BRCA1 overexpression attenuates breast cancer cell growth and migration by regulating the pyruvate kinase M2-mediated Warburg effect via the PI3K/AKT signaling pathway
- Published
- Accepted
- Received
- Academic Editor
- Bernardo Franco
- Subject Areas
- Cell Biology, Genetics, Molecular Biology, Histology
- Keywords
- BRCA1, PKM2, Breast cancer cell, Glycolysis, PI3K/Akt
- Copyright
- © 2022 Liu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. BRCA1 overexpression attenuates breast cancer cell growth and migration by regulating the pyruvate kinase M2-mediated Warburg effect via the PI3K/AKT signaling pathway. PeerJ 10:e14052 https://doi.org/10.7717/peerj.14052
Abstract
This work explored the mechanism of the effect of breast-cancer susceptibility gene 1 (BRCA1) on the metabolic characteristics of breast cancer cells, including the Warburg effect and its specific signaling. We transfected MCF-7 cells with a BRCA1-encoding LXSN plasmid or PKM2 siRNA and examined cancer cell metabolism using annexin V staining, inhibitory concentration determination, Western blotting, glucose uptake and lactic acid content measurements, and Transwell assays to assess glycolytic activity, cell apoptosis, and migration, and sensitivity to anti-cancer treatment. The BRCA1-expressing MCF-7 cells demonstrated low PKM2 expression and decreased glycolytic activity (downregulated hexokinase 2 (HK2) expression, upregulated isocitrate dehydrogenase 1 (IDH1) expression, and reduced O2 and glucose consumption and lactate production) via regulation of PI3K/AKT pathway compared with the empty LXSN group. BRCA1 transfection slightly increased apoptotic activity, decreased cell migration, and increased the IC50 index for doxorubicin, paclitaxel, and cisplatin. Inhibiting PKM2 using siRNA attenuated the IC50 index for doxorubicin, paclitaxel, and cisplatin compared with the control. Inhibiting PKM2 activated PI3K/AKT signaling, increased apoptosis, and decreased MCF-7 cell migration. Our data suggest that BRCA1 overexpression reverses the Warburg effect, inhibits cancer cell growth and migration, and enhances the sensitivity to anti-cancer treatment by decreasing PKM2 expression regulated by PI3K/AKT signaling. These novel metabolic findings represent a potential mechanism by which BRCA1 exerts its inhibitory effect on breast cancer.
Introduction
Cancer cells are hypermetabolic and rely heavily on “aerobic glycolysis”, which is demonstrated to be the metabolic hallmark of cancer cell (Li et al., 2020a). The conversion of glucose to lactate, which occurs under hypoxic condition in normal cellular environment, is exhibited in cancer cells despite the presence of oxygen, which normally suppresses glycolytic activity. Aerobic glycolysis, known as the Warburg effect, is one of the main metabolic signatures of cancer cells. Recent work have demonstrated that continuous aerobic glycolysis in cancer cell triggers oncogene development or the loss of tumor-suppressor genes. Recent discoveries have identified tumor-suppressor genes related to metabolic pathways, including citrate metabolism, such as sdha/c in paragangliomas and idh1/2 in gliomas (Thompson, 2009). Oncogenes and tumor-suppressor genes regulate cancer metabolism, reflecting the importance of discovering metabolic biomarkers and therapeutic targets (Morvan & Demidem, 2007). Breast-cancer susceptibility gene 1 (BRCA1) is a major tumor-suppressor gene associated with several anti-carcinogenic pathways. BRCA1 is downregulated in many sporadic cases of breast cancer (Miki et al., 1994; Narod & Foulkes, 2004). The molecular profiles of BRCA1-mutation breast cancer are featured by the lack of expression of estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (Yin et al., 2020). Immunohistochemical profiles of the BRCA1-mutation breast cancer are featured by expression of cytokeratin (CK) 5/6 and CK14, contributing to the increasing risk of breast cancer (Rigakos & Razis, 2012). BRCA1 affects the cellular response to stress and senses DNA damage, as well as participates in DNA repair, cell cycle regulation, transcription, ubiquitination, apoptotic activity, and resistance to anticancer drugs (Yarden & Papa, 2006).
In the Warburg effect, pyruvate kinase M2 (PKM2) regulates metabolic pathways. Increased PKM2 expression in cancer cells can enhance glycolytic activity and provide sufficient energy for the rapid growth of cancer cells. In numerous human cancers, PKM2 expression is higher than that in normal tissues (Liu et al., 2018). Knocking down PKM2 in human hepatocellular carcinoma cells inhibited their function and proliferation and increased apoptosis (Xu et al., 2015).
However, the role and mechanism of BRCA1 in cancer cell metabolism remain unclear. BRCA1 has been demonstrated to regulate de novo fatty acid synthesis (Moreau et al., 2006), and protect tumor cells against oxidative stress (Bae et al., 2004). BRCA1 is reported to induce major energetic metabolism reprogramming in breast cancer cells (Privat et al., 2014). To explore this, metabolic and other functional profiles were measured in breast cancer cells expressing BRCA1. Furthermore, this work explored the specific signaling pathway of BRCA1 and whether the glycolytic enzyme PKM2 is involved in the effects of BRCA1.
Materials and Methods
Biological materials
Michigan Cancer Foundation-7 (MCF-7) human breast cancer cells (No. TCHu 74; cell bank of Typical Culture Preservation Committee of Chinese Academy of Science, Shanghai, China) were incubated in high-glucose Dulbecco Modified Eagle Medium (DMEM, No. 11965092; Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, No. 16140071; Gibco, Waltham, MA, USA) at 37 °C under 5% CO2. MCF-7-BRCA1 was stably transfected using a BRCA1-encoding LXSN plasmid (Shanghai GeneChem Co., Ltd., Shanghai, China) (Privat et al., 2009). While control cells (MCF-7-LXSN cell) were transfected with the LXSN empty vector. The transfection effect was measured for BRCA1 level by Western blotting. All experimental procedures related to the treatment of animals were performed adhere to the Animal Care and Use Committee of Ji Ning First People’s Hospital (JNRM-2022-DW-006).
Apoptotic activity
The percentage of apoptotic cells was evaluated via the Annexin V-PE apoptosis detection kit (BD Pharmingen, Franklin Lakes, NJ, USA). After treatment, the specimens were washed using phosphate buffered saline (PBS), treated with annexin V and propidium iodide for 30 min, and measured using flow cytometry (BD FACS Calibur, San Jose, CA, USA).
Inhibitory concentration
The MTS assay was used to measure inhibitory concentrations adhere to the instruction of the CellTiter96® Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA). MCF-7 cells (1 × 104) were planted on 96-well microplates containing DMEM with 1% FBS and LXSN plasmid or siRNA, simultaneously with an anti-cancer agent. The MTS assay was performed after 24 h. The IC50 was determined by extrapolating the log concentration vs cell viability.
Western blotting
MCF-7 cells were lysed in rapid immunoprecipitation assay (RIPA) solution with phosphatase and protease inhibitors, and the concentration of proteins was evaluated via the bicinchoninic acid (BCA) method. Proteins were separated via 8–10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and then loaded onto the nitrocellulose membrane. The membranes were treated with 5% non-fat milk for 90 min and then treated by the primary antibodies (anti-BRCA-1 (No. ab238983, 1:1,000; Abcam, Cambridge, UK), anti-PKM2 (No. ab85555, 1:1,000; Abcam, Cambridge, UK), anti-hexokinase 2 (HK2) (No. ab209847, 1:1,000; Abcam, Cambridge, UK), anti-isocitrate dehydrogenase 1 (IDH1) (No. ab172964, 1:1,000; Abcam, Cambridge, UK), anti-phospho-AKT(Thr308) (No.#13038, 1:1,000; Cell Signaling Technology, Danvers, MA, USA), anti-AKT (No.#4691, 1:1,000; Cell Signaling Technology, Danvers, MA, USA) and anti-β-actin (No. ab8227, 1:1,000; Abcam, Cambridge, UK)) overnight, then incubated with the secondary antibodies (anti-mouse IgG (No. 7076; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) or anti-rabbit IgG (No. 7074; 1:1,000; Cell Signaling Technology, Danvers, MA, USA)) for 1 h. Bands were evaluated using an imaging system (Bio-Rad, Hercules, CA, USA).
Glucose uptake and lactate measurement
Cells were plated in six-well plate (1 × 105/well) at 37 °C under 5% CO2 for 1 day. After washing the wells with PBS, the culture medium was replaced with medium without FBS, and then the supernatant was collected. A glucose and lactate kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was applied to measure glucose consumption and lactic acid release.
Transwell assay
To assess migration, MCF-7 cells were trypsinized and placed in transwell cell culture inserts (Corning Inc., Corning, NY, USA), including non-coated membranes. Then, 1% FBS (600 μL) was added into the lower chamber for 1 day, the upper surface of the membranes was wiped with a cotton tip, and the lower surface were incubated for 30 min using 0.1% crystal violet.
Small interfering RNA (siRNA)
PKM2-specific siRNA was used to knockdown PKM2. In line with the manufacturer’s instruction, MCF-7 cells were transfected using 100 pmol PKM2 siRNA via Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA). At 6 h after transfection, the medium was changed by medium with 5% FBS for 1 day.
Statistical analysis
All results (mean ± SD) were analyzed using SPSS 21.0 (SPSS, Chicago, IL, USA). Two groups with normal distributions were compared via t-tests. Statistical significance was defined as P < 0.05.
Results
BRCA1 overexpression decreased PKM2 expression and increased apoptosis in MCF-7 cells
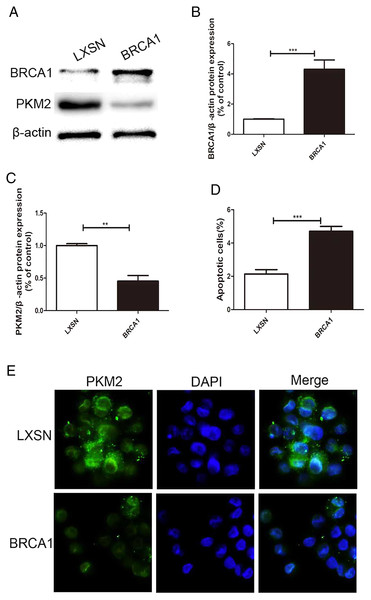
MCF-7 cells were transfected via a LXSN plasmid expressing the BRCA1 protein and showed higher expression of BRCA1 (Figs. 1A, 1B), while Control cells (MCF-7-LXSN cell) were transfected with the LXSN empty vector. The BRCA1-expressing MCF-7 cells exhibited lower PKM2 expression compared with the empty-LXSN group (Figs. 1A–1C), which is consistent with the immunofluorescence results (Fig. 1E). The MCF-7-BRCA1 group showed slightly enhanced apoptotic activity compared with the MCF-7-LXSN group (Fig. 1D).
Figure 1: BRCA1 overexpression decreased the expression of PKM2 and increased apoptosis in MCF-7 cell.
(A–C) Level of BRCA1 and PKM2 was tested by Western blot and quantification analysis. (D) Number of apoptotic cells was measured at 2 days via the annexin V assay. (E) Immunofluorescence imaging of the PKM2 expression and the nucleus (DAPI, blue). Values are represented as mean ± SD, n = 3 (**P < 0.01 and ***P < 0.001).BRCA1 decreased glycolysis
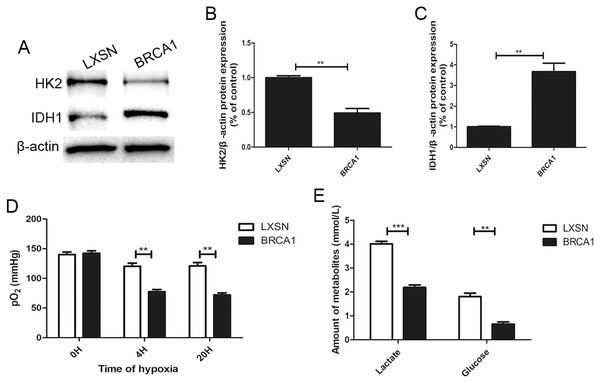
To explore the regulation of glycolysis by BRCA1, the expression of two key glycolytic proteins, HK2 and IDH1, was measured by Western blotting (Figs. 2A–2C). In the BRCA1 group, HK2 expression was downregulated and IDH1 expression upregulated. We analyzed respiration in MCF-7 cells by measuring pO2 in cell culture medium (Fig. 2D). Oxygen is consumed exclusively via the mitochondrial respiratory chain. Under hypoxic conditions, the MCF-7-BRCA1 group consumed more O2 compared with the MCF-7-LXSN group. More glucose was consumed and more lactate released in the MCF-7-LXSN group than in the MCF-7-BRCA1 group (Fig. 2E), indicating that BRCA1 overexpression reduced glycolysis.
Figure 2: BRCA1 decreased activity of glycolysis.
(A–C) Western blotting and quantification analysis of HK2 and IDH1 enzymes. (D) The pO2 was tested in the culture medium under the hypoxic condition for the indicated time. (E) Lactate release and glucose consumption were evaluated in cell culture media by enzymatic colorimetric assays. Values are represented as mean ± SD, n = 3 (**P < 0.01 and ***P < 0.001).BRCA1 overexpression attenuated MCF-7 cell migration
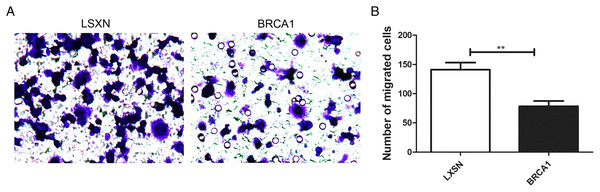
The Transwell assays were used to evaluate the migration ability of MCF-7. Our results showed greater migration in the MCF-7-LXSN group compared with the MCF-7-BRCA1 group (Figs. 3A, 3B), indicating that BRCA1 overexpression attenuates MCF-7 cell migration.
Figure 3: BRCA1 overexpression attenuated the migration of MCF-7 cells.
(A and B) The effects of BRCA1 on migration of MCF-7 cells was measured via transwell method. Values are represented as mean ± SD, n = 3 (**P < 0.01).BRCA1 overexpression in MCF-7 cells resulted in greater sensitivity to anti-cancer agents
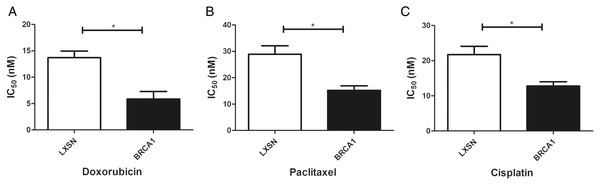
Our work aimed to clarify the role of BRCA1 in the sensitivity to anti-cancer drugs (doxorubicin, paclitaxel, and cisplatin). BRCA1 overexpression significantly increased the IC50 index for doxorubicin, paclitaxel, and cisplatin compared with MCF-7-LXSN cells, indicating that BRCA1 promotes the sensitivity of MCF-7 cells to anti-cancer treatment (Fig. 4).
Figure 4: BRCA1 overexpression in MCF-7 cell shows Higher Sensitivity for anti-cancer treatment.
LXSN group was demonstrated to be less response compared to BRCA1-MCF-7 group to (A) Doxorubicin, (B) Paclitaxel, (C) Cisplatin. LXSN and BRCA1 MCF-7 cells response to treatment was meassured by IC50 for 24h with anti-cancer agents. Values are represented as mean ± SD, n = 3 (*P < 0.05).BRCA1 overexpression reduced glycolytic flux via regulation of PI3K/AKT signaling
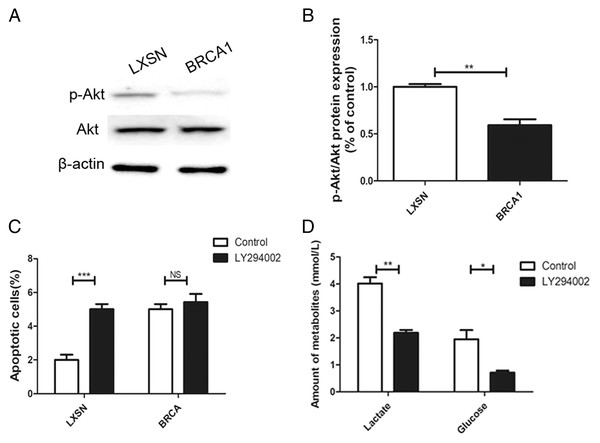
The AKT oncogene promotes the transcription of numerous genes encoding proteins involved in glycolytic signaling (Robey & Hay, 2009). p-AKT level was up-regulated in the MCF-7-LXSN cells than in the MCF-7-BRCA1 cells (Figs. 5A, 5B). AKT inhibition by LY294002 increased the apoptotic MCF-7-LXSN cell population slightly, but not that of MCF-7-BRCA1 cells (Fig. 5C). Inhibiting AKT signaling using LY294002 attenuated lactate release from MCF-7 cells. In the MCF-7-BRCA1 group, inhibiting AKT using LY294002 had no significant effect on glucose consumption or lactate production (Fig. 5D). The results indicated that BRCA1 overexpression contributes to regulation of the PI3K/AKT pathway, decreasing glycolytic flux.
Figure 5: BRCA1 overexpression reduced glycolytic flux by regulation of PI3K/AKT signalling.
(A and B) The PI3K/AKT signaling was measured by Western blot. (C) Number of apoptotic cells was measured at 2 days. (D) Lactate release and glucose consumption were evaluated in cell culture media. Values are represented as mean ± SD, n = 3 (*P < 0.05, **P < 0.01 and ***P < 0.001).Depletion of PKM2 suppressed PI3K/AKT signaling
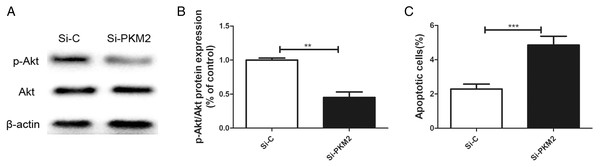
Previously, we reported that BRCA1 reduces PKM2 expression. To explore the effects of PKM2 on cancer-related signaling pathways, we used siRNA to knockdown PKM2 in MCF-7 cells. A lower p-AKT level was seen in cells transfected with PKM2 siRNA compared with control siRNA (Figs. 6A, 6B). Depletion of PKM2 resulted in increased MCF-7 cell apoptosis (Fig. 6C). All results suggested that the PKM2 is involved in apoptotic activity via regulation of PI3K/AKT signaling.
Figure 6: Inhibiting PKM2 activated the PI3K/AKT signalling.
(A and B) The PI3K/AKT signaling was measured by Western blot. (C) Number of apoptotic cells was measured at 2 days via the Annexin V assay. Values are represented as mean ± SD, n = 3 (**P < 0.01 and ***P < 0.001).MCF-7 cells were more sensitive to anti-cancer agents after depletion of PKM2
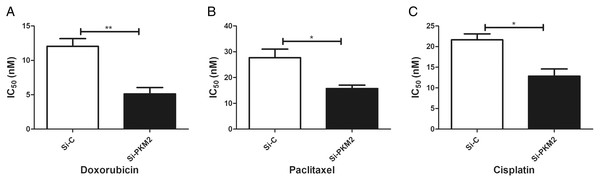
To examine the effect of PKM2 depletion on MCF-7 cells, the sensitivity to anti-cancer agents was measured in cells transfected with PKM2 siRNA. Our results suggest that siRNA-mediated depletion of PKM2 markedly reduced the IC50 index of doxorubicin, paclitaxel, and cisplatin, indicating that PKM2 attenuated the sensitivity of MCF-7 to anti-cancer drugs (Fig. 7).
Figure 7: Inhibiting PKM2 in MCF-7 cell shows higher sensitivity for anti-cancer treatment.
Si-C group is demonstrated to be less response as compared to Si-PKM2 MCF-7 group to (A) Doxorubicin, (B) Paclitaxel, (C) Cisplatin. Si-C and Si-PKM2 MCF-7 cells response to treatment was measured by IC50 for 24 h with anti-cancer agent. Values are represented as mean ± SD, n = 3 (*P < 0.05, **P < 0.01).Depletion of PKM2 attenuated MCF-7 cell migration
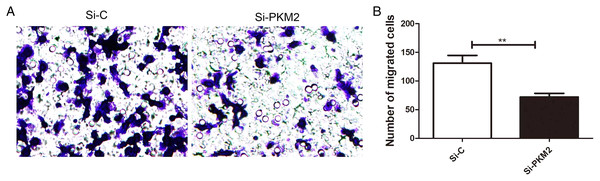
To examine the effect of PKM2 depletion on migration ability of MCF-7 cells, the Transwell assay was measured in cells transfected with PKM2 siRNA. Our results showed that PKM2 siRNA decreased MCF-7 cell migration compared with the control siRNA (Fig. 8), suggesting that PKM2 induces breast cancer cell migration.
Figure 8: Inhibiting PKM2 attenuated the migration of MCF-7 cells.
(A and B) The role of PKM2 in migration of MCF-7 cells was evalutated by transwell method. Values are represented as mean ± SD, n = 3 (**P < 0.01).Discussion
BRCA1 is a major breast cancer suppressor gene with a high mutation rate in hereditary breast cancer. BRCA1 expression is reduced in sporadic breast cancer (Li et al., 2020b; Miki et al., 1994; Narod & Foulkes, 2004). This enzyme orchestrates cellular responses to stress and DNA injury. BRCA1 is involved to DNA repair, cell cycle regulation, transcription, ubiquitination, apoptotic activity, and sensitivity to anti-cancer drugs (Yarden & Papa, 2006). In the 1920s, Warburg found that the rates of glucose uptake and glycolysis were higher in cancer cells than normal cells (Warburg, 1956). The glycolysis product pyruvate is converted mainly into lactic acid, which is discharged from cells to produce a small amount of energy (Warburg, 1956). This phenomenon is called the Warburg effect and is an inefficient way for cells to generate energy. Some researchers believe that the use of glycolysis rather than a more efficient metabolic pathway in cancer cells is closely involved in the occurrence and development of cancer (Zhang, Darshi & Sharma, 2018). Cancer cells acquire energy mainly via the Warburg effect or aerobic glycolysis. Reversing or weakening the Warburg effect has become a focus of cancer prevention and treatment. The PK enzyme controls the rate of the last step of glycolysis. With the lactate dehydrogenase, PKM2 enables the production of lactic acid from pyruvate, promotes aerobic glycolysis, contributes to the biosynthesis of lipids, nucleic acids and proteins, and provides favorable conditions for the proliferation and metastasis of cancer cells (Xiaoyu et al., 2018; Ye et al., 2019). PKM2 deletion in the mammary glands of a BRCA1-loss-driven cancer model did not postpone carcinogenesis (Israelsen et al., 2013). HK2 is upregulated in cancer cells and is the major gene contributing to Warburg glycolysis (Privat et al., 2014). Some glycolysis suppressors targeting HK2 have been used to treat cancer (Mathupala, Ko & Pedersen, 2009). Increased glucose uptake provides energy for glycolysis in cancer cells to compensate for the lack of ATP produced by glycolysis; as a result, cancer cells produce high levels of lactic acid, thereby decreasing the pH of the cell microenvironment (Jia et al., 2018; Ye et al., 2019). The lactic acid content reflects the level of cell glycolysis occurring. We showed that BRCA1-MCF-7 cells expressed less PKM2 and exhibited decreased glycolysis (downregulated HK2 expression, upregulated IDH1 expression, increased O2 and glucose consumption, and increased lactate production) compared with the empty LXSN group. BRCA1 transfection also contributed to enhanced apoptotic activity and decreased cell migration. Chemotherapeutic method is the most predominant strategy for treating solid tumor particularly in metastatic manner (Sharifi et al., 2014). Anti‑mitotic chemotherapy is performed as the first‑line treatment for breast cancer. Paclitaxel combined with B-tubulin and triggers apoptotic signaling via stabilizing microtubule and inducing cell-cycle arrest (Fabbri et al., 2006). Cisplatin interferes with DNA replication, which contributes to the death of fastest proliferating cell. One important issue regarding chemotherapeutic agents is the emergence of chemo resistance to the drug regimen which in turn reduces the efficacy of chemotherapy (Sadava & Kane, 2013). Previous study have demonstrated that reduction of BRCA1 in breast cancer cell can increase the sensitivity of cisplatin and also lead to the resistance of anti-microtubule drugs (such as paclitaxel and vincristine) (Lafarge et al., 2001). BRCA1 is involved in the regulation of cell cycle and apoptotic activity via the c-Jun N-terminal kinase signaling, suggesting that these mechanisms may be related to the resistance of cisplatin (Kennedy et al., 2004). In present work, BRCA1 overexpression significantly increased the IC50 index of doxorubicin, paclitaxel, and cisplatin. These data suggest that BRCA1 has anticancer effects by inhibiting PKM2-mediated glycolysis.
Several pathways are likely involved in the effects of BRCA1 on glycolysis. One is AKT signaling. BRCA1 inactivates the oncoprotein AKT (Xiang et al., 2008), which induces glycolysis via multiple mechanisms, including promoting the expression and activation of hexokinases (e.g., HK2) (Robey & Hay, 2009). The present data suggested that BRCA1 overexpression reduces AKT phosphorylation. The use of LY294002 to inhibit AKT signaling resulted in decreased lactate release from MCF-7 cells. Transfection of BRCA1 in MCF-7 cells led to reduced glycolysis via regulation of the PI3K/AKT pathway.
Genetic research on cancer susceptible constitutions and high-throughput sequencing of cancer cell genomes have shown that metabolic enzyme mutations are critical for the development of cancer (Qi et al., 2018). PKM2 is a key enzyme in glycolysis, which supplies energy for the synthesis of macromolecules required for cell proliferation, such as proteins, lipids, and nucleic acids. PKM2 catalyzes the conversion of phosphoenolpyruvate to pyruvate. Four PK isozymes are known: PKL, PKR, PKM1, and PKM2. Of these, PKM2 is up-regulated in most cancer cells and acts independently of the cancer tissue source (Mazurek, 2011). PKM2 affects the occurrence and development of cancer. Christofk et al. (2008) confirmed that a high PKM2 level promotes aerobic glycolysis in cancer cells. Inhibiting PKM2 using shRNA in a human lung cancer cell line reduced its carcinogenicity, confirming the important role of PKM2 in cancer cell growth (Christofk et al., 2008). Microarray analysis also showed that PKM2, the rate-limiting enzyme of glycolysis, was one of the most upregulated genes in cancer cells (Altenberg & Greulich, 2004). Recent studies have found that the PKM2 level is related to multidrug resistance (Israelsen et al., 2013). For example, cancer cells exposed to oxaliplatin showed upregulated PKM2 expression, which led to oxaliplatin-resistant cancers. Therefore, PKM2 can also be used as an indicator of resistance to anti-cancer drugs such as oxaliplatin (Martinez-Balibrea et al., 2009). PKM2 is a very sensitive cancer marker and may become an important target of anti-cancer drugs. The present work showed that depleting PKM2 using siRNA markedly reduced the IC50 index of doxorubicin, paclitaxel, and cisplatin. Depleting PKM2 also suppressed PI3K/AKT signaling and increased MCF-7 cell apoptosis. Transwell migration assays showed that PKM2 siRNA attenuated the migration of MCF-7 cells compared with control siRNA. Therefore, PKM2 may become a new marker for diagnosing cancer, evaluating treatment efficacy, and determining prognosis.
The limitation is that the details of the interaction between BRCA1 and PKM2 needs further study, and we will continue to explore the mechanism of PKM2 in depth.
Conclusion
Our results suggest that BRCA1 overexpression inhibits the Warburg effect, decreases cancer cell growth and migration, and enhances sensitivity to anti-cancer treatments via downregulation of PKM2 regulated by PI3K/AKT signaling. These novel metabolic effects of BRCA1 are a potential mechanism by which it inhibits breast cancer.