Ability to detect antibodies to beak and feather disease virus in blood on filter paper decreases with duration of storage
- Published
- Accepted
- Received
- Academic Editor
- Laura Brannelly
- Subject Areas
- Molecular Biology, Veterinary Medicine, Virology, Zoology
- Keywords
- Platycercus elegans, BFDV, PBFD, Parrots, Crimson rosella, Haemagglutination inhibition, HI, Serology, Wildlife disease
- Copyright
- © 2021 Blanch-Lázaro et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Ability to detect antibodies to beak and feather disease virus in blood on filter paper decreases with duration of storage. PeerJ 9:e12642 https://doi.org/10.7717/peerj.12642
Abstract
Background
Beak and feather disease virus (BFDV) is a circovirus that infects captive and wild psittacine birds, and is of conservation concern. The haemagglutination inhibition (HI) assay is used to determine antibody titres against BFDV, and the use of dried blood spots (DBS) on filter paper stored at room temperature has been suggested to be an equally valid technique to the use of frozen serum. However, research on other pathogens has found variable results when investigating the longevity of antibodies stored on DBS at room temperature. Consequently, we aimed to test the temporal stability of antibodies to BFDV in DBS samples stored long-term at room temperature. A further goal was to add to the current knowledge of antibody response to naturally acquired BFDV infection in crimson rosellas (Platycercus elegans).
Methods
Blood was collected from wild P. elegans in Victoria, Australia, that had been live-trapped (n = 9) or necropsied (n = 11). BFDV virus load data were obtained from blood stored in ethanol by real-time quantitative PCR (qPCR); antibody titres were obtained by HI assay from either DBS or serum samples, which had been collected concurrently. All HI assays were performed commercially by the Veterinary Diagnostic Laboratory (VDL) in Charles Sturt University, Australia, who were blind to BFDV blood status.
Results
HI titres from DBS stored at room temperature declined significantly over time (~80 weeks). By contrast, frozen serum samples assayed after 80 weeks in storage all had high HI titres, only varying up to one dilution step from the initial HI titres obtained from DBS at 3–6 weeks after sampling. Weak HI titres from DBS samples all came back negative when the test was repeated only nine weeks later. Novel high HI titres were reported in P. elegans, and while most birds with high antibody titres had corresponding negative qPCR results, a single subadult presented with high HI titres and virus load simultaneously.
Conclusion
Detection of antibodies on filter paper stored at room temperature decreases over time, increasing the chances of false negatives in these samples, and in repeated testing of samples with weak HI titres. Consequently, serum should be the preferred sample type to use for seroepidemiological studies on BFDV in parrots and other bird species. When not possible, it may help to store DBS on filter paper at −20 °C or lower. However, prompt testing of DBS samples (e.g., <6 weeks in storage) is recommended pending further research on antibody temporal stability. We also show that P. elegans, especially adults, can produce high antibody titres against BFDV, which may help them resist infection.
Introduction
Beak and feather disease virus (BFDV) is a small single-stranded circular DNA virus from the Family Circoviridae (Todd, 2000). It causes psittacine beak and feather disease (PBFD), which in some bird species may present as a chronic and eventually fatal disease. Common clinical signs include symmetric feather loss with growing of abnormal feathers, beak and claw deformity, and immunosuppression (Pass & Perry, 1984). PBFD threatens psittacine birds in captivity and in the wild, and is considered of conservation concern in Australia and globally (Australian Department of the Environment & Heritage, 2005; Raidal, Sarker & Peters, 2015).
Since initial description of BFDV in Australia (Pass & Perry, 1984), several diagnostic tests have been developed and used to detect either virus or antibodies to BFDV in birds (Ritchie et al., 1991; Ypelaar et al., 1999; Fogell, Martin & Groombridge, 2016). Although serum samples are considered more reliable for antibody testing, dried blood spots (DBS) have become widely used for serological studies in a range of different pathogens (including BFDV) and in both wildlife (Khalesi et al., 2005; Wasniewski et al., 2014; Carlsson et al., 2019; Martens et al., 2019) and humans (Corran et al., 2008; Aston et al., 2014; Smit et al., 2014). A key reason is that they facilitate easy and cost-effective field collection, storage and transport (e.g., do not require refrigeration) (Curry et al., 2011). Whilst the effect of storage time and conditions on detecting antibodies on DBS has been studied for several pathogens, such as avian infectious bronchitis virus (Lana, Marquardt & Snyder, 1983), Onchocerca volvulus (Rodríguez-Pérez et al., 1999), avian influenza virus (Dusek et al., 2011) and Yersinia pestis (Bevins et al., 2016), it has not been tested for BFDV. Findings from such studies on pathogens other than BFDV have validated the use of DBS for antibody detection, but have provided conflicting evidence concerning the extent to which antibody longevity is affected by storage duration and conditions (Smit et al., 2014; Amini et al., 2021). For example, Rodríguez-Pérez et al. (1999), using an enzyme-linked immunosorbent assay (ELISA) to detect antibodies to the parasite Onchocerca volvulus, reported that DBS stored for 3 months at different temperatures (room temperature, 4 °C, −20 °C or −70 °C) had the same detection rate as frozen serum. However, they found that the ability to detect antibodies on DBS, started to decrease after 7 months of storage (longest storage tested), as two out of 21 samples stored at room temperature, and one out of 21 at −70 °C, fell below the detection threshold when tested again. These results were similar to Dusek et al. (2011) who reported a consistent detection rate of antibodies against avian influenza using frozen sera and DBS at room temperature after 3 months of storage, but at the same time detected a decrease in the absorbance ratio by the blocking ELISA in DBS compared to sera when tested both at 1 and 3 months post collection. In contrast, Bevins et al. (2016), using a passive hemagglutination assay to detect antibodies to the bacteria Yersinia pestis in coyotes, found that DBS stored at room temperature began to degrade 10 weeks post-collection. Antibodies were still detectable in some DBS samples stored at room temperature at week 104, but none were detected after that at weeks 130 and 155. However, DBS stored at −20 °C with a desiccant pack still had detectable antibody even when tested on week 155 (~3 years). Some studies suggest that when storing samples for longer than a few weeks for serology testing, freezing DBS at –20 °C might help extend sample integrity (Corran et al., 2008; Bevins et al., 2016; Amini et al., 2021). Variation between pathogens and assays might be the reason behind such variation, and therefore it is important to study the influence of storage duration and conditions for each pathogen and serology assay.
We used the haemagglutination inhibition (HI) assay, which is currently the leading assay to detect antibodies to BFDV (Fogell, Martin & Groombridge, 2016). Although a blocking ELISA was developed to aid with serodiagnosis of BFDV (Shearer et al., 2009), this method is yet to be widely adopted and HI remains the gold standard for this virus (Fogell, Martin & Groombridge, 2016). Using DBS on filter paper has been suggested to be an equally valid technique to using serum, for detecting and quantifying antibodies to BFDV via HI (Riddoch, Raidal & Cross, 1996). Furthermore, it has been widely assumed that storage time for DBS samples at room temperatures does not affect the antibody results. However, this assumption has not been tested for BFDV, despite its important implications. In studies investigating antibody titres to BFDV, findings from DBS have been used interchangeably with those from serum (Khalesi et al., 2005; Shearer et al., 2009). None of these studies mentioned how long DBS were stored for, which further hampers understanding of the effects of storage duration on HI results and underscores the tendency to neglect this issue in BFDV research. We set out to investigate this using samples from wild crimson rosellas (Platycercus elegans) collected as part of a broader study on BFDV ecology in this host species (Eastwood et al., 2014; Martens et al., 2019).
P. elegans is an abundant parrot occurring in eastern and south-eastern Australia (Higgins, 1999). High BFDV prevalence has been reported in apparently healthy wild P. elegans in Australia (Eastwood et al., 2014, 2015; Martens et al., 2020a), but only two studies (Eastwood et al., 2014; Martens et al., 2019) have documented their immune response to BFDV infection, due largely to the difficulty and expense of the HI assay needed. Currently in Australia the HI assay is only performed by the Veterinary Diagnostic Laboratory (VDL) at Charles Sturt University (CSU). The above two studies, using that laboratory, reported that P. elegans lack or only produce low antibody titres against BFDV (Eastwood et al., 2014; Martens et al., 2019). According to previous work on different psittacine species, most clinically normal birds with BFDV infection have a positive HI titre (Raidal, Sabine & Cross, 1993), while clinically sick birds do not have detectable antibodies on serum, and only occasional sick birds developed low titres (Riddoch, Raidal & Cross, 1996). Moreover, seroprevalence of up to 94% has been reported in wild and clinically normal psittacine birds (Raidal, McElnea & Cross, 1993).
Given the uncertainty regarding the influence of storage duration at room temperature for antibody detection using DBS, and the ubiquity of HI testing using this sample type for BFDV research and diagnostics, the main aim of this study was to use HI assay to assess the stability of antibodies stored long-term as DBS on filter paper at room temperature. A further goal was to add to the current knowledge of antibody response to naturally acquired BFDV infection in P. elegans.
Materials & methods
Sample collection
This work was conducted under Australian Bird and Bat Banding Scheme authority number 2319 and complied with the animal ethics permit (B32-2018) approved by Deakin University and the research permit approved by the Victorian Department of Environment, Land, Water and Planning (permit number 10009105).
Blood was collected from 20 wild crimson rosellas (Platycercus elegans) from south-east Australia. Of these, nine were live-trapped, sampled and released between 12 April and 28 August 2019 from field sites in Bellbrae (S38°33′ E144°18′, n = 7) and Meredith (S37°85′ E144°11′, n = 2), Victoria, Australia, using baited walk-in traps (Martens et al., 2020a). One bird was recaptured once 4 months after the first capture (B3) and thus sampled a second time (B3r); all other live-trapped birds were sampled once (thus giving n = 10 samples). Each bird was weighed, banded, aged and blood was collected from the brachial vein (Eastwood et al., 2014). Blood spots were collected on filter paper (Whatman®; Sigma Aldrich, St. Louis, MO, USA) for HI testing. When sufficient blood volume was available, paired serum samples were also collected (n = 8 samples; Table S1) for HI testing; for this, blood was stored at 4 °C and centrifuged within 3 h of collection to obtain serum, which was then stored at −80 °C until testing (65–84 weeks later) (Stokes et al., 2020). To test the presence of circulating virus in each bird at the time of capture, approximately 100 µL of blood was also collected and stored in 90% ethanol at room temperature for testing with real-time quantitative PCR (qPCR) (Owen, 2011; Eastwood et al., 2015).
An additional 11 recently killed birds were collected from licensed culling of P. elegans as orchard pests in central Victoria, within 200 km of the other field sites, between 17 May 2019 and 24 June 2020. These carcasses were stored individually in resealable plastic bags and refrigerated at 4 °C until necropsy, which was done within 30 h of death (Table S1). At time of necropsy, blood was collected directly from the heart and stored both on filter paper and in ethanol, as described above, for HI and qPCR assays. Collection of serum samples was not possible from the 11 necropsied birds.
To minimize the chance of virus transmission and sample cross-contamination, all sampling materials were single-use, all cotton bags used to hold live birds were autoclaved in between uses, and F10 SC Veterinary Disinfectant (Health and Hygiene Pty. Ltd., Randburg, South Africa) was used to sanitize equipment used after each bird to reduce transmission risk (Stanford, 2006; Martens et al., 2019).
DBS storage and HI assays
For each sampling event (n = 21), we collected three to five blood spots on filter paper (Whatman®; Sigma Aldrich, St. Louis, MO, USA) which were then air dried and stored in individual zip lock plastic bags in a dark storage box at constant room temperature (~20 °C, in the laboratory at Deakin University) until HI testing. DBS samples were stored between 3 and 70 weeks before being tested by HI for the first time (initial assay). DBS samples were grouped by the date they were tested at the VDL using HI, where HI assay 1 was tested on 24th May 2019, HI assay 2 on 21st September 2020 and HI assay 3 on 26th November 2020 (Table S1). All samples initially tested during HI assay 1 (live-trapped birds) or HI assay 2 (necropsied birds) were retested during HI assay 3 (November 2020). During HI assay 3, we also tested four DBS samples (birds B3r and B7-9) for the first time, after 65–84 weeks in storage, where we used paired serum samples to compare the results with DBS at that time point. When retesting samples during HI assay 3, we always used a different blood spot that had been collected during the same collection event and on the same filter paper. While samples from live-trapped birds were retested ~79 weeks after HI assay 1 (May 2019), DBS from necropsied birds were only retested ~9.5 weeks after HI assay 2 (September 2020). When available, paired frozen serum samples were also tested during HI assay 3 (Table S1).
To investigate the presence of BFDV antibodies, DBS on filter paper and available paired serum samples were sent to the Veterinary Diagnostic Laboratory (VDL), Charles Sturt University, in Wagga Wagga, New South Wales, Australia and processed commercially. Filter papers were transported at room temperature, while serum samples were transported overnight by courier from Deakin University in Geelong, Victoria, Australia, packed with frozen gel packs. After the samples arrived, HI assays were carried out by staff at the VDL, as described in Riddoch, Raidal & Cross (1996) for DBS and in Raidal, Sabine & Cross (1993) for serum. In brief, serum was obtained from eluting a DBS in 100 µL of 5% acid washed kaolin for 1 h at room temperature or overnight at 4 °C. Then, 50 µL of serum (from either sample type) were added to 50 µL of 10% normal galah erythrocytes and left to haemadsorb at 4 °C overnight. The haemadsorbed serum was added to 50 µL of phosphate buffered saline (PBS) in a microtitre plate well (Eppendorf), and serial dilutions were made across each row, and including a positive and negative control row in each batch. HI titres were obtained from twofold serial dilutions, starting at 1:20. Results were reported as the highest dilution of BFDV antibodies that cause complete inhibition of the hemagglutination. We considered a sample negative when antibody titres were undetectable (<1:20). Samples were supplied to VDL with a unique identifier and assayed blind to storage time and BFDV infection status by qPCR.
DNA extraction and real-time quantitative PCR
For blood samples stored in ethanol that were obtained from the live-trapped birds, DNA was extracted using an ammonium acetate extraction method (Eastwood et al., 2015). For blood samples collected from necropsied birds, DNA was extracted using the Mechanical Lysis Module of the MagMAX™ CORE Nucleic Acid Purification Kit (Applied Biosystems, Austin, TX, USA). We followed the low-input workflow from the manufacturer’s protocol for the Mechanical Lysis Module, with a slight modification of the starting product, using instead a mix of 0.1 mL of blood in ethanol with 0.3 mL of ultrapure water. These samples were run in the KingFisher™ Flex (ThermoScientific, Waltham, MA, USA) extraction robot with the “MagMAX Core Flex with heat” script. To ensure DNA had been properly extracted from each sample, the purified nucleic acid was quantified using the QIAxpert system (Qiagen, Hilden, Germany). To each extraction plate we added a known BFDV positive sample and PBS as positive and negative extraction controls, respectively. The positive control was obtained from a sulphur-crested cockatoo (Cacatua galerita) that was diagnosed with PBFD based on advanced clinical signs and subsequently confirmed by qPCR.
For BFDV detection we followed the probe-based qPCR method described by Eastwood et al. (2015), with minor modifications for the necropsied subset. Primers, probe and master mix were the same as described in previous studies (Eastwood et al., 2015; Martens et al., 2020a). The final reaction volume was 10 µL and contained 2 µL of extracted DNA, 1 µL of each primer (9 µM stock forward and 3 µM stock reverse; Applied Biosystems, Waltham, MA, USA), 5 µL of Brilliant Multiplex qPCR master mix (Agilent Technologies, USA) and 1 µL of probe (1 µM stock; Integrated DNA Technologies, Coralville, IA, USA). A standard curve with known BFDV positive quantities and molecular grade water were used as positive and negative controls, respectively. The thermal cycling conditions were followed as described by Eastwood et al. (2015), which includes an initial denaturation of 10 min at 95 °C, followed by 40 cycles of 30 s at 95 °C, 1 min at 60 °C and 30 s at 72 °C, and a final extension step of 5 min at 72 °C. Each sample was run in duplicate, and repeated if the quantification cycle (Cq) value differed more than one cycle between duplicates (Eastwood et al., 2015, 2017; Martens et al., 2019). We set a conservative detection limit for BFDV, where Cq values greater than 36 were considered negative, following Martens et al. (2019, 2020b). All qPCR assays were performed before DBS were sent to VDL.
Statistical analysis
Statistical analyses were performed in R (v4.0.0) on RStudio (v1.3.959). We ran a generalized linear model (GLM) for the live-trapped subset. The response variable was the HI titre value in log2-form, and predictors were duration of storage in weeks (scaled), sample type (paper or serum), the two-way interaction between duration and sample type, and a factor which denoted whether an assay was the initial (i.e., results from HI assays 1 and 2) or repeat (i.e., results from HI assay 3) date a sample was assayed. Bird ID was not included as a random factor as variance was close to zero. Post-hoc tests were performed to compare sample type and initial/repeat assay, for which bird ID was added as random effect factor only when comparing results from filter paper from HI assay 1 and serum, as variance was higher. For the data from necropsied subset, we ran a generalised linear mixed model (GLMM). The response variable was the HI titre value in log2-form, and predictors were “duration of storage in weeks” (scaled), initial/repeat assay and the two-way interaction. Bird ID was included as a random factor. We used a backward stepwise procedure, starting by removing the interaction and followed by the duration of storage. For all models, we used gamma distribution (link identity). Graphs were created using GraphPad Prism (v8.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com).
Results
Detection of BFDV by qPCR
Amongst the live-trapped birds (n = 9), we had one bird recaptured and sampled a second time 17 weeks later (B3r); thus, we had n = 10 samples collected from n = 9 individuals. BFDV circulating in blood was only detected in one bird from this subset by qPCR (Cq = 25). Amongst the necropsied birds (n = 11), three birds were BFDV positive in blood by qPCR (Table S1).
Detection of antibodies by HI
DBS on filter paper were assayed for antibodies by HI from all n = 21 P. elegans samples. Only eight serum samples, out of the 10 collection events from live-trapped birds, were available for HI assays (Table S1).
Antibodies in samples from live-trapped birds
The effect of duration of storage was significantly different depending on whether the sample type was DBS or serum (GLM: t = 2.28, p = 0.034). DBS from six birds were tested within 3–6 weeks after collection during HI assay 1 (initial assay). They were all positive and had high antibody titres, between 1:160 and 1:640 (Fig. 1, Table S1). Titres from a second blood spot on the same filter paper and stored for longer at room temperature, together with four extra samples (B3r and B7-9) collected between April-August 2019, declined significantly with the duration of storage (GLM: t = −11.03, p < 0.001). At HI assay 3 (repeat assay), after being stored for 65–85 weeks (Fig. 1, Table S1), all DBS samples came back negative.
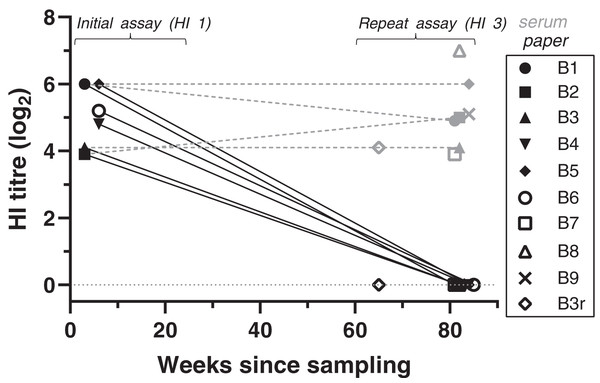
Figure 1: Antibody titres from haemagglutination inhibition (HI) assays in relation to duration of storage for samples from live-trapped birds.
Each symbol indicates an individual bird tested at one time point. B3r indicates samples obtained during recapture of B3. Antibody titres are shown in log2 form (e.g., 1:20 = log21, 1:40 = log22) for both filter paper (black) and serum (grey) samples tested; the lines between samples indicate that the samples belong to the same bird, rather than a linear decline of antibody titres. “Weeks since sampling” indicates the number of weeks that each sample was stored for prior to testing. For “initial assay” only filter paper samples were tested (3–6 weeks post-sampling); for “repeat assay” both serum (when available) and filter paper samples were tested (81–85 weeks post-sampling). Single dots represent samples tested only once in “repeat assay” (for both filter paper and serum) where we compared the results between filter paper stored at room temperature and frozen serum samples, both stored for the same duration (~80 weeks).At HI assay 3, we also tested the available frozen serum samples from eight of the same birds and the same collection event. HI results for serum samples were all strong positives, with titres ranging from 1:160 to 1:1280 (Fig. 1, Table S1). Titres were significantly higher in serum samples compared to the filter paper that was tested at the same time (GLM: t = 19.78, p < 0.001). Antibody titres obtained from frozen serum during HI assay 3 were either the same or varied up to a twofold dilution step from the antibody titres obtained from the DBS samples at the initial assay (GLMM: t = 0.47, p = 0.637).
BFDV blood status by qPCR and HI titres for the recaptured bird (B3r) did not vary between collection events, which were 17 weeks apart. Bird B7 was the only subadult tested in the live-trapped subset, and it stood out from the rest as it showed a high antibody response (1:160) at the same time that qPCR results showed a high amount of virus circulating in blood (Cq = 25) (Table S1).
Antibodies in samples from necropsied birds
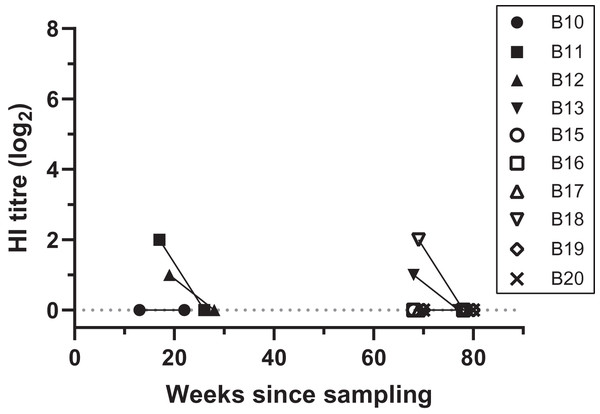
At HI assay 2 (initial assay), DBS from 11 birds necropsied during 2019–2020 were assayed by HI at VDL. Therefore, the storage duration prior to the HI assay ranged between 13–19 weeks for 2020 birds, and 68–70 weeks for 2019 birds (Table S1). Four birds (4/11, 36.4%) had low antibody titres, ranging between 1:20 and 1:40. From these positive birds, two DBS samples that came back with a 1:20 and 1:40 titre had been stored for 68 and 69 weeks, respectively (Fig. 2; Table S1). All samples came back negative for antibody titres when retested 9 weeks later during HI assay 3 (GLMM: t = −2.06, p = 0.039) (Fig. 2; Table S1). Three of the 11 necropsied birds had detectable BFDV by qPCR, including bird B13 that had a low virus load (Cq = 35) at the same time as a low antibody titre (1:20) was detected in HI assay 2 (Table S1).
Figure 2: Antibody titres from haemagglutination inhibition (HI) assays in relation to duration of storage for samples from necropsied birds.
Each symbol indicates an individual bird tested at one time point. Antibody titres are shown in log2 form (e.g., 1:20 = log21, 1:40 = log22) for filter paper samples assayed in each HI assay date; the lines between samples indicate that the samples belong to the same bird, rather than a linear decline of HI titres, and connects samples between HI assays 2 and 3. “Weeks since sampling” indicates the number of weeks that each sample was stored for prior to HI testing. Each HI assay tested samples collected both during 2019 (13–28 weeks post-sampling) and 2020 (68–80 weeks post sampling); HI assay 2 and 3 were performed 9.5 weeks apart.Discussion
For accurate seroepidemiological research it is necessary to ensure the quality and viability of samples. When working with wildlife in the field it can be beneficial to collect samples in ways that do not require a centrifuge or a portable freezer, which are essential for preserving serum samples. Consequently, alternative methods like the storage of DBS on filter paper have been developed to cover such needs (Corran et al., 2008; Smit et al., 2014; Wasniewski et al., 2014). This method is widely used in the study of BFDV by HI assay, and the assumption is widely made that storage duration does not affect the ability to detect antibodies on filter paper. Our study provides the first assessment of the effect of long-term storage of DBS at room temperature in detecting antibodies to BFDV by HI assay in P. elegans.
Using samples from wild caught P. elegans, and the only laboratory in Australia where HI assay is done commercially, we investigated if the results of HI testing varied with duration of storage of DBS at room temperature. Contrary to what was widely assumed, and what we expected by following published literature on antibody testing for BFDV using DBS (Riddoch, Raidal & Cross, 1996; Khalesi et al., 2005; Shearer et al., 2009; Martens et al., 2019), we found that known positive DBS samples for antibodies to BFDV had undetectable antibody titres (<1:20) after storage at room temperature for ~80 weeks or longer. This suggests that DBS samples stored at room temperature for similar periods or longer are likely unreliable for seroepidemiological studies on BFDV. However, it is important to notice that much shorter storage periods may also be unreliable when considering titre or initially weak positive samples. Although we had two DBS samples with low antibody titers at 68 and 69 weeks (1:20 and 1:40 respectively), another known positive sample returned negative after 65 weeks of storage. DBS samples tested within 3 to 6 weeks of collection had high antibody titres (1:160 to 1:640) to BFDV. Taken together, this suggests that our methods (blood collection and initial storage at room temperature) were unlikely to be the cause of negative results during HI assay 3, when we retested DBS samples after long-term storage at room temperature.
Based on the data we currently have and arising from the lack of testing between HI assays 1 and 3, we can only conclude that there was a decline of antibody detection in DBS on filter paper that had occurred by ~65–80 weeks of storage; antibodies were not reported in any of the DBS samples tested at that time (HI assay 3). Although we lacked serum data from the initial assay, HI titres hardly varied between DBS from HI assay 1 and serum from the HI assay 3, which suggests that frozen serum was a reliable sample to compare with the DBS after long-term storage. Based on available information, we would suggest the use of frozen serum whenever possible, and that any DBS samples should be tested within 6 weeks of collection. If using DBS, it may help to store filter paper at −20 °C or lower (Corran et al., 2008; Bevins et al., 2016; but see Rodríguez-Pérez et al., 1999), and/or using desiccants to reduce potential degradation damage caused by excess humidity (Aston et al., 2014; Smit et al., 2014). Our results should therefore be interpreted with some caution, and further work is needed to determine which DBS storage length and conditions provide the best quality antibody results for BFDV when using serum is not feasible.
This is also the first report, to our knowledge, of high antibody titres (up to 1:1280) against BFDV in P. elegans. Previous work in this species reported either lack of antibody production, or weak antibody titres ranging between 1:20 and 1:80 (Eastwood et al., 2014; Martens et al., 2019). Both those studies used DBS on filter paper and the same VDL at CSU for HI testing. Consequently, the duration of filter paper storage might explain their low reported levels of antibody titres. Although storage duration was not indicated in those papers, going back to records in our group it seems that Eastwood et al. (2014) had storage at room temperature of >120 weeks, and Martens et al. (2019) had storage of 40–120 weeks. Thus, our interpretation above about the duration after which DBS may be unreliable is consistent with their results. Our findings reported here show that P. elegans can produce high antibody titres, which may help fight and clear infection without developing clinical signs. If this is the case, then P. elegans may be more similar than previously supposed to other psittacine species, such as sulphur-crested cockatoos and galahs (Eolophus roseicapillus), which can have high HI titres and be clinically normal, while clinically PBFD affected birds usually have high virus loads or haemagglutination (HA) titres without detectable antibodies (Raidal & Cross, 1994). However, P. elegans still appears distinct from those species, in that clinical signs of disease are not observed in the wild. One hypothesis is that P. elegans are able to produce an immune response to BFDV, and thereby recover from BFDV infection, as suggested for other psittacine species (Raidal, McElnea & Cross, 1993); another hypothesis could be that higher mortality of birds infected with BFDV (as juveniles and subadults) results in the lower BFDV prevalence reported in adults (Eastwood et al., 2014, 2019). Further work needs to be done to distinguish between these hypotheses.
We note the striking results of one apparently healthy subadult P. elegans (B7), which had high virus load (Cq = 25) in blood and a high antibody titre (1:160). The high levels of both antigen and antibody detected in this bird could indicate that there are high levels of circulating immune complexes, with an excess of free antibodies to BFDV. While immune complexes are considered a natural and effective response of the immune system required to clear pathogens, an excess of antibodies can trigger the deposit within capillary walls in various tissues and contribute to disease (Wellenberg et al., 2004; Wang & Ravetch, 2015). Based on our current data we cannot conclude if there was something unique to this animal, or if this result might be generalizable to more subadults. Further studies on the immune response to BFDV infection are needed to better understand how P. elegans, and potentially other psittacine species, respond to BFDV infection.
For the necropsied birds, only weak antibody titres were detected using DBS on filter paper. The general low antibody titres from this subset could be explained by: (1) the antibodies degraded during long-term storage on filter paper at room temperature; (2) the antibodies degraded faster in blood collected from the heart due to potential microbial contamination; or (3) the antibodies degraded faster due to potential accumulation of humidity during storage of the samples. Further work is needed to test these hypotheses. Samples from necropsied birds were only tested for the first time after 13 weeks of storage for samples collected in 2020, and after 68 weeks of storage for samples collected in 2019, which means we did not have initial antibody titres (i.e., tested within the first 6 weeks of storage) from these birds. Consequently, we could only test for an effect of HI assay number (initial/repeat assay), but not duration of storage. While we can trust the low antibody titres were true positives, we cannot exclude the possibility that seronegative birds had had detectable antibody titres at time of collection, which had degraded with time. The one bird (B13) that presented with low positive antibody titre and virus load in blood, suggests that a normal antibody response may have been developing simultaneously while virus was still circulating in blood (Martens et al., 2019).
Conclusions
Contrary to the assumptions prevailing over the last three decades of BFDV testing, our results show that stability of antibodies to BFDV in DBS on filter paper decreases over time of storage at room temperature. We show that if DBS on filter paper is stored for lengthy periods (~65–80 weeks, and possibly shorter periods) at room temperature, false negative or weak positive results can arise. We suggest that antibody titres will be most reliable when tested using relatively fresh DBS, or better still, using serum that has been stored at −20 °C or below. We recommend that samples collected on filter paper, with the aim of HI testing, should be tested as soon as possible for best HI results. Alternatively, for longer storage periods, freezing samples on filter paper may be considered, as suggested for other systems by Corran et al. (2008) and Bevins et al. (2016), although this remains to be tested for BFDV and the HI assay. Our results also show that P. elegans are capable of producing high BFDV antibody titres, and therefore might be more similar to other psittacine species than previously suggested. Further studies are needed to better understand the impact of storage conditions on DBS sample viability and provide validated storage guidelines to detect antibody titres to BFDV. Doing so should help improve the capacity for future studies to advance knowledge of BFDV immunology, a virus with globally significant conservation and animal welfare implications.
Supplemental Information
HI assay results for both live-trapped and necropsied birds.
Antibody titres are shown for both filter paper and serum samples. Blank cells indicate those samples were not tested at that date. “Time death to sampling” indicates the time that the bird was dead before samples were collected from it (not applicable to live-trapped birds). “BFDV in blood” indicates qPCR results for blood stored in ethanol (Cq values shown for positive samples (Cq<36), and “neg” indicates BFDV DNA was not detected). “Weeks in storage” shows the weeks that each sample was stored for prior to HI testing. In grey and bold we highlight where antibody titres were detected in at least one sample type for each HI assay. B3r indicates samples obtained during recapture of B3. Each sample (except for B3r and B7-9) was assayed in either HI assay 1 or 2 (initial assay), and retested in HI assay 3 (repeat assay). Serum, when available, was only tested during HI assay 3 (“-“ indicates serum was not available).