Fig. 4). The mortality of the 1st, 2nd, 3rd and 4th instar larvae infected by C. cateniannulata were 43.33%, 38.33%, 30%, 30% at 1 d, respectively. No dead larvae were observed in the 5th and 6th instar larvae at this time. As time went on, the mortality rate increased.
Figure 4: Mortality rate (%±SE) of the 3th instars larvae of A. luctifer treated with C. cateniannulata by using spraying and impregnating methods.
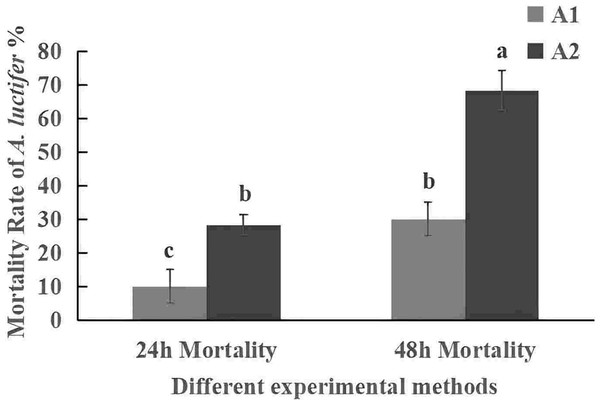
All data in the figure are mean ± standard error. The same lowercase letters indicated that the same difference was not significant (P > 0.05), and different lowercase letters indicated that the difference was not significant (P < 0.05). A1, impregnation method; A2, spray method.The mortality rate of the 3rd instar larvae infected with spray method was higher than that of impregnation method (Fig. 5). The mortality rate within 24 h was 30% in sprayring treatment, and only 10% in impregnating treatment. At 48 h, mortality rate was 63.3% by spray method and 28.3% by impregnation method. The difference may be due to spores not only attaching to the larval body surface but also to leaves during spraying. After the larvae fed on the leaves, the spores entered their body. The spray method is commonly used in the field, so it was used for subsequent field experiments.
Figure 5: Mortality rate (%±SE) of A. luctifer larvae in different instars treated with C. cateniannulata.
All data in the figure are mean ± standard error. The same lowercase letters indicated that the same difference was not significant (P > 0.05), and different lowercase letters indicated that the difference was not significant (P < 0.05). A1, impregnation method; A2, spray method.Symptoms of the larvae infected by the strain 19GZAl-1
Figure 6 shows the infection symptoms observred in different instar larvae infected by the strain 19GZAl-1 of C. cateniannulata. Across all instars, the symptoms were generally consistent: after being infected by the fungus, the larvae would eat petioles and move slowly, with no obvious tilting upon stimulation. The consumption rate (RCR) of larvae decreased gradually after infection until it reached zero before death. Initially, the amount of defecation increased, then diminished until almost no food residue remained in the intestines. The head color gradually shifted from dark brown to tan or yellow, while the body color gradually changed from green to light yellow (Fig. 6A). On the first day after death, the larvae typically adopted a “C” shape (Fig. 6A) or a “1” shape (Fig. 6B), and began to stiffen and melanize after 4 d (Fig. 6B). After 7 d, hyphae began to emerge from the integument, causing the larvae to atrophy (Fig. 6C). After 10 d, the larvae were completely covered with hyphae and conidia (Fig. 6D).
Figure 6: Infection symptoms of different instars larvae by C. cateniannula.
(A) Symptoms of infected larvae in the early stages of death; (B) symptoms of infected larvae about 4 d after death; (C) symptoms of infected larvae about 7 d after death; (D) symptoms of infected larvae about 10 d after death. Scale bars: 1 mm.Infection/growth behaviors of the submerged conidia on different instars larvae
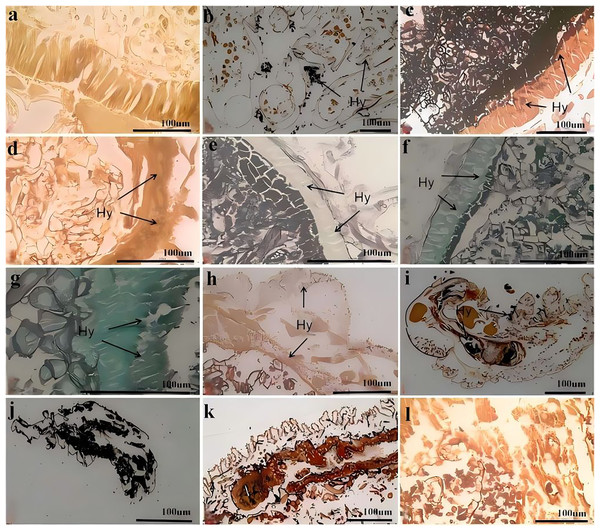
Figure 7 shows the process of C. cateniannulata infecting different instar larvae of A. luctifer, revealing no obvious difference across instars. The normal integument of A. luctifer larvae consists of the cuticle, epidermis and basement membrane. The midgut wall generally includes the peritrophic membrane, cell layer (midgut cells), and musal layer. The normal midgut cells were arranged neatly and regularly (Fig. 7A). Upon treated with a spore suspension, spores primarily adhered to the larval body surface, mainly on the baenopoda and integument folds (internode folds) at 0 h (Fig. 7B). At 2 h, the attached spores germinated, producing appressoria that began to invade the haemocoele (Fig. 6C). Rapid hyphal proliferation occurred within 4–8 h, with the hyphae invading the intestinal tract through the wall and causing gradual decomposition of midgut cells (Figs. 7D, 7E). At 12 h, cell arrangement loosened with distinct vacuoles, which might be due to the production of toxins and metabolites during conidia germination and mycelial growth of C. cateniannulata, changing the physical and chemical properties of the blood, interfering the immune system of the host cells, and disrupting normal metabolic function and morphology of the host (Pu & Li, 1996; Li, 2007). The intestinal wall of the larvae showed varying degrees of melanization, resisting hyphal invasion and leading to tissue atrophy (Fig. 7F). At 24 h, hyphal proliferation continued, causing disntegratioin of the columnar cell midgut wall and separation of midgut cells from the muscular layer (Fig. 7G). At 48 h, midgut cells completely separated from the muscular layer, the entire digestive tract disintegrated, and hyphae began to extend outward (Fig. 7H), attributed to mycelial proliferation that inhibited host cell phagocytosis and disrupted normal fluid circulation. In addition, physiological starvation, caused by the depletion of host nutrients, likely accelerated the mechanical destruction of self-organizing cells (Wang, 2015).
Figure 7: Infection process of C. cateniannulata into the larvae.
(A) Cross section of the control larvae treated with sterile water; (B) submerge conidia adhered to larvae after treatment; (C) germ tube of the submerged conidia begin to infect into the hemolymph, at 2 h; (D) the infected intestinal canal by the fungus, at 4 h; (E) the cells in the midgut wall decomposed, at 8 h; (F) infection at 12 h; (G) infection at 24 h; (H) infection at 48 h; (I) infected the 2nd instar larvae at 12 h; (J–L) the 1st, 4th, 6th instar larvae infected at 72 h; Hy (Hyphae).At 0 h, a greater number of spores were observed to attach to the 1st–2nd instar larvae compared to other instars. At 12 h, it was observed that C. cateniannulata spores infected the larvae not only via integument invasion but also through the digestive tract as the spores were ingested during feeding. As the hyphae consumed nutrients from the body fluids and tissues of the 1st–2nd instar larvae, the tissue structure became severely damaged (Fig. 7I). At 24 h, vacuolation of columnar cells in midgut walls of infected 6th instar larvae was observed, occurring later than in other instars. At 72 h, the hyphae of the 1st–2nd instar larvae filled the entire body and began to extend outward, penetrating the body wall to form conidiophores (Fig. 7J). At this stage, significant tissue structure damage was noted in the 3rd–4th instar larvae. The hyphae continued to grow externally (Fig. 7K). In the 5th–6th instar larvae, the peritrophic membranes were decomposed by hyphae, leading to the loosening and detachment of columnar cells. The hyphae filled the intestinal tract and continued to proliferate (Fig. 7L).
Discussion
Common identification methods for entomogenous fungi include morphological and molecular identification (Zhou et al., 2020). The macroscopic and microscopic morphological features, rDNA- ITS sequence alignment, and phylogenetic analysis of strain 19GZAl-1 in this study were consistent with C. cateniannulata (Wang et al., 2014; Zhou et al., 2020). Although slight differences in measurements of the micro-conidia and phialides, as well as in growth characteristics of the strain, may arise due to different media (Zhuang et al., 2020; Montesinos-Matías et al., 2021), the collected fungus 19GZAl-1 is possibly a new isolate of C. cateniannulata.
The pathogenicity of the EPF to pests varies with different pest species and fungal species or strains (Zhang et al., 2013, 2014; Rehman et al., 2019; Montes-Bazurto, Bustillo-Pardey & Medina-Cárdenas, 2020; Folgarait, Goffré & Osorio, 2020; Dhanapal et al., 2020; Nawaz & Freed, 2022). Our results, consistent with previous studies demonstrate for the first time that C. cateniannulata has a significant pathogenic effect on the larvae of A. luctifer, In studies by Zhang et al. (2022) and Yang et al. (2024), it was also shown that C. cateniannulata not only promoted growth but also had the potential to control pests and diseases, although the pathogenicity to larvae varies with different strains. This variation might be attributed to differences in key enzymes and their activities, such as protease and chitinase, which are essential for epidermis degradation and differ among fungal species and strains (Wang et al., 2019; Geng, 2016). The differences within the same species may also arise due to intraspecific genetic variation, affecting the morphological characteristics of the strain and its pathogenicity to host insects (Han et al., 2012; Maistrou et al., 2020).
Under the same conditions, the pathogenicity of entomopathogenic fungi is negatively correlated with larval instar, with younger larvae being more susceptible to infection than older larvae (Sufyan et al., 2019; Manzoor et al., 2020). Fite et al. (2020) found that the mortality rate of 2nd instar larvae (69%) of Helicoverpa armigera was higher than that of 3rd instar larvae (56.5%) when treated with a spore concentration of 109 spores/mL of Beauveria bassiana. Other studies (Niu et al., 2022; Wang et al., 2016) found that increasing inoculation time of B. bassiana, LC50 decreased, and the dose effect gradually improved, the younger the larval age, the higher the mortality rate. The present study has also found that within 48 h, the mortality of the 1st instar larvae reached the highest level of 85%, gradually decreasing with the increase in instar. No “zombie” phenomenon was found in the 6th instar larvae, which was attributed to differences in immunity among larval instars, as the humoral and cellular immune responses of larvae enhance with the increase of instars (Li, 2019).
Different application methods directly affect the lethal effect of entomopathogenic fungi on pests (Djouhri et al., 2022; Lv & He, 2010). The research found that, under room temperature and natural light, Compared to the feeding method (Ren et al., 2014), the spraying method had a more significant effect on the control of C. cateniannulata. Under the conditions of 25 °C, RH90% and L:D = 14 h:10 h, the pathogenicity of B. bassiana to the 2nd instar larvae of Plutella xylostella was better when treated with the impregnation method compared to the spraying method. However, for the 3rd instar larvae, the spraying method was more effective; Under laboratory conditions, the mortality rate with the spraying method was 35% higher than with the impregnation method. This may be because that different parts of the insect have different susceptibilities to pathogenic entomopathogenic fungi (Cai et al., 2009). Paraffin section showed that spores were more likely to invade the body through areas such as base of baenopoda and integument folds where cuticle is thin. In the spraying method, commonly used in field, the residual spores sprayed on buckwheat leaves could increase infection potential as larvae feed and move, laying the foundation for field tests to control A. luctifer larvae.
All larvae infected by entomopathogenic fungi showed symptoms of infection. Studies have found that the larvae of Sesamia nonagrioides (Mantzoukas & Grammatikopoulos, 2019) and Spodoptera exigua (Song et al., 2006) infected by B. bassiana exhibited slowed motion and decreased food intake. Similar to the findings in this study, the larvae of A. luctifer exhibited slow movements and no obvious tilting when stimulated after 24–48 h of infection with C. cateniannulata. After 48 h, the color of the larval head gradually changed from dark brown to tan or yellow, and the color of the body gradually shifted from green to light yellow and stiff. The change many be attributed to the prolific growth of invading C. cateniannulata hyphae, which consumed large amounts of nutrients, blocked circulation, and affected the physiological activities of the larvae. After the death of larvae due to infection, tissues in the body were digested and decomposed, resulting in a pale yellow surface color.
Entomopathogenic fungi primarily infect pests via two main pathways: one is by penetrating insect integument, mostly at stomata, internode membrane and wounds, and the other is by penetrating internal walls via respiratory and digestive tract (Wang et al., 2008). The results in this study are consistent with those reported by previous studies, indicating that C. cateniannulata could infect A. luctifer larvae through integument, respiration, and feeding. The spores primarily attach to the thorax foot and folds of the larvae, degrade the outer epidermis, penetrate the inner epidermis and intestinal wall, and then enter the body cavity. This also proves once again that the death of host by contacting the body wall is advantageous for the development of entomogenous fungi as biocontrol agents (Holder & Keyhani, 2005; Li, 2015; Wang et al., 2017).
The host exhibits an antifungal effect on pathogenic microorganisms primarily through melanization, which is the main mode of action of humoral immunity. It could produce a large amount of phenol oxidase and melanin through the phenol oxidase-serine cascade reaction or through encapsulation, acting directly on pathogenic microorganisms (Butt et al., 2016). The study found that the blood darkening accelerated with an increase in instar (Lv, 2014), and as the instar of silkworm increased, the expression levels of storage proteins with antifungal and anti-melanization properties continuously improved (Li, 2019). After Bombyx Mori was infected with Escherichia coli, the change trend coincided well among dose groups within the same instars but varied between different instars, implying that the ability of the circulatory system to maintain homeostasis in immunity was positively correlated with the developmental stage, and immunological characteristics varied at different developmental stages (Li et al., 2019). Similar to the findings in the present study, melanization occurred earlier in the larvae as instar increased, which might be due to older larvae having stronger immune defense against the fungus, suggesting differences in the immunological characteristics between developmental stages.
The results of this experiment revealed that C. cateniannulata strain 19GZAl-1 could infect the 1st–5th instar larvae of A. luctifer within 2 d by spraying, with younger larvae exhibiting higher mortality rates. Therefore, it can be concluded that the C. cateniannulata has a good application effect on the pests before the 3rd instar, providing a foundation for further research on C. cateniannulata as a biocontrol agent against A. luctifer.