Exogenous silicon induces aluminum tolerance in white clover (Trifolium repens) by reducing aluminum uptake and enhancing organic acid secretion
- Published
- Accepted
- Received
- Academic Editor
- Piotr Dąbrowski
- Subject Areas
- Agricultural Science, Plant Science, Soil Science, Environmental Contamination and Remediation, Environmental Impacts
- Keywords
- White clover, Aluminum toxicity, Silicon, Organic acids, Mineral distribution
- Copyright
- © 2024 Yang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Exogenous silicon induces aluminum tolerance in white clover (Trifolium repens) by reducing aluminum uptake and enhancing organic acid secretion. PeerJ 12:e17472 https://doi.org/10.7717/peerj.17472
Abstract
Excessive aluminum (Al) in acidic soils is a primary factor that hinders plant growth. The objective of the present study was to investigate the effect and physiological mechanism of exogenous silicon (Si) in alleviating aluminum toxicity. Under hydroponic conditions, 4 mM Al significantly impeded the growth of white clover; however, pretreatments with 1 mM Si mitigated this inhibition, as evidenced by notable changes in growth indicators and physiological parameters. Exogenous silicon notably increased both shoot and root length of white clover and significantly decreased electrolyte leakage (EL) and malondialdehyde (MDA) content compared to aluminum treatments. This positive effect was particularly evident in the roots. Further analysis involving hematoxylin staining, scanning electron microscopy (SEM), and examination of organic acids (OAs) demonstrated that silicon relieved the accumulation of bioactive aluminum and ameliorated damage to root tissues in aluminum-stressed plants. Additionally, energy-dispersive X-ray (EDX) analysis revealed that additional silicon was primarily distributed in the root epidermal and cortical layers, effectively reducing the transport of aluminum and maintaining the balance of exchangeable cations absorption. These findings suggest that gradual silicon deposition in root tissues effectively prevents the absorption of biologically active aluminum, thereby reducing the risk of mineral nutrient deficiencies induced by aluminum stress, promoting organic acids exudation, and compartmentalizing aluminum in the outer layer of root tissues. This mechanism helps white clover alleviate the damage caused by aluminum toxicity.
Introduction
Soil acidity is exacerbated by the substantial influx of H+, NH4+, NO3−, and SO42− ions into the soil. Acid precipitation (H+), the overuse of chemical fertilizers, and the emission of acidifying gases (such as NO3 and SO2) are the causes of this increase (Bojórquez-Quintal et al., 2017). The substitution of H+ and Al3+ ions for exchangeable base cations such as magnesium (Mg), potassium (K), and zinc (Zn) is one of the most significant effects of soil acidification (Kochian et al., 2015). In acidic soils, an excess of aluminum (Al) becomes the primary inhibitor of crop growth and often leads to crop death. The presence of aluminum in acidic soil not only negatively affects crop production but also poses annual obstacles to economic growth (Dong et al., 2018; Hao et al., 2022). The suppression of root growth is a defining sign of Al poisoning (Hodson & Evans, 1995). Previous studies have shown that aluminum imposes significant stress on the root tips (Hao et al., 2022). Elevated concentrations of bioactive aluminum can inhibit the absorption of water and mineral ions, as well as prevent root elongation and the accumulation of reactive oxygen species (ROS) (Hodson & Evans, 2020; Sun et al., 2020a).
Most plants benefit from silicon, which is also known to be efficient in reducing biotic and abiotic stress in a variety of ways (Anderson et al., 2022; Jonas et al., 2022; Zahra et al., 2021). According to research, plants can accumulate silicon at levels ranging from 1 to 100 g·kg−1 on average, depending on their species and capacity (Ma, 2007). Many silicon transporters have been reported in monocots because they can absorb and accumulate silicon, whereas dicots typically accumulate silicon less frequently (Coskun et al., 2021). Research findings indicate that the utilization of silicon in monocotyledonous crops, such as maize (Zea mays), can notably improve their resistance to aluminum (Sousa et al., 2019; Wang, Angelika & Walter, 2004) and rice (Oryza sativa) (Xiao et al., 2021). Regarding whether silicon can efficiently detoxify aluminum in forage crops, especially in leguminous forages, there is a significant research gap (Pontigo et al., 2017; Bhat et al., 2019).
White clover (Trifolium repens) is widely recognized as one of the world’s best perennial legumes because of its prostrate growth habit and excellent feed quality (Li et al., 2018). White clover is widely cultivated for grazing in many countries. In regions with elevated soil acidification, like in south-western China, the growth and utilization of white clover are significantly hindered (Pan, Zhu & Cheng, 2008). Recent studies have significantly enhanced our comprehension of how various crops alleviate aluminum stress through silicon induction. However, we still lack a comprehensive understanding of the underlying mechanisms of silicon-mediated aluminum tolerance in white clover. Therefore, the aim of our study was to investigate the feasibility of exogenous silicon supplementation under aluminum stress by evaluating growth indices, physiological parameters, endogenous silicon and aluminum ion concentrations, and organic acid levels in white clover leaves and root systems. At the same time, the internal mechanism of silicon-induced aluminum resistance was elucidated.
Materials and Methods
White clover culture and Al stress treatments
We used ‘Haifa’ seeds from Barenbrug China Company as our experimental material in this study. Prior to being used, the seeds were sterilized by rinsing them three times with distilled water after immersing them in a 0.1% mercuric chloride solution for 5 min. A meticulously regulated growth chamber (GHP-500; Jiangnan Instrument Factory, Ningbo, China) with a 12-h photoperiod, 75% relative humidity, and a day/night temperature of 23 °C was used throughout the entire experiment. There was 750 μmol m−2 s−1 of light. After 7 days of germination, the seedlings were carefully transferred into plastic trays and cultivated in Hoagland’s solution at a 1/4 strength for 3 days. They then spent an additional 23 days growing in the solution at half strength. Before being exposed to aluminum stress, the white clover plants were first subjected to a 4-day pretreatment with either 0 or 1 mM K2SiO3. Using 4 mM AlCl3 dissolved in 1/2 strength Hoagland’s solution, aluminum stress was induced. Four distinct treatment groups were formed, namely: (1) CK (control): plants were kept in 1/2 concentration of Hoagland’s solution for 19 days without receiving any additional Al or Si; (2) Si (exogenous Si treatment): plants were pretreated with Si for 4 days before being exposed to 1/2 concentration of Hoagland’s solution for 15 days; (3) Al (Al stress): plants were exposed to 1/2 concentration of Hoagland’s solution for 4 days, followed by 15 days of Al stress, with pH kept at 4.5; and (4) Al+Si (exogenous Si treatment followed by Al stress): plants were pretreated with Si for 4 days before being exposed to 15 days of Al stress, with pH kept at 4.5. Every therapy was administered in growth chambers at random.
Measurement of growth index
Ten independent biological replicates were assigned to each treatment group after dividing the plants from the four treatment groups into shoot and root sections for length measurement. The shoot and root tissues were then placed in an oven (DHG-2200B; Sheng Yuan Instrument Co., Ltd., Zhengzhou, China) at 65 °C until they reached a constant dry weight.
The physiological parameters
Four independent biological replicates were assigned to each treatment, and meticulous measurements of physiological indices were conducted on the second leaves of multiple stems and the entire root system of each plant. 0.1 g of leaves and roots were carefully placed in centrifuge tubes with 35 ml of distilled water to test for electrolyte leakage (EL). The tubes were then incubated for a full day. Then, a conductivity meter (DDS-307; Lei-Ci, China) was used to measure the initial conductance (Ci). The tubes were then autoclaved for 20 min at 140 °C, and the maximum conductance (Cmax) was determined. Finally, the formula (%) = Ci/Cmax × 100 was used to calculate EL (Blum & Ebercon, 1981). The formula used to compute the relative water content (RWC) of leaves is RWC (%) = ((FW − DW)/(SW − DW)) × 100%. Here, FW stands for the weight of the leaves when they were fresh, SW for their weight after they were saturated after soaking in ultrapure water for 24 h, and DW for their weight when they were dried in an oven for 24 h at 80 °C to a consistent weight. 0.15 g of fresh tissue was cleaned before being submerged in 15 ml of dimethyl sulfoxide solution. It was then left in the dark at room temperature for 8 h, or until the leaves turned translucent, to extract chlorophyll (Chl). The extract’s absorbance was then measured using a spectrophotometer (Spectronic Instruments, Rochester, NY, USA) at 663 and 645 nm (Barnes et al., 1992). Using the Chlorophyll Fluorescence System (Pocket PEA, Hansatech, UK), white clover leaf samples were dark-adapted for 20 min. PIABS and PSII Fv/Fm measurements were taken. PIABS serves as a comprehensive photosynthetic index and a plant health status indicator. In order to extract malondialdehyde (MDA), 0.1 g of leaves and roots were first crushed separately using liquid nitrogen. Following this, the mixture was centrifuged for 30 min at 12,000 rpm and 4 °C using 2 mL of phosphate buffer (50 mM, pH 7.8). One ml of reaction solution containing 0.5% thiobarbituric acid and 20% trichloroacetic acid was mixed with the supernatant. After centrifugation, 1 mL of the reaction solution containing 20% trichloroacetic acid and 0.5% thiobarbituric acid was mixed with 0.5 mL of the supernatant. Following centrifugation, 1 mL of reaction solution containing 20% trichloroacetic acid and 0.5% thiobarbituric acid is mixed with 0.5 mL of supernatant, and the mixture is heated for 15 min at 95 °C. The mixture was quickly cooled in an ice water bath before being centrifuged for 10 min at 8,000 rpm. The OD600 and OD532 values were subtracted to determine the absorbance of the supernatant (Dhindsa, Pamela & Thorpe, 1981).
Measurement of Al and Si content
After 15 days of aluminum stress, the leaves and root tissues of white clover were carefully placed in an oven at 65 °C. Samples with a dry weight of 1 g were digested with 5 mL of hydrofluoric acid (HF) and 2 mL of hydrogen peroxide (H2O2). Each treatment comprised four independent biological replicates, and the aluminum and silicon content was determined using inductively coupled plasma mass spectrometry (ICP-MS).
Root staining
The plant roots were carefully submerged in deionized water for 5 min, irrespective of whether they had been exposed to aluminum stress for 15 days. The samples were subsequently rinsed in deionized water for an additional 15 min after being stained with hematoxylin staining solution for 15 min. The staining solution consisted of 0.1% hematoxylin, 0.01% KIO3, and 0.2 mM NaOH (Delhaize, Ryan & Randall, 1993). The LEICA MC 190 HD stereomicroscope was used to examine the stained roots’ hematoxylin staining patterns after they had been placed on a level surface.
Measurements of internal and external OAs
Regardless of whether they had received Si pretreatment or not, white clover plants were transferred to trays filled with 1/2 strength Hoagland’s solution containing either 0 or 4 mM AlCl3 and left for a full day. Then, 0.2 g of root tips were collected to measure the concentration of citrate and malate, and the solution was collected to measure the exudation of these two compounds. A spectrophotometer was used to measure the exudation and concentration of citrate and malate. G0864W and G0862W kits (Suzhou Geruisi Bio-Technology Co. Ltd., Suzhou, China) were used to measure the OD470 and OD450 readings, respectively.
Micro-distribution and compartmentation of ions in root
The elemental distribution and mineral ion compartmentation in the root samples were carefully investigated using an energy dispersive X-ray (EDX) analyzer attached to a scanning electron microscope (SEM). The plant root tips were carefully cut into 1 cm pieces with a sharp knife, rapidly submerged in liquid nitrogen, and then freeze-dried for 24 h at −80 °C. For each treatment, three distinct samples were meticulously collected for X-ray microanalysis. After deducting background X-ray counts, the net K-shell X-ray peak counts were used to automatically quantify the relative weights of the mineral ions in the root’s epidermal, cortical, and stelar layers (Javaid et al., 2019).
Statistical analyses
In this study, all data were presented as the mean ± standard error (SE) of at least three replicates. Using SPSS version 25, the Levene’s test for homogeneity of variance was conducted, followed by Tukey’s post hoc multiple comparison correction technique. In particular, the process included selecting “Data Analysis,” conducting a one-way ANOVA test, and then performing multiple comparisons afterward. Both the Tukey and LSD approaches were selected, with a significance threshold of 0.05. Principal component analysis (PCA) was conducted using Origin 2021 software. Principal component analysis was chosen as the method, and a 2D component plot was selected. All other settings were left at their default values.
Results
Growth and physiological response of white clover plants under treatments
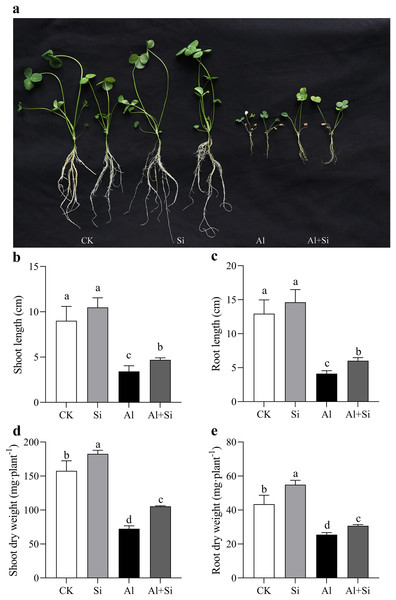
Figure 1A illustrates the growth phenotype of white clover under various treatments. It is clear that exogenous silicon had a mitigating impact on aluminum stress, but aluminum clearly hindered the growth of white clover. The measurements of length and dry weight under various treatment conditions further confirmed this observation. Both the length and dry weight significantly decreased under Al stress (54% and 41%, respectively, compared to the control in shoots and roots, and 62% and 68%, respectively, compared to the shoots and roots). On the other hand, silicon treatments substantially reduced the inhibition of dry weight (45% increase in shoots and 20% in roots) and length (37% increase in shoots and 46% in roots) compared to aluminum treatments. Furthermore, under normal conditions, additional silicon had a favorable effect on white clover growth, as evidenced by a significant increase in the dry weight of both shoots and roots (Figs. 1D, 1E).
Figure 1 : Changes of growth parameters on white clover in response to Al toxicity.
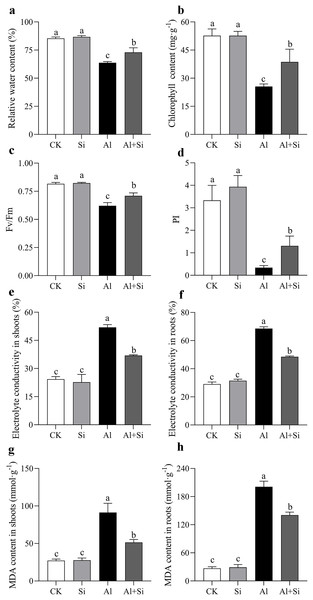
The phenotype of white clover under Al and Si treatments was taken (A), and the length of shoots (B) and roots (C), as well as the dry weight of shoots (D) and roots (E) were measured after 15 days Al toxicity with or without Si pretreatment. Data are mean ± SE, n ≥ 3.We carefully examined the physiological characteristics of the plants to confirm the impact of silicon on aluminum stress. There were no appreciable differences in the phenotypic or physiological indices between the silicon-treated plants and the untreated control group (Figs. 2A–2H). Nevertheless, exposure to aluminum stress resulted in a significant drop in the leaves’ relative leaf water content (RGH) and chlorophyll levels, as well as a decrease in photosynthetic efficiency and overall health index, irrespective of silicon treatment. However, by the nineteenth day of aluminum stress, plants treated with silicon showed significant increases of 1.15 times for relative leaf water content (Fig. 2A), 1.4 times for chlorophyll content (Fig. 2B), and 3.88 times and 1.14 times for photosynthetic efficiency and health index, respectively (Figs. 2C, 2D). Furthermore, aluminum stress induced a significant increase in conductivity and MDA content in both leaves and roots of the plants. Conversely, under silicon treatment conditions, conductivity decreased by 15.27% in leaves and 18.32% in roots (Figs. 2E, 2F), while MDA content decreased by 42.21% in leaves and 67.23% in roots (Figs. 2G, 2H).
Figure 2 : Changes of physiological parameters on white clover in response to Al toxicity.
Electrolyte leakage in shoots (A) and roots (B), malondialdehyde content in shoots (C) and roots (D) were determined after 15 days Al toxicity with or without Si pretreatment. Data are mean ± SE, n ≥ 3. Different letters indicate significant differences at P < 0.05.Exogenous Si pretreatment increased the endogenous Si concentration and decreases Al accumulation under Al stress
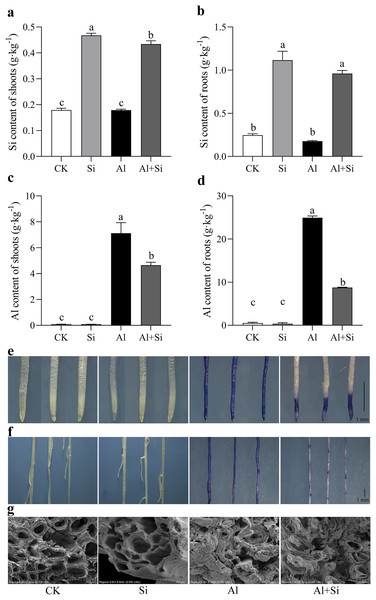
Endogenous silicon (Si) content in shoots and roots increased by 60% and 74%, respectively, in Si-treated shoots and roots compared to the control. However, there were no significant differences in endogenous silicon content between the control and aluminum treatments, as well as between the silicon and Al+Si treatments (Figs. 3A, 3B). Aluminum (Al) stress was found to cause a substantial increase in Al content in shoots and roots (80- and 48-fold increases, respectively, compared to the control), with Al predominantly accumulating in the roots (over 24 g·kg−1). Additionally, compared to aluminum-stressed plants, the aluminum concentration in the roots and shoots of the Al+Si treatments was significantly lower by 65% and 29%, respectively (Figs. 3C, 3D). Hematoxylin staining, an indicator of aluminum absorption, was used to confirm the uptake of Al in the roots under various treatments (deeper staining indicating a higher concentration of physiologically active Al). Compared to the roots in Al treatments, the roots in Al+Si treatments exhibited a lighter hematoxylin stain. In contrast, only the lateral root development sites in Al+Si treatments were darkly colored, while the entire root surface in Al treatments appeared dark (Figs. 3E, 3F). SEM analysis of root cross-sections revealed that aluminum damaged the cortical, stellar, and epidermal tissues of the roots, leading to a reduction in the xylem area and eventual collapse. In contrast to aluminum treatments, silicon priming resulted in a larger xylem area and reduced collapse (Fig. 3G).
Figure 3 : Effect of exogenous Si on the content of endogenous Si and Al and the structures of root apices under Al stress.
The Si content in the shoot (A) and root (B), and the Al content in the shoot (C) and root (D) were measured using ICP-MS analysis after 15 days Al stress. Data are mean ± SE, n = 4. Different letters indicate significant differences at P < 0.05. To observe the accumulation of Al, the root apices (E) and whole roots (F) were stained with hematoxylin, and the structures of root apices (G) were taken by SEM after 15 days Al stress.Exogenous Si improves the exudation and concentration of OAs under Al toxicity
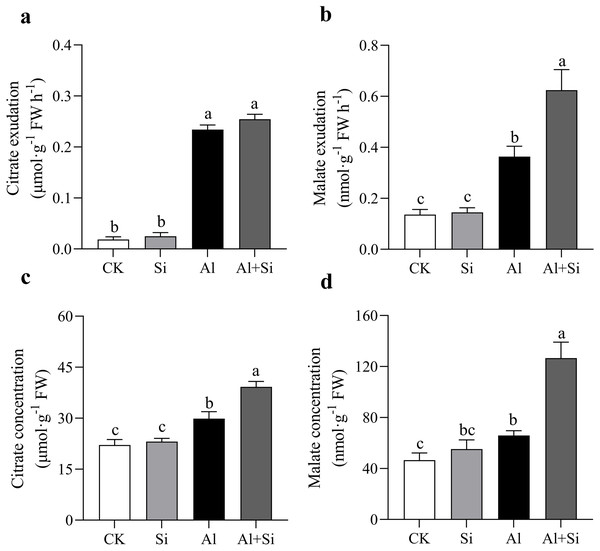
In our studies, aluminum dramatically increased the exudation of malate and citrate. When compared to normal plants, the exudation of citrate and malate increased by approximately 12 and 2 times, respectively, in Al-stressed plants. Furthermore, there was no discernible difference in citrate exudation between the two treatments, but malate exudation was 71% higher in the Al+Si treatment than in the Al treatment (Figs. 4A, 4B). In the Al and Al+Si treatments, compared to the control, the concentrations of citrate and malate increased by 35% and 41%, and 77% and 172%, respectively. These increases indicate that malate was more sensitive to silicon stimulation than citrate in response to aluminum stress in white clover (Figs. 4C, 4D).
Figure 4 : Effect of Si on external and internal citrate and malate concentration with Al stress.
The exudation of citrate (A) and malate (B), and the concentration of citrate (C) and malate (D) in roots were measured at OD470 and OD450 with a spectrophotometer by using citrate and malate kits (G0864W, G0862W) after 24 h Al stress. Data are mean ± SE, n ≥ 3. Different letters indicate significant differences at P < 0.05.Micro-distribution and compartmentation of ions in white clover root tips
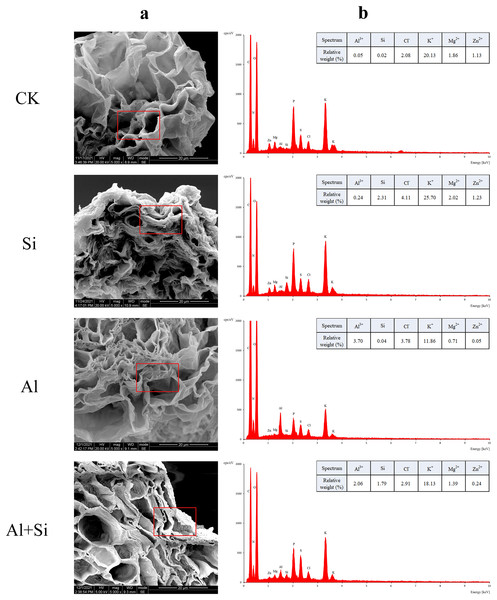
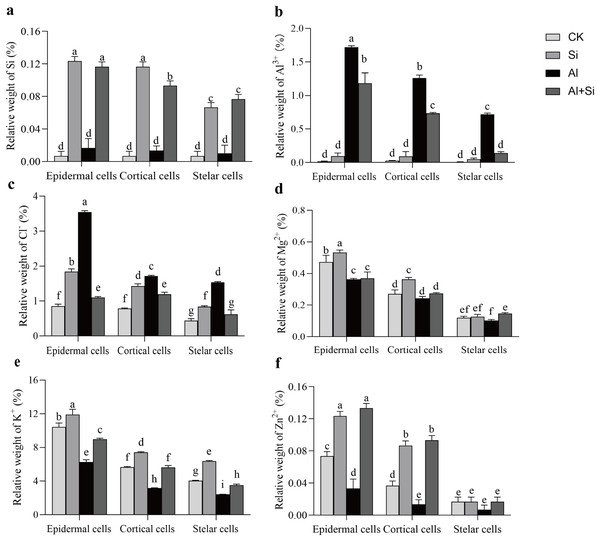
In order to understand how Si affects the distribution of mineral elements (Si2+, Al3+, Cl−, Mg2+, K+, and Zn2+) in roots across four treatments, energy-dispersive X-ray (EDX)-based mineral element microanalysis was conducted by scanning the cross-section of root tips and analyzing energy spectra. The cross-sectional image of the root revealed that the cortical and epidermal regions were the primary areas where newly added silicon accumulated, with the smallest xylem area observed in the aluminum treatments (Fig. 5A). When white clover plants were treated with exogenous silicon, the concentration of Si peaked, and the relative contents of Cl−, Mg2+, K+, and Zn2+ increased by 97%, 8%, 28%, and 9%, respectively, compared with the control. The relative weights of Mg2+, K+, and Zn2+ were all lower under Al stress (62%, 41%, and 95% lower than under control conditions, respectively). On the other hand, Al+Si treatments protected the xylem from Al stress and decreased the relative concentration of Al3+ by 44%. Additionally, in comparison to aluminum stress, they increased the relative weight of Mg2+, K+, and Zn2+ by 96%, 53%, and 356%, respectively (Fig. 5). Using EDX-based methods, mineral element microanalysis was conducted in the epidermal, cortical, and stelar layers of the root, as further reported (Fig. 6). The relative weight of silicon in the epidermal, cortical, and stelar layers increased dramatically in the silicon treatments compared to the control, reaching 18.5-fold, 17.5-fold, and 10.0-fold, respectively. Significant silicon buildup was observed in the epidermal and cortical layers, which was found to have a positive effect on the accumulation of Cl−, Mg2+, K+, and Zn2+. In contrast, aluminum stress significantly decreased the relative weight of Mg2+, K+, and Zn2+ in the cortical layers by 10%, 44%, and 64%, in the stelar layers by 139%, 40%, and 60%, and in the epidermal layers by 23%, 40%, and 55% compared to the control. The relative weight of Al3+ in the cortical, stelar, and epidermal layers increased by 1.72%, 1.26%, and 0.72%, respectively, compared to the control, as predicted. The epidermal layers acquired the highest amount of Al3+. In the meantime, K+ levels in the root epidermal, cortical, and stelar layers significantly increased in the Al+Si treatments, and the relative weight of Zn2+ in the root epidermal and cortical layers similarly increased. In contrast to aluminum treatments, the administration of silicon did not significantly alter the relative weight of Mg2+ in either the cortical or epidermal layers (Figs. 6D–6F).
Figure 5 : EDX-based microanalysis of mineral elements in the root of white clover.
For each measurement, root samples from three independent experiments were selected to analyze the energy spectra of different mineral elements. The representative images of typical cryo-planed transverse sections in white clover roots were shown, the red rectangles were the areas of EDX analyses (A) for energy spectra of different mineral elements (B).Figure 6 : Relative weight (%) of different mineral elements in epidermal, cortical and stelar layers of the root in white clover plants.
Root samples were selected to analyze the energy spectra of different mineral elements in epidermal, cortical and stelar layers, respectively. Data are mean ± SE, n = 3. Different letters indicate significant differences at P < 0.05.Principal component analysis of the impact of different treatments on the growth of white clover
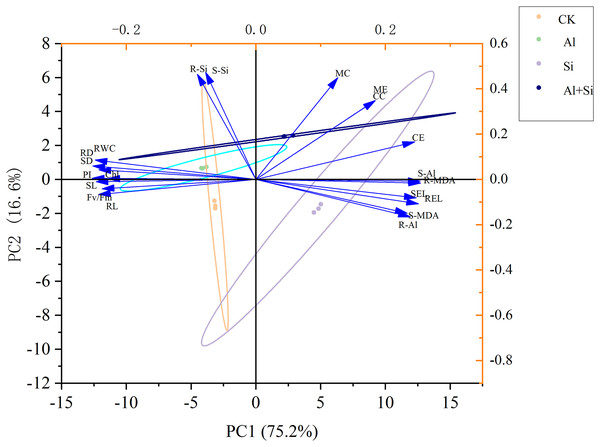
The morphological and physiological traits of seedlings under various treatments were thoroughly examined using principal component analysis (PCA) to evaluate the mitigating effect of exogenous silicon under aluminum stress. PC1 and PC2, which clearly separated the various treatments, accounted for 75.2% and 16.6% of the overall variance, respectively, as shown in Fig. 7. Notably, the treatments with aluminum stress alone and in combination with exogenous silicon were precisely distributed in the first and fourth quadrants, respectively, while the control (CK) treatment was clearly located in the third quadrant and the exogenous silicon treatment in the second quadrant. Additionally, in PC1, there were noteworthy positive relationships between the following parameters: electrolyte conductivity of the shoots and roots; MDA content in the shoots; Al content in the shoots; and concentrations of citrate, malate, citrate exudate, and malate exudate. Significantly and favorably correlated with citrate concentration, malate concentration, citrate exudation, and malate exudation, respectively, was the silicon content in shoots and roots in the analyses that included PC2. This indicates that exogenous silicon treatments were successful in promoting the secretion of organic acids, thereby significantly reducing the toxicity of aluminum. The silicon content of shoots and roots was also significantly negatively correlated with the MDA content of shoots, the Al content of roots, the Al content of shoots, and the electrolyte conductivity of both. This demonstrates how the administration of exogenous silicon greatly reduces oxidative damage, enhances plant resilience to aluminum stress, and significantly decreases the uptake and translocation of aluminum in white clover (Fig. 7).
Figure 7 : Principal component analysis of the impact of different treatments on the growth of white clover.
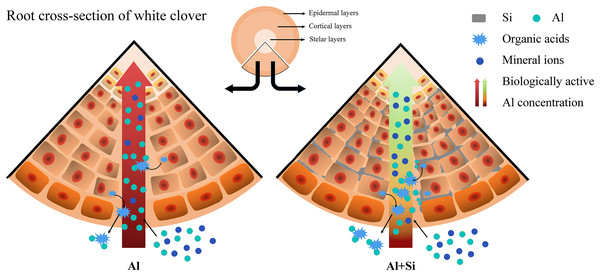
SL, shoot length; RL, root length; SD, shoot dry weight; RD, root dry weight; RWC, relative water content; Chl, chlorophyll content; Fv/Fm, maximum quantum yield of photosystem II photochemistry; PI, PIABS; SEL, electrolyte conductivity in shoots; REL, electrolyte conductivity in roots; S-MDA,MDA content in shoots; R-MDA, MDA content in roots; S-Al, Al content in shoots; R-Al, Al content in roots; S-Si, Si content in shoots; R-Si, Si content in roots; CC, citrate concentration; MC, malate concentration; CE, citrate exudation; ME, malate exudation.We present a well-developed conceptual model that clearly illustrates the essential role of silicon in reducing the toxicity of aluminum, drawing on insights obtained from comprehensive investigations. When white clover is exposed to aluminum stress, exogenous silicon pretreatment plays a critical role in reducing the negative effects. It accomplishes this by greatly boosting the production of organic acids and carefully controlling the absorption of vital mineral elements (Fig. 8).
Figure 8 : Effect of Si in white clover root with Al stress. Extra Si deposition in root tissues forms a barrier.
Firstly, Si will reduce Al accumulation by combining with the biologically active Al, when excess Al is coming. Then, Si will alleviate Al toxicity by balancing the absorption of mineral nutrients and promoting organic acid secretion, when Al is working. The compartmentalized Al with no biological activity caused by Si in the outer layer of root tissues could protect white clover from Al toxicity.Discussion
With the increasing amount of research being conducted on exogenous additives to reduce abiotic stress, it is possible that environmentally friendly exogenous supplements, such as silicon, will be widely utilized in various industries (Jonas et al., 2022; Lamia et al., 2022; Renjuan et al., 2021; Yue et al., 2022). Determining how silicon functions in various plant species to reduce aluminum toxicity is crucial. This study investigated how different treatments affected the physiological function and growth of white clove. According to the study’s findings, aluminum stress significantly impedes the normal growth of white clover. A significant increase in conductivity and MDA concentration in roots and aboveground tissues, along with a decrease in aboveground biomass, health index, and photosynthetic efficiency, were indicative of this situation. These results imply that white clover suffered significant oxidative damage due to aluminum stress (Figs. 1, 2). On the other hand, exogenous silicon treatment significantly reduced the conductivity and MDA concentration in Al-stress conditions, along with oxidative damage and the Al-stress-induced inhibition of root elongation. On the other hand, photosynthetic efficiency and the relative water content of above-ground leaves both markedly increased. According to earlier studies, silicon reduces aluminum-induced root rot and promotes root growth in rice, thereby alleviating aluminum stress. Silicon also inhibits aluminum absorption and translocation in roots (Xiao et al., 2022). To mitigate the inhibition of rice root growth, silicon encourages the elongation of the transition zone cell wall in the transition zone of root tips and reduces the accumulation of aluminum in the cell wall (Jiang et al., 2022). Furthermore, our research revealed that exogenous silicon treatment greatly decreased aluminum accumulation in both above-ground and root tissues under aluminum stress conditions (Fig. 3). This suggests that silicon can further maintain normal plant growth by mitigating aluminum-induced oxidative damage by reducing aluminum absorption and translocation in plants. Research on the interaction between silicon and aluminum in dicotyledonous plants has attracted significant interest (Hodson & Evans, 2020; Khan et al., 2021; Xiao et al., 2021). In contrast to monocotyledonous plants like rice, white clover has a very limited capacity for absorbing silicon (0.74 g·kg−1 Si accumulation). White clover is considered a non-accumulator of silicon due to its low Si accumulation (Ma & Yamaji, 2006; Ma, 2007). On the other hand, white clover’s silicon efficiently reduces aluminum toxicity (Fig. 3). White clover, on the other hand, primarily accumulates silicon in its roots, which likely shields them from the toxic effects of aluminum. Monocotyledons typically accumulate silicon in their shoots (Pan, Zhu & Cheng, 2008). Hematoxylin staining in our studies showed that Si+Al treatment resulted in shorter and lighter root tip staining. This indicates that Si limits the accumulation of physiologically active Al after Al uptake. Interestingly, following silicon treatment, we observed that the root protrusion sites exhibited darker staining compared to other sites. This could mean that, as lateral root development progresses, the root protrusion sites absorb more aluminum than other sites. This provides a theoretical basis for dicotyledonous plants, especially leguminous forages, to detoxify aluminum using silicon. Aluminum toxicity is a significant factor that hinders plant growth in acidic soil. Plants have evolved a variety of defense mechanisms, such as internal tolerance and external exclusion, to enable them to survive and flourish in harsh environments. These systems also help plants grow in acidic soil. To reduce aluminum toxicity, the tolerance mechanism primarily involves sequestering aluminum during transportation and storage in metabolically less active compartments such as vacuoles and the epidermis (Goodwin & Sutter, 2009). The mechanisms through which aluminum is excluded externally include the release of ligands (such as organic acids and phosphates) that can chelate aluminum, cell wall binding of aluminum, stimulation of the rhizosphere pH barrier, and active efflux of Al3+ out of cells (Tolra et al., 2005; Kochian et al., 2015). One common mechanism by which plants protect themselves against aluminum (Al) toxicity is the exudation of organic acids. However, the reasons behind the different types of organic acids that various plant species secrete remain unknown (Li et al., 2009, 2021; Ma, 2000; Ryan et al., 2009). So far, it has been determined that the primary organic acid anions released by plant roots in response to aluminum stress are citrate, malate, and oxalate (Sun et al., 2020b). Prior research has indicated a connection between increased aluminum resistance and the regular release of high concentrations of organic acids (Zheng, Ma & Matsumoto, 1998). Furthermore, studies have shown that enhancing the accumulation of malate and citrate salts in the roots, followed by their exudation, decreases the concentration of aluminum in both roots and leaves, thereby alleviating aluminum toxicity (Yang et al., 2020; Ryan et al., 2009). In our study, we found that when white clover was subjected to aluminum stress, there was a notable increase in the secretion of citrate and malate (Fig. 4). Moreover, exogenous silicon therapy significantly increased the quantities of organic acids, thereby reducing the harmful effects of aluminum poisoning. It is interesting to note that silicon-treated plants exhibited a more pronounced increase in malate secretion under aluminum stress. Previous research has suggested that different mechanisms control the patterns of citrate and malate secretion induced by aluminum, with organic acid anion channels facilitating the rapid secretion of organic acids (Ma, Taketa & Yang, 2000; Li, Ma & Matsumoto, 2000). Additionally, the synergistic impact of Si+B greatly improves the regulation of the ALMT1 and ALMT2 aluminum-activated malate transporter protein genes (Bilal et al., 2022). An anion channel protein unique to plants, the aluminum-activated malate transporter (ALMT) is essential for the efflux of malate to chelate excess Al3+ (Kobayashi et al., 2013). Thus, we speculate that the timely release of malate induced by silicon could be associated with the upregulation of transporter protein gene expression related to organic acid anion channels during silicon absorption by the plant. This, in turn, accelerates the exudation of malate.
According to EDX tests, silicon mainly accumulated in the cortical and epidermal layers, significantly reducing the concentration of physiologically active aluminum and impeding the transit of aluminum (Fig. 5). According to certain research, silicon was transported to the apoplast where it formed silica barriers that helped prevent salt from entering root cells (Khan et al., 2021). Consistently, the primary detoxification process for aluminum involves the formation of hydroxyaluminosilicates (HAS) or aluminosilicates in the cytoplasm (Exley & Birchall, 1993). Additionally, research revealed that aluminum hampered the absorption of elements, causing the concentrations of Ca2+, Mg2+, K+, and P in plant roots to drop (Javaid et al., 2019). Moreover, these inorganic ions were crucial for Al’s detoxification process. According to the study, Mg2+ and K+ can help release organic acid anions and maintain electrolyte balance (Kibria, Barton & Rengel, 2021). By increasing Mg uptake, the overexpression of the Mg transporter gene AtMGT1 reduced Al toxicity (Deng et al., 2006). Zn2+ could stimulate the synthesis of IAA, which is beneficial in reducing Al toxicity and promoting root elongation. It could also control reactive oxygen species (ROS) homeostasis and reduce the formation of peroxidation (Su et al., 2020). In our experiment, we found that under Al stress, the relative weights of Mg2+, K+, and Zn2+ in plant roots also dramatically dropped. Si, on the other hand, partially offset the reduction of ion absorption during Al stress and increased the absorption of exchangeable cations (Mg2+, K+, and Zn2+) under normal conditions (Fig. 6). Similar outcomes were shown when Si was applied to the salt-stressed plant, improving K+, Ca2+, and Mg2+ absorption both with and without salt stress (Javaid et al., 2019). It was clear from this that white clover’s ability to tolerate Al may be linked to the ions balance brought on by more Si.
Conclusion
This study demonstrates that when white clover is subjected to aluminum stress, the application of exogenous silicon provides significant protective benefits and elicits a favorable response. White clover’s normal growth patterns are severely impacted by aluminum toxicity, which is especially noticeable in physiological changes such as relative water content (RWC), cellular membrane stability, Fv/Fm ratios, photosynthetic efficiency, and PIABS measurements. Exogenous silicon treatment significantly increases intrinsic silicon levels in white clover plants exposed to aluminum stress, reduces aluminum buildup, and significantly lessens the damage caused by aluminum exposure to the roots’ epidermis and cortical tissues. Furthermore, in plants exposed to aluminum toxicity, this treatment increases the rate and concentration of malate and citrate exudation. These compounds can bind free aluminum ions and reduce the harmful effects of aluminum poisoning. The use of exogenous silicon has a positive impact on reducing aluminum toxicity by altering the distribution of mineral elements in the tips of white clover roots. Exogenous silicon administration is crucial for managing white clover’s tolerance to aluminum, all things considered. The results of this study suggest the potential of utilizing silicon in a synergistic way to enhance white clover productivity and growth under aluminum stress.
Supplemental Information
Changes of growth parameters on white clover in response to Al toxicity.
Measurements of shoot length, root length, shoot DW and root DW.
Physiological parameters.
Measurements of RWC, Chl, Fv/Fm, PI and MDA
Exogenous Si improves the exudation and concentration of OAs under Al toxicity.
EDX-based microanalysis of mineral elements in the root of white clover.
Relative weight (%) of different mineral elements in epidermal, cortical and stelar layers of the root in white clover plants.